Abstract
Several triazole-containing chemicals have previously been shown to act as efficient inhibitors of cytochrome P450 monooxygenases. To discover a strigolactone biosynthesis inhibitor, we screened a chemical library of triazole derivatives to find chemicals that induce tiller bud outgrowth of rice seedlings. We discovered a triazole-type chemical, TIS13 [2,2-dimethyl-7-phenoxy-4-(1H-1,2,4-triazol-1-yl)heptan-3-ol], which induced outgrowth of second tiller buds of wild-type seedlings, as observed for non-treated strigolactone-deficient d10 mutant seedlings. TIS13 treatment reduced strigolactone levels in both roots and root exudates in a concentration-dependent manner. Co-application of GR24, a synthetic strigolactone, with TIS13 canceled the TIS13-induced tiller bud outgrowth. Taken together, these results indicate that TIS13 inhibits strigolactone biosynthesis in rice seedlings. We propose that TIS13 is a new lead compound for the development of specific strigolactone biosynthesis inhibitors.
Keywords: Chemical library, Inhibitor, P450, Rice, Strigolactone, Triazole
Introduction
Strigolactones (SLs) were first identified in root exudates of plants as seed germination stimulants of parasitic weeds such as Orobanche and Striga (Cook et al. 1966), and since then they have been recognized as a group of terpenoid lactones. SLs also induce hyphal branching in arbuscular mycorrhizal fungi that form symbiotic associations with the roots of >80% of land plants (Akiyama et al. 2005). More recently, two groups reported that SLs, or their metabolites, inhibit shoot branching (Gomez-Roldan et al. 2008, Umehara et al. 2008).
Genetic analysis of a series of branching mutants, more axillary growth (max) mutants of Arabidpsis, ramosus (rms) mutants of pea, particular dwarf (d) or high tillering dwarf (htd) mutants of rice and decreased apical dominance (dad) mutants of petunia, revealed that SL biosynthesis involves two carotenoid cleavage dioxygenases, CCD7 (MAX3, RMS5 D17/HTD1, DAD3) and CCD8 (MAX4, RMS1, D10, DAD1), one cytochrome P450 monooxygenase (MAX1) and one novel iron-containing protein (D27) (Drummond et al. 2009, Beveridge and Kyozuka 2010). However, the SL biosynthesis pathway still needs to be uncovered (Rani et al. 2008).
Biosynthetic inhibitors of biologically active substances can control their endogenous levels in various plant species, occasionally in a developmental stage- and tissue-specific manner. In addition, even if a mutation or targeted knock-out of an individual gene in sets of paralogous genes could hardly affect phenotypes, biosynthetic inhibitors can overcome such gene redundancy in many cases. Therefore, the use of specific biosynthesis inhibitors is an alternative and valuable way to determine the physiological functions of endogenous substances. For example, abamine, an inhibitor of 9-cis-epoxycarotenoid dioxygenase in ABA biosynthesis, significantly contributed to the recent findings that ABA plays a role in the control of the number of nodules on the roots of leguminous plants (Han et al. 2004a, Suzuki et al. 2004) and in the high-light response in Arabidopsis (Galvez-Valdivieso et al. 2009). Studies using fluridone, an inhibitor of the carotenoid biosynthesis pathway, have shown that carotenoid biosynthesis is necessary for the biosynthesis of normal levels of SLs (Matusova et al. 2005, López-Ráez et al. 2008). However, fluridone inhibits the biosynthesis of all carotenoids and carotenoid-derived metabolites and causes photodestruction of chlorophyll and lethal damage. Therefore, fluridone is not an ideal inhibitor to study the biological roles of SLs in plants. As has been the case with brassinosteroid (BR) and ABA biosynthesis inhibitors (Asami et al. 2000, Asami et al. 2003, Han et al. 2004b, Kitahata et al. 2006), specific SL biosynthesis inhibitors would be useful tools for biochemical studies of SL biosynthesis and for better understanding of the biological roles of SL in plants. Moreover, SL biosynthesis inhibitors can be applicable for the identification and characterization of new SL signal transduction and/or biosynthesis mutants. In this context, we have started to search for SL biosynthesis inhibitors.
Recombinant AtCCD7 cleaves multiple carotenoid substrates (Booker et al. 2004, Schwartz et al. 2004). Recently, it was reported that some hydroxamic acid analogs inhibited the activity not only of AtCCD7 but also of many other CCDs, in Escherichia coli (Sergeant et al. 2009). In addition, CCD7 also plays a role in the production of some mycorrhiza-induced apocarotenoids in tomato (Vogel et al. 2010), suggesting that CCD7 inhibitors may affect the production of other apocarotenoids. Besides CCDs, another target enzyme class for developing SL biosynthesis inhibitors is cytochrome P450 monooxygenases (P450s); at least one P450 (CYP711A) is involved in SL biosynthesis. Towards this goal, we screened a chemical library consisting of potential inhibitors of P450s for SL biosynthesis inhibitors and discovered a new lead compound that is able to decrease SL levels in rice seedlings.
Results
Screening for triazole-type chemicals inducing SL-deficient mutant-like morphology in rice
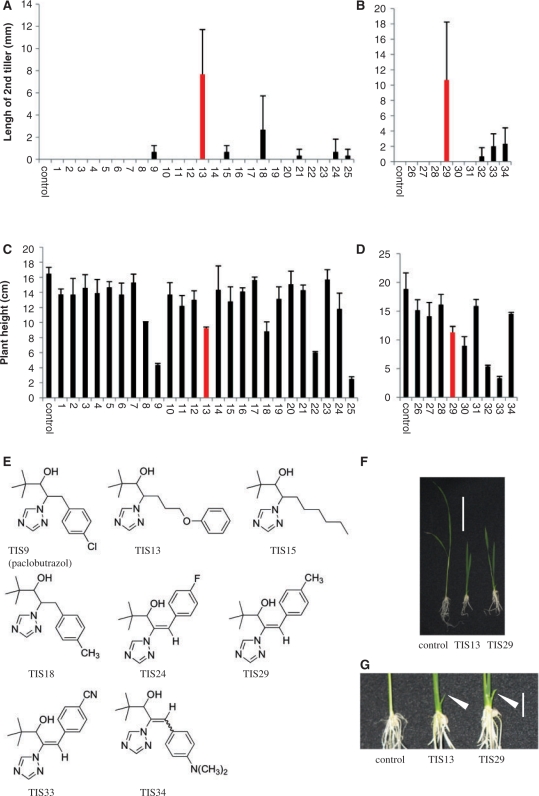
1H-1,2,4-triazole derivatives such as uniconazole-P and paclobutrazol inhibit a variety of cytochrome P450s. In the mode of action of these chemicals the triazole group is a key component because the nitrogen atom in the triazole group is essential for the binding to heme iron in cytochrome P450. In the SL biosynthesis pathway in Arabidopsis, at least one P450 (CYP711A) is likely to be involved (Booker et al. 2005) and there are five CYP711 family members in rice (Nelson et al. 2004). As an attempt to find a new SL biosynthesis inhibitor, we screened a chemical library of triazole derivatives constructed in our laboratory by Min et al. (1999) and Sekimata et al. (2001, 2002). In hydroponically grown rice seedlings, first and second tiller buds of SL-deficient mutants such as d10 and d17 grow out, while those of wild-type plants remain dormant (Umehara et al. 2008). Therefore, our chemical library was screened for chemicals that induce the first and second tiller bud outgrowth as candidates for SL biosynthesis inhibitors. Unfortunately, none of the chemicals tested induced the outgrowth of the first tiller bud. However, some chemicals induced second tiller bud outgrowth, many of which were found also to reduce plant height. Under our growth conditions, SL-deficient mutants do not show a significant difference in plant height from the wild type (data not shown), so this reduction in plant height caused by chemical treatments could be due to inhibition of other pathway(s). A likely explanation was the inhibition of gibberellin biosynthesis, because, with the exception of TIS13 and TIS15, all chemicals that induced second tiller bud outgrowth were gibberellin biosynthesis inhibitors or their analogs: paclobutrazol (TIS9), paclobutrazol analog (TIS18) and uniconazole analogs (TIS24, TIS29, TIS33 and TIS34) (Fig. 1A–E). Among the tested compounds, TIS13 and TIS29 were the most effective in inducing second tiller bud outgrowth (Fig. 1F, G).
Fig. 1.
Screening of the chemicals that induce outgrowth of second tiller bud in 2-week-old rice seedlings. (A and B) Length of the second tiller in seedlings treated with 10 μM of the chemicals. (C and D) Plant height of seedlings treated with 10 μM of the chemicals. The data are means ± SD of three samples. (E) Structures of chemicals that induced tiller bud outgrowth in A and B. (F and G) Two-week-old rice seedling treated with or without chemicals (TIS13 or TIS29). Scale bars in F and G indicate 5 and 1 cm, respectively. White arrowheads indicate second tillers.
Analysis of SL levels in chemical-treated rice
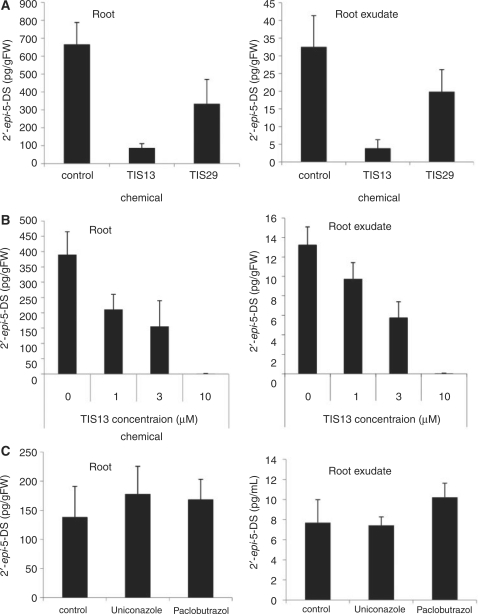
Although rice seedlings treated with TIS13 or TIS29 showed second tiller bud outgrowth, plant height was remarkably reduced (Fig. 1). Paclobutrazol (TIS9), a gibberellin biosynthesis inhibitor, and its analogs showed reduced plant height and second tiller bud outgrowth (Fig. 1). A rice gibberellin-deficient mutant which overexpresses gibberellin 2-oxidase has reduced plant height and increased tiller bud outgrowth (Lo et al. 2008). In this context we thought that second tiller bud outgrowth on seedlings treated with TIS13 or TIS29 could be induced by inhibiting gibberellin biosynthesis. To determine whether or not these chemicals inhibit SL biosynthesis, we analyzed the level of 2′-epi-5-deoxystrigol (epi-5DS), an endogenous SL in rice, in roots and root exudates by liquid chromatography–mass spectrometry (LC-MS/MS). Because SL levels in root exudates of rice seedlings are elevated when inorganic phosphate is depleted in the medium (Umehara et al. 2008), we examined the effect of chemical treatments on SL levels under phosphate deficiency. TIS13 strongly reduced the levels of epi-5DS in both roots and root exudates in a dose-dependent manner within the concentration range of 1–10 μM (Fig. 2); however, TIS29 reduced the level of epi-5DS in both roots and root exudates less effectively than TIS13 (Fig. 2A). The reduction in the levels of epi-5DS in both roots and root exudates by TIS13 and TIS29 implies that these chemicals do not inhibit SL secretion (Fig. 2A, B). Importantly, the gibberellin biosynthesis inhibitors, uniconazole-P and paclobutrazol did not change the levels of epi-5DS in either roots or root exudates (Fig. 2C). These results suggest that TIS13 and TIS29 induced tiller bud outgrowth by inhibiting SL biosynthesis, while uniconazole-P and paclobutrazol did not. Because TIS13 is a more effective SL biosynthesis inhibitor than TIS29 (Fig. 2A), we investigated the property of TIS13 in more detail in the subsequent studies.
Fig. 2.
epi-5DS levels in roots and root exudates of chemical-treated rice seedlings determined by LC-MS/MS. (A) Effect of 10 μM TIS13 and TIS29. (B) Effect of varying concentrations of TIS13. (C) Effect of 10 μM uniconazole and paclobutrazol. The data are means ± SD of three samples.
Co-application of GR24 with TIS13
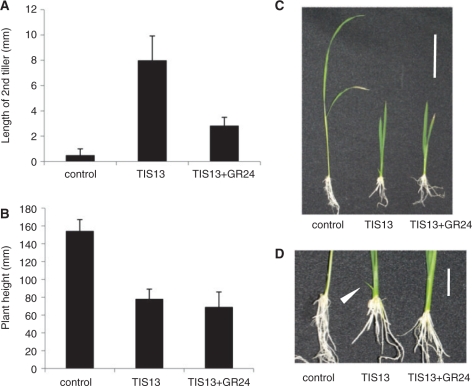
SL biosynthesis mutants show more tillers and reduced plant height than wild-type plants (Ishikawa et al. 2005, Zou et al. 2006, Arite et al. 2007). These phenotypes are rescued by application of SLs (Gomez-Roldan et al. 2008, Umehara et al. 2008). To determine whether the TIS13-induced phenotypes are due to SL deficiency in plants, GR24, a synthetic analog of SL, was co-applied to rice with TIS13. Second tiller bud outgrowth was partially suppressed by co-application of 1 μM GR24 with TIS13 (Fig. 3A, D), but plant height was not rescued (Fig. 3B, C). These results indicate that TIS13 induces second tiller bud outgrowth by inhibiting SL biosynthesis, but TIS13-treated plants show dwarfism due to a side effect of this chemical.
Fig. 3.
Effect of GR24 on tillering of 2-week-old rice seedlings treated with TIS13. (A) The lengths of second tillers in 3 μM TIS13-treated rice with or without 1 μM GR24. (B) Plant height in 3 μM TIS13-treated rice with or without 1 μM GR24. The data presented are means ± SD of six samples. (C and D) Two-week-old rice seedlings. Scale bars in C and D indicate 5 and 1 cm, respectively. The white arrowhead in D indicates a second tiller.
Striga germination assay
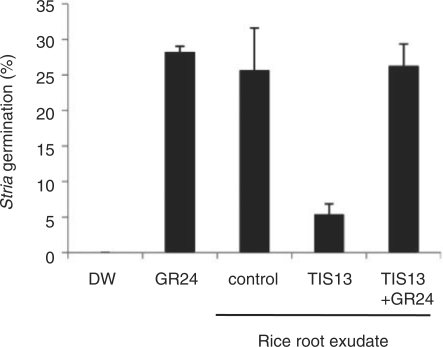
SLs are not only branching inhibitors but also seed germination stimulants for the parasitic weeds Striga and Orobanche (Cook et al. 1966). We employed a highly sensitive germination assay using Striga hermonthica seeds as a first step to evaluate TIS13 as a chemical that controls parasitic weed germination. In agreement with the result of epi-5DS analysis by LC-MS/MS, the culture medium of TIS13-treated rice seedlings contained less germination-stimulating activity than that of control plants (Fig. 4). The result shown in Fig. 4 demonstrates that the reduced germination-stimulating activity of TIS13-treated root exudates is not due to a direct inhibition of Striga germination, because the co-application of 1 μM GR24 with the TIS13-treated culture medium did not inhibit Striga germination (Fig. 4). These results indicate that the SL biosynthesis inhibitor selected as an inducer of tiller bud outgrowth could have the potential to be applied to control parasitic weed germination.
Fig. 4.
Estimation of germination stimulant levels in culture media of 2-week-old seedlings using Striga seeds. DW, distilled water; GR24, 1 μM GR24; control, non-treated rice culture media; TIS13, culture media of TIS13-treated plants; TIS13 + GR24, culture media co-incubated with 1 μM GR24 and TIS13. The data are means ± SD of three samples.
Discussion
To develop novel SL biosynthesis inhibitors that target P450s, azole-type chemicals inducing second tiller bud outgrowth were screened from a target-oriented chemical library constructed in our laboratory (Fig. 1). Some chemicals induced second tiller bud outgrowth in 2-week-old rice seedlings. One of these chemicals, TIS13 [2,2-dimethyl-7-phenoxy-4-(1H-1,2,4-triazol-1-yl)heptan-3-ol], when applied at 10 μM, reduced the levels of SL in both roots and root exudates (Fig. 2). TIS13-induced second tiller bud outgrowth was suppressed by co-application of 1 μM GR24. In addition, the root exudates of rice treated with 10 μM TIS13 had less germination-stimulating activity on the root parasitic weed, S. hermonthica than those of control plants. These results strongly suggest that TIS13 inhibits SL biosynthesis in rice, and that TIS13 is a useful lead compound for developing specific and potent SL biosynthesis inhibitors in the future.
Hydroxamic acid analogs inhibit the activity of many CCDs, including AtCCD7, increase the number of branches of Arabidopsis plants at 100 μM (Sergeant et al. 2009) and reduce the level of SL (López-Ráez et al. 2010). TIS13 decreases the level of SL and induces the outgrowth of tiller buds in rice seedlings at a lower concentration (3–10 μM) (Figs. 1–3). Thus, TIS13 appears to be a more potent inhibitor than hydroxamic acid analogs, although these two type of inhibitors need to be tested under the same assay conditions to compare their effectiveness.
Many chemicals that were selected in this study are analogs of uniconazole or paclobutrazol, both of which are gibberellin biosynthesis inhibitors. Gibberellin-deficient mutants have been shown to have an increased tiller number (Lo et al. 2008). In addition, paclobutrazol-treated rice plants exhibited second tiller bud outgrowth in this study (Fig. 1A). These results show that many chemicals inducing second tiller bud outgrowth in rice might not inhibit SL biosynthesis, but rather gibberellin biosynthesis. Actually, uniconazole or paclobutrazol treatment at 10 μM does not reduce the levels of epi-5DS (Fig. 2C1).
SL biosynthesis mutants of rice show many tillers and dwarfism, and these morphological abnormalities are nearly fully recovered by the application of 1 μM GR24 to the medium (Umehara et al. 2008). TIS13 treatment also promoted tiller bud outgrowth and dwarfism in rice seedlings. Tiller bud outgrowth was partially suppressed by co-application of 1 μM GR24 with 3 μM TIS13, but the dwarfism was not rescued by this treatment (Fig. 3). P450s are involved in the biosynthesis and metabolism of many plant hormones such as gibberellin, BR, auxin, cytokinin, jasmonic acid and ABA. Inhibition of P450s in the gibberellin or BR biosynthesis pathway induces dwarf phenotypes and this dwarfism was recovered by a co-application of gibberellin or BR, respectively, but not by any other hormones (Asami et al. 2000). We speculate that TIS13 inhibits not only SL biosynthesis but also that of other plant hormones such as gibberellin and/or BR and/or an uncharacterized P450 that affects tiller growth and plant height, and therefore TIS13-induced second tiller and dwarfism could not be perfectly recovered by 1 μM GR24 treatment.
TIS13 reduces the level of SL, but its target site(s) is still unknown. Given the fact that at least one P450 (CYP711A) is involved in SL biosynthesis and that TIS13 is a triazole-type inhibitor and has the potential to show affinity for P450s, the target site(s) of TIS13 could be CYP711A. However, several steps in the SL biosynthesis pathway still have to be elucidated (Rani et al. 2008) and there may be other P450s involved in SL biosynthesis. In the future, it will be necessary to identify the target site of TIS13.
SLs are germination stimulants of host plants for parasitic weeds as well as being shoot branching inhibitors (Cook et al. 1966, Gomez-Roldan et al. 2008, Umehara et al. 2008). Parasitic weeds are responsible for large-scale crop devastation worldwide. Germination of parasitic weeds is initiated by recognition of SL secreted by the roots of the host plant. Without this recognition, parasitic weeds cannot germinate and remain dormant in the soil. Until now, several ways to control parasitic weeds have been suggested (Humphrey et al. 2006), but effective ways have not yet been discovered. We demonstrate here that TIS13 is an SL biosynthesis inhibitor that can reduce the ability of root exudates to stimulate germination of Striga seeds. This SL biosynthesis inhibitor can be a new tool for controlling parasitic weeds.
In this report we demonstrate that TIS13 reduces the levels of SL and induces morphological changes in rice. Although TIS13 probably has side effects, we can find specific inhibitors for SL biosynthesis through structure–activity relationship studies on TIS13 as has been successful for the development of brassinazole and abamine which are BR and ABA biosynthesis inhibitors, respectively (Min et al. 1999, Han et al. 2004b). As SL-deficient mutants are known only in Arabidopsis, rice, petunia, pea and tomato at present (Koltai et al. 2010), SL biosynthesis inhibitors will play an important role in investigations into the function of SLs not only in other plants, but also in tissues, organs and biochemical processes. Moreover, varying the concentration of TIS13 can change the concentration of SLs in plants. This may make it possible to titrate the minimum concentration of SLs for suppression of branching in plants by comparing the concentration of SLs in TIS13-treated and untreated plants. Moreover, the ability to select a group of new mutants using CYP711A biosynthesis inhibitors (Jacobsen and Olszewski 1993, Nambara et al. 1994) and BR biosynthesis inhibitors (Wang et al. 2002, Yin et al. 2002, Komatsu et al. 2010) suggests that SL biosynthesis inhibitors can provide a way to find a new SL pathway or other novel mutants. Other than their use in basic science, SL biosynthesis inhibitors can be developed as a new commercial plant growth regulator.
Materials and Methods
Chemicals
GR24 was synthesized as described previously (Mangnus et al. 1992) to give four stereoisomers. We used (±)-(3aR*,8bS*,2′R*)-GR24 with the same relative stereochemistry as (±)-strigol. Triazole-type chemicals were synthesized by Min et al. (1999) and Sekimata et al. (2001, 2002).
Rice hyproponic culture
We used the rice normal cultivar, Shiokari. Rice seeds were sterilized in 2.5% sodium hypochlorite solution containing 0.01% Tween-20 for 30 min, rinsed with sterile water and incubated in sterile water at 25°C in the dark for 2 d. Germinated seeds were transferred into hydroponic culture medium (Umehara et al. 2008) solidified with 0.6% agar and cultured at 25°C under fluorescent white light with a 14 h light/8 h dark photoperiod for 6 d. Each seedling was transferred to a glass vial containing a 12 ml sterilized hydroponic culture solution with or without chemicals, and grown under the same conditions for 6 d.
For the SL analysis, the 1-week-old seedlings were transferred to a glass vial containing a 12 ml sterilized hydroponic culture solution and grown under the same conditions. After 5 d, seedlings were transferred to a new glass vial containing the same culture solution with or without chemicals for 1 d.
SL analysis
SL analysis was performed according to a previously described method (Umehara et al. 2008). Briefly, the hydroponic culture media (10 ml) were extracted with ethyl acetate twice after adding d1-epi-5DS (200 pg) as an internal standard. The organic layer was dried under nitrogen gas and dissolved in 1 ml of ethyl acetate : n-hexane (15 : 85). The solutions were loaded onto a Sep-Pak Silica 1 ml cartridge (Waters), washed with the same solution twice, eluted with ethyl acetate : n-hexane (35 : 65) three times and concentrated in vacuo. The roots were homogenized in acetone containing d1-epi-5DS (200 pg). The filtrates were dried under nitrogen gas and dissolved in water. The solutions were extracted with ethyl acetate twice, dried and dissolved in 10% acetone. The extracts were loaded onto Oasis HLB 3 ml cartridges (Waters), washed with water twice, eluted with acetone twice and dried under nitrogen gas. The concentrates were dissolved in 1 ml of ethyl acetate : n-hexane (15 : 85) and loaded onto a Sep-Pak Silica 1 ml cartridge, washed, eluted and concentrated in the same way. The epi-5DS-containing fractions from the culture media and roots were dissolved in 50% acetonitrile and subjected to LC-MS/MS analysis.
Striga germination assay
The germination assay using S. hermonthica was performed as described previously (Sugimoto et al. 2008). For bioassay, de-ionized water and GR24 solution were used as negative and positive controls, respectively.
Funding
Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) [grant to T.A.]; Japan Society for the Promotion of Science Fellows [to S.I.].
Glossary
Abbreviations
- BR
brassinosteroid
- CCD
carotenoid cleavage dioxygenase
- epi-5DS
2′-epi-5-deoxystrigol
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- P450
cytochrome P450 monooxygenase
- SL
strigolactone.
References
- Akiyama K., Matsuzaki K., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Arite T., Iwata H., Ohshima K., Maekawa M., Nakajima M., Kojima M., et al. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007;51:1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- Asami T., Min YK., Nagata N., Yamagishi K., Takatsuto S., Fujioka S., et al. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 2000;123:93–100. doi: 10.1104/pp.123.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T., Nakano T., Nakashita H., Sekimata K., Shimada Y., Yoshida S. The influence of chemical genetics on plant science: shedding light on functions and mechanism of action of brassinosteroids using biosynthesis inhibitors. J. Plant Growth Regul. 2003;22:336–349. doi: 10.1007/s00344-003-0065-0. [DOI] [PubMed] [Google Scholar]
- Beveridge C.A., Kyozuka J. New genes in the strigolactone-related shoot branching pathway. Curr. Opin. Plant Biol. 2010;13:34–39. doi: 10.1016/j.pbi.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Booker J., Auldridge M., Wills S., McCarty D., Klee H., Leyser O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004;14:1232–1238. doi: 10.1016/j.cub.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Booker J., Sieberer T., Wright W., Williamson L., Willett B., Stirnberg P., et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell. 2005;8:443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Cook C.E., Whichard L.P., Turner B., Wall M.E., Egley G.H. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- Drummond R.S., Martínez-Sánchez N.M., Janssen B.J., Templeton K.R., Simons J.L., Quinn B.D., et al. Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE7 is involved in the production of negative and positive branching signals in petunia. Plant Physiol. 2009;151:1867–1877. doi: 10.1104/pp.109.146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Valdivieso G., Fryer M.J., Lawson T., Slattery K., Truman W., Smirnoff N., et al. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell. 2009;21:2143–2162. doi: 10.1105/tpc.108.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.P., et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Han S.Y., Kitahata N., Saito T., Kobayashi M., Shinozaki K., Yoshida S., et al. A new lead compound for abscisic acid biosynthesis inhibitors targeting 9-cis-epoxycarotenoid dioxygenase. Bioorg. Med. Chem. Lett. 2004b;14:3033–3036. doi: 10.1016/j.bmcl.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Han S.Y., Kitahata N., Sekimata K., Saito T., Kobayashi M., Nakashima K., et al. A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol. 2004a;135:1574–1582. doi: 10.1104/pp.104.039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey A.J., Galster A.M., Beale M.H. Strigolactones in chemical ecology: waste products or vital allelochemicals? Nat. Prod. Rep. 2006;23:592–614. doi: 10.1039/b512776a. [DOI] [PubMed] [Google Scholar]
- Ishikawa S., Maekawa M., Arite T., Onishi K., Takamure I., Kyozuka J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005;46:79–86. doi: 10.1093/pcp/pci022. [DOI] [PubMed] [Google Scholar]
- Jacobsen S.E., Olszewski N.E. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata N., Han S.Y., Noji N., Saito T., Kobayashi M., Nakano T., et al. A 9-cis-epoxycarotenoid dioxygenase inhibitor for use in the elucidation of abscisic acid action mechanisms. Bioorg. Med. Chem. 2006;14:5555–5561. doi: 10.1016/j.bmc.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Koltai H., LekKala S. P., Bhattacharya C., Mayzlish-Gati E., Resnick N., Wininger S., et al. A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions. J. Exp. Bot. 2010;61:1739–1749. doi: 10.1093/jxb/erq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T., Kawaide H., Saito C., Yamagami A., Shimada S., Nakazawa M., et al. The chloroplast protein BPG2 functions in brassinosteroid-mediated post-transcriptional accumulation of chloroplast rRNA. Plant J. 2010;61:409–422. doi: 10.1111/j.1365-313X.2009.04077.x. [DOI] [PubMed] [Google Scholar]
- Lo S.F., Yang S.Y., Chen K.T., Hsing Y.I., Zeevaart J.A., Chen L.J., et al. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell. 2008;20:2603–2618. doi: 10.1105/tpc.108.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez J.A., Charnikhova T., Gómez-Roldán V., Matusova R., Kohlen W., De Vos R., et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 2008;178:863–874. doi: 10.1111/j.1469-8137.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- López-Ráez J.A., Kohlen W., Charnikhova T., Mulder P., Undas A.K., Sergeant M.J., et al. Does abscisic acid affect strigolactone biosynthesis? New Phytol. 2010 doi: 10.1111/j.1469-8137.2010.03291.x. In press [Epub ahead of print] (doi: 10.1111/j.1469-8137.2010.03291.x) [DOI] [PubMed] [Google Scholar]
- Matusova R., Rani K., Verstappen F.W., Franssen M.C., Beale M.H., Bouwmeester H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y.K., Asami T., Fujioka S., Murofushi N., Yamaguchi I., Yoshida S. New lead compounds for brassinosteroid biosynthesis inhibitors. Bioorg. Med. Chem. Lett. 1999;9:425–430. doi: 10.1016/s0960-894x(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Nambara E., Keith K., McCourt P., Naito S. Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol. 1994;35:509–513. [PubMed] [Google Scholar]
- Nelson D.R., Schuler M.A., Paquette S.M., Werck-Reichhart D., Bak S. Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol. 2004;135:756–772. doi: 10.1104/pp.104.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani K., Zwanenburg B., Sugimoto Y., Yoneyama K., Bouwmeester H.J. Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol. Biochem. 2008;46:617–626. doi: 10.1016/j.plaphy.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Schwartz S.H., Qin X., Loewen M.C. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J. Biol. Chem. 2004;279:46940–46945. doi: 10.1074/jbc.M409004200. [DOI] [PubMed] [Google Scholar]
- Sekimata K., Han S.Y., Yoneyama K., Takeuchi Y., Yoshida S., Asami T. A specific and potent inhibitor of brassinosteroid biosynthesis possessing a dioxolane ring. J. Agric. Food Chem. 2002;50:3486–3490. doi: 10.1021/jf011716w. [DOI] [PubMed] [Google Scholar]
- Sekimata K., Kimura T., Kaneko I., Nakano T., Yoneyama K., Takeuchi Y., et al. A specific brassinosteroid biosynthesis inhibitor, Brz2001: evaluation of its effects on Arabidopsis, cress, tobacco, and rice. Planta. 2001;213:716–721. doi: 10.1007/s004250100546. [DOI] [PubMed] [Google Scholar]
- Sergeant M.J., Li J.J., Fox C., Brookbank N., Rea D., Bugg T.D., et al. Selective inhibition of carotenoid cleavage dioxygenases: phenotypic effects on shoot branching. J. Biol. Chem. 2009;284:5257–5264. doi: 10.1074/jbc.M805453200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Akune M., Kogiso M., Imagama Y., Osuki K., Uchiumi T., et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004;45:914–922. doi: 10.1093/pcp/pch107. [DOI] [PubMed] [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Vogel J.T., Walter M.H., Giavalisco P., Lytovchenko A., Kohlen W., Charnikhova T., et al. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010;61:300–311. doi: 10.1111/j.1365-313X.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Nakano T., Gendron J., He J., Chen M., Vafeados D., et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- Yin Y., Wang Z.Y., Mora-Garcia S., Li J., Yoshida S., Asami T., et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Zou J., Zhang S., Zhang W., Li G., Chen Z., Zhai W., et al. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006;48:687–698. doi: 10.1111/j.1365-313X.2006.02916.x. [DOI] [PubMed] [Google Scholar]