Abstract
Reward-seeking behavior is controlled by neuronal circuits that include the basolateral nucleus of amygdala (BLA), medial prefrontal cortex (mPFC), nucleus accumbens (NAc) and ventral tegmental area. Using a discriminative stimulus (DS) task in which an intermittently presented cue (DS) directs the animals to make an operant response for sucrose, we previously demonstrated that dopamine receptor antagonism in the NAc reduced reinforced cue responding, whereas general inactivation of the NAc increased behavioral responding in the absence of the cue. Because they send major glutamatergic projections to the NAc, the BLA and mPFC may also contribute to reward-seeking behaviors modulated by the NAc. In this study we compare the effects of BLA and mPFC inactivation on the performance of a DS task. BLA inactivation by combined GABAA and GABAB agonists impaired cue responding with minimal effects on operant behavior in the absence of cues. Dorsal mPFC (dmPFC) inactivation also inhibited cue-evoked reward-seeking. In contrast, ventral mPFC (vmPFC) inactivation disinhibited responding to unrewarded cues with less influence on reinforced cue responding. These findings demonstrate that the BLA and dmPFC facilitate cue-evoked reward-seeking, whereas, in the same task the vmPFC exerts inhibitory control over unrewarded behaviors.
Keywords: basolateral nucleus, infralimbic cortex, nucleus accumbens, prelimbic cortex, cingulate cortex, reward-seeking behavior
Cue-evoked reward-seeking behaviors require recognition of learned cues that predict reward availability and selection of appropriate motor responses. Performance of reward-seeking behavior requires integrated functions of multiple brain areas including the basolateral nucleus of the amygdala (BLA), medial prefrontal cortex (mPFC), nucleus accumbens (NAc) and ventral tegmental area (VTA). The BLA and mPFC play crucial roles in reward-related behaviors and are major sources of glutamatergic afferents to the NAc (McGeorge and Faull, 1989; Brog et al., 1993; Zahm, 2000). The NAc, which receives excitatory inputs from the BLA and mPFC and projects via both direct and indirect paths to output nuclei of the basal ganglia, has been proposed to function as an interface between corticolimbic and motor areas (Mogenson et al., 1980). The VTA provides a dense dopaminergic projection to the NAc (Swanson, 1982), and this projection is critical for the performance of learned appetitive responses to reward predictive cues (Nicola et al., 2000; Yun et al., 2004b; Fields et al., 2007; Nicola, 2007).
Electrophysiological studies have reported that BLA and mPFC neurons as well as VTA neurons respond to reward-predictive cues during many reward-seeking behaviors (Ljungberg et al., 1992; Muramoto et al., 1993; Schultz et al., 1993; Schoenbaum et al., 1998, 1999; Jodo et al., 2000). Different subpopulations of NAc neurons are excited or inhibited by discriminative stimuli (DSs) and other cues that predict reward availability (Nicola et al., 2004b; Ghitza et al., 2003; Wan and Peoples, 2006; Day et al., 2006). VTA inactivation selectively abolishes the responses of NAc neurons evoked by reward-predictive cues (Yun et al., 2004b). These findings have led to a model of neuronal circuits involving the NAc, VTA and corticolimbic structures (BLA and mPFC). According to this model (Nicola, 2007), dopamine released in the NAc at the time of cue presentation facilitates the firing of a subpopulation of neurons that are activated by corticolimbic structures and promote specific reward-seeking behaviors. Since the VTA is the sole source of dopamine input to the NAc, the fact that dopamine antagonists injected into the NAc reduce responding to DSs supports this model (Yun et al, 2004a,b). Strikingly, however, responding to incentive cues in the same task is not reduced by general inactivation of NAc neurons with glutamate receptor antagonists or tetrodotoxin. Instead, general inactivation of the NAc results in increased behavioral responding that is not appropriate for reward seeking, such as responding to a stimulus that does not predict reward, or uncued responding on levers during the inter-trial interval (Yun et al., 2004a). This finding suggests that the firing of some NAc neurons normally inhibits behaviors that are inappropriate to the learned task. Thus, corticolimbic input to the NAc can either inhibit or dopamine-dependently excite reward-seeking behavior. An important question, then, is which specific inputs promote and which inhibit reward-seeking.
The BLA plays a crucial role in reward representation and stimulus-reward learning (Baxter and Murray, 2002). Previous lesion studies demonstrated that BLA-lesioned rats showed deficits in appetitive Pavlovian second-order conditioning and Pavlovian-instrumental transfer (Hatfield et al., 1996; Blundell et al., 2001). On the other hand, the mPFC has been implicated in a variety of cognitive and executive processes. Lesions and inactivation of the mPFC induce deficits in formation of action-outcome associations, movement initiation, inhibition of responses, behavioral flexibility and working memory tasks (Granon et al., 1994; Ragozzino et al., 1999; Passetti et al., 2002; Risterucci et al., 2003; Salazar et al., 2004; Ostlund and Balleine, 2005; Narayanan et al., 2006). Furthermore, the mPFC can be functionally and anatomically subdivided into dorsal and ventral divisions (dmPFC and vmPFC, respectively) (Passetti et al., 2002; Heidbreder and Groenewegen, 2003). The dmPFC, including the anterior cingulate and dorsal prelimbic cortex, is particularly involved in the temporal organization and shifting of behavioral sequences, while the vmPFC, including ventral prelimbic cortex, infralimbic and medial orbital cortex, appears to be specifically responsible for maintaining behavioral flexibility and motor preparation of conditioned responses (Passetti et al., 2002; Heidbreder and Groenewegen, 2003; Risterucci et al., 2003).
Although both the BLA and mPFC participate in reward-related behaviors, the mPFC is likely to contribute to reward-associated action selection, whereas the BLA appears to be implicated in processing information about emotional or motivational significance. Because the tasks used previously to draw these conclusions differ from the DS task we have employed in our behavioral and electrophysiological studies, it is difficult to predict which, if any, nucleus (BLA or mPFC) provides the critical input to the NAc that dopamine-dependently facilitates behavioral and neuronal responses to learned reward predictive cues, and which provides the input that results in behavioral inhibition. Therefore, in the present study, we asked how the BLA and mPFC contribute to performance of a DS task similar to the one we used earlier to explore the contribution of NAc neurons and dopamine to cue-evoked reward-seeking. Parts of these data have been presented in abstract form (Ishikawa et al., 2007).
Experimental procedures
Subjects
Thirty-two male Long-Evans rats (~350 g on arrival) were individually housed in a colony room maintained on a 12-h light, 12-h dark cycle. All experiments occurred during the light portion of the cycle. After receipt, rats were allowed at least 1 week of ad libitum food and water, followed by 1 week of restricted food and water before training. Animals were fed 13 g of BioServ formula F-173 pellets (1 g each) and 30 ml of water per day for the duration of the experiments. Animals were weighed daily, and those showing weight loss >10% of free feeding weight were given additional food until their weight stabilized. All procedures were approved by the Ernest Gallo Clinic & Research Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Apparatus
Experimental sessions were conducted in standard operant chambers (29.5 cm long, 25 cm wide and 25 cm high) enclosed within a sound- and light-isolating plastic outer chamber (MED Associates, St. Albans, VT, USA). Two retractable response levers (6 cm above the grid floor) were situated on one wall of the operant chamber, with a reward receptacle between them. The reward receptacle contained a small well into which a liquid 10% sucrose reward was pumped using a syringe pump. Two white houselights were on throughout experiments, and white noise (65 dB) was present at all times. An additional speaker was used to present two auditory stimuli (85 dB). One was an intermittent 15 kHz tone that was on for 120 ms and off for 270 ms, and the other was a constant 5 kHz tone.
Behavioral tasks
Twenty-four animals were trained on the DS task. In the DS task, two auditory stimuli (up to 10 s long) were presented as tone cues: a DS, which predicted reward delivery after a correct lever-press during DS presentation, and a non-rewarded stimulus (NS), during which lever-pressing did not trigger reward delivery. For half the animals, the DS was the intermittent tone and the NS was the constant tone; the opposite relation held for the other half. The left lever was designated the active lever for half the animals, and the right lever was designated active for the other half. Responses on the inactive lever had no programmed consequence at any time. A response on the active lever during DS presentation always terminated the DS and resulted in delivery of 50 μl of 10% sucrose into the reward receptacle. Cues were presented on a variable interval schedule with an average interval (from the end of the previous cue to the onset of the next) of 29.4 sec; the DS or NS was randomly presented at the end of each interval.
Eight additional rats were trained on a simpler uncued fixed ratio 1 (FR1) task in which there was no explicit auditory stimulus. As in the DS task, the left lever was active for half the animals, and the right lever was active for the other half. A single press on the active lever caused the delivery of 50 μl of 10% sucrose reward. After reward delivery, additional reward was immediately available contingent on an active lever press. In both tasks, test sessions lasted for 1 h.
Training procedures
Animals progressed through several stages of training before undergoing surgical implantation of cannulae for drug microinjections. In stage 1 of DS task training, food-restricted animals were introduced to the chamber. Entry into the reward receptacle or pressing either of the two levers triggered delivery of 50 μl of a 10% sucrose solution. A 20 sec time-out was imposed after reward delivery, during which reward could not be earned. After animals learned to obtain all 100 available rewards in < 1 h, they were advanced to stage 2. This stage consisted of a two-lever FR1 task in which a response on either lever triggered reward delivery followed by a 3 s time-out. Animals remained at this stage until they learned to obtain 100 rewards in < 1 h. In stage 3, animals were advanced to a one-lever FR task in which pressing the active lever (left or right, depending on the animal) during cue presentation triggered reward delivery followed initially by a 10 s timeout. The cue was presented at the end of the timeout and remained on until an active lever press. The timeout was increased to 20 s and then 30 s when animals obtained more than 100 rewards during the session. Lever-pressing in the absence of the cue was not rewarded. Animals were advanced to stage 4 (the final DS task) when their latency to press the lever was less than 15 s after cue presentation. Training procedures for the uncued FR1 task began with stages 1 and 2 as described for the DS task. Animals were then advanced to the uncued FR1 task.
Animals were trained on the DS task until the NS response probability averaged ~20% and the DS response probability averaged > 90%. For animals on the uncued FR1 task, training continued until animals performed more than 600 active lever-presses per session in 3 consecutive sessions. When animals met these criteria, surgery for cannula implantation was performed.
Surgery
Animals were anesthetized with isoflurane (0.5-2.0%) and placed in a stereotaxic apparatus. Nineteen rats (11, DS task; 8, uncued FR1 task) were implanted bilaterally with two sets of 27 gauge microinjection guide cannulae (Plastics One, Roanoke, VA, USA), one set in the BLA and the other in the mPFC. BLA coordinates were AP −3.0, ML ±4.8, DV −7.0 mm relative to bregma and mPFC coordinates were AP +3.2, ML ±0.75, DV −1.5 mm. In 5 additional rats trained on the DS task, guide cannulae were implanted bilaterally in both the caudate putamen (CPu) (AP −2.3, ML ±5.0, DV −4.5 mm) and motor cortex (MC) (AP +3.2, ML ±2.0, DV −1.5 mm). A final group of 8 rats on the DS task was implanted with cannulae only in the mPFC (AP +3.2, ML ±0.75, DV −2.0 mm) to compare the effects of dmPFC and vmPFC inactivation. Guide cannulae were secured to the skull with bone screws and dental acrylic, and wire obturators were inserted into the guide cannulae; the ends of the obturators were flush with the ends of the guide cannulae. Rats were given at least 7 days of recovery before they were retrained on their respective tasks and habituated to the close handling necessary for the microinjection procedure.
Microinjections
After retraining, animals were injected bilaterally in the BLA, mPFC, CPu or MC prior to every other session with either drug or vehicle (saline). The drug solution consisted of a mixture of muscimol and baclofen (M/B) (GABAA and GABAB agonists) dissolved in saline. The doses of muscimol and baclofen were 12.5, 25 and 50 ng of each drug for BLA injection; 25, 50 and 100 ng for mPFC injection; 12.5 ng for CPu injection; and 25 and 50 ng for the MC, dmPFC and vmPFC. Doses indicate the amount delivered per hemisphere. Injection volume was always 0.5 μl per side.
To inject animals, the obturators were removed and 30 gauge injector cannulae were bilaterally inserted into the guides. Injectors extended 1 (MC and dmPFC), 1.5 (BLA and CPu), 2 (mPFC) and 3 mm (vmPFC) below the end of the guides for final injection targets of AP −3.0, ML ±4.8, DV −8.5 mm (BLA), AP +3.2, ML ±0.75, DV −3.5 mm (mPFC), AP −2.3, ML ±5.0, DV −6.0 mm (CPu), AP +3.2, ML ±2.0, DV −2.5 mm (MC), AP +3.2, ML ±0.75, DV −3.0 mm (dmPFC) and AP +3.2, ML ±0.75, DV −5.0 mm (vmPFC). After a 1 min pre-injection period the entire volume was injected over 2 min. After a 1 min post-injection wait, the injectors were removed, the obturators were replaced and the animal was immediately placed into the behavioral chamber and the session began.
Animals with cannulae in both the BLA and mPFC received injections in these structures on alternating days (although animals were run on the task every day, 5 days per week), except that 3 DS-trained rats received BLA injections prior to mPFC injection The order of drug doses applied for each brain site was randomly chosen for each animal except that injection of 12.5 ng M/B into the BLA for 5 animals was the last treatment. In the 8 rats receiving dmPFC and vmPFC inactivation, injection into the vmPFC was performed after all doses of M/B and saline were applied to the dmPFC.
Because each injection site received several injections over the course of the experiment, it is possible that cumulative tissue damage could have affected behavioral performance. To determine whether this was the case, we examined the animals’ performance on the last (uninjected) session, one day prior to the last injection received. Performance at that time was similar to the animals’ initial criterion performance. Specifically, in the 16 DS-trained rats (8 for BLA or mPFC injection; 8 for dmPFC or vmPFC injection), the DS ratio was 93.7 ± 1.0 and the NS ratio was 19.3 ± 2.3.
Data analysis
For the DS task, several behavioral measures were examined: the DS and NS response ratios (proportion of these cues during which the animal made a response on the active lever during presentation of the cue), the DS response latency, and the rate of uncued responding on the active levers (the rate of responding in the absence of DS and NS). For the uncued FR1 task, the number of active lever presses in the 1-h session was determined. The effects of the drug were analyzed using paired t-test or repeated measures ANOVA followed by Fisher’s PLSD post hoc test, in which significance was defined as P < 0.05.
Histology
At the end of experiments, animals were deeply anesthetized with sodium pentobarbital and perfused with saline and 10% formalin. Sections (50 μm) from each brain site were cut on a cryostat and stained with neutral red, and cannula positions were determined. Data from improper placements were excluded from the analysis. Final N values were 8 DS-trained rats that received BLA and mPFC inactivation, 5 DS-trained rats receiving CPu and MC inactivation, 8 rats trained on FR1 that received BLA and mPFC inactivation, and 8 DS-trained rats receiving dmPFC and vmPFC inactivation.
Results
The role of the BLA in reward-seeking behaviors
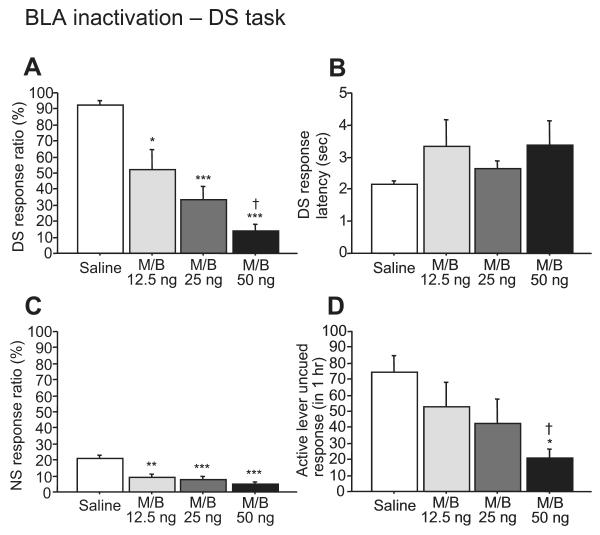
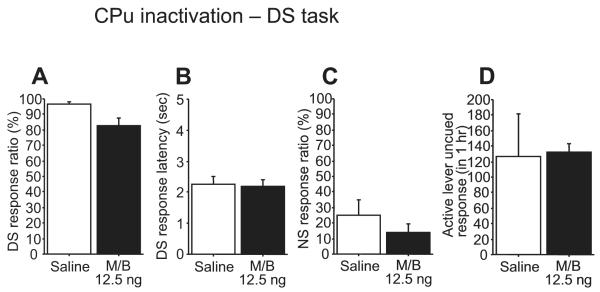
Inactivating the BLA with GABAA and GABAB agonists (M/B) impaired cue responding behavior (Fig. 1). M/B induced a dose-dependent decrease in DS response ratio (F3,21 = 22.77, P < 0.0001; Fig. 1A). Responding to the NS was also reduced by BLA inactivation (F3,21 = 12.31, P < 0.0001; Fig. 1C). Although the DS response latency tended to increase following BLA inactivation, there was no significant difference among groups (F3,21 = 1.58, P = 0.23; Fig. 1B). The rate of uncued responding on the active lever was reduced when M/B was injected at a dose of 50 ng (F3,21 = 4.17, P < 0.05; Fig. 1D). As the injected drugs may diffuse away from the BLA and affect neighboring brain areas over time, we implanted a separate group of animals with cannulae directed towards the CPu and monitored their performance on the DS task. M/B (12.5 ng) infused into the neighboring CPu had no effect on any behavioral measures (DS response ratio, t = 2.59, P = 0.06; NS response ratio, t = 1.26, P = 0.28; DS response latency, t = 0.24, P = 0.82) (Fig. 2).
Fig. 1.
The effects of BLA inactivation on DS behaviors. Bilateral microinjection of M/B into the BLA dose-dependently reduced the DS response ratio (A), although there was no significant difference in DS response latency (B). BLA inactivation decreased the NS response ratio (C). Uncued responses on the active lever were attenuated by 50 ng of M/B (D). *P < 0.01, **P < 0.001 and ***P < 0.0001 compared with saline. †P < 0.05 compared with 12.5 ng of M/B.
Fig. 2.
Microinjection of M/B into the CPu. CPu inactivation had no effect on DS or NS response ratio (A, C), DS response latency (B) and active lever uncued response (D).
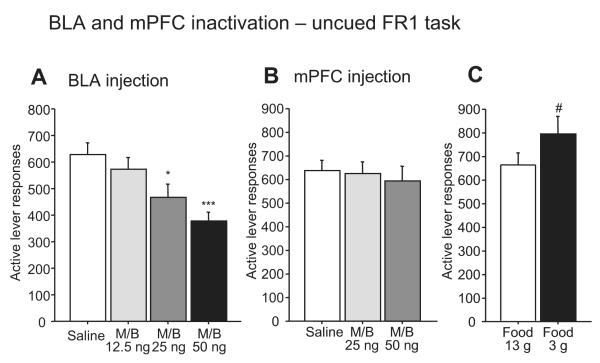
To determine whether the behavioral effects of BLA inactivation could be due to a gross motor deficit or a reduction in the animals’ motivation for reward, we determined the effects of BLA inactivation on lever-pressing in the uncued FR1 task (Fig. 5A). Although 25 and 50 ng of M/B microinjected into the BLA significantly reduced active lever responses, the 12.5 ng dose did not affect responding (F3,21 = 12.61, P < 0.0001). In contrast, the lowest (12.5 ng) dose of M/B reduced the DS response ratio by ~50% (Fig. 1A). Therefore, although the reduction in responding in the DS task by higher doses of M/B injected into the BLA may be due in part to a motor or motivational deficit, these factors cannot entirely explain the impaired cue responding observed in the DS task. Instead, elements of the DS task structure that differ from the FR1 task (e.g., a cue is presented at relatively long inter-trial intervals) likely cause the DS task to be more dependent on the BLA than the FR1 task.
Fig. 5.
Effects of BLA (A) and mPFC (B) inactivation on the performance of an uncued FR1 task. There was no significant difference in active lever response between saline and 12.5 ng M/B injection into the BLA, although M/B injection of 25 and 50 ng reduced active lever responses (A). M/B injection into the mPFC had no effect on active lever responses (B). Increased food restriction (3 g pellets and 15 ml water) for one day induced a significant increase in active lever responses, indicating that the absence of an increase in lever pressing due to M/B injection was not the result of a ceiling effect (C). *P < 0.01 and ***P < 0.0001 compared with saline. #P < 0.01 compared with animals given 13 g of food.
The role of the mPFC in reward-seeking behaviors
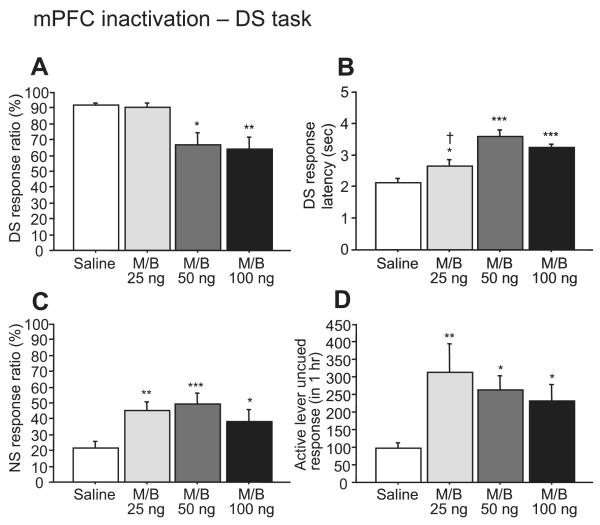
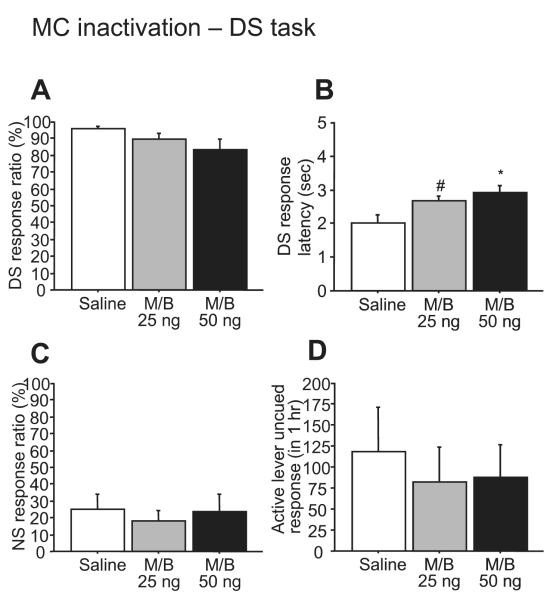
In contrast to BLA inactivation, microinjection of M/B into the mPFC (between the dmPFC and vmPFC injection sites described in the next section) induced significant increases in NS response ratio (F3,21 = 9.59, P < 0.001; Fig. 3C) and in uncued responses on the active lever (F3,21 = 7.74, P < 0.01; Fig. 3D). At higher doses (50 and 100 ng), M/B injected into the mPFC also reduced the DS response ratio (F3,21 = 8.75, P < 0.001; Fig. 3A). The DS response latency increased after all doses of M/B (F3,21 = 24.77, P < 0.0001; Fig. 3B). To exclude the possibility that the effectiveness of M/B injected into the mPFC depends upon diffusion to neighboring brain areas, we examined the effects of MC inactivation on performance in the DS task. Inactivation of the neighboring MC with M/B had no effect on the DS response ratio (F2,8 = 2.76, P = 1.22 or NS response, F2,8 = 1.18, P = 0.36), although it did increase DS response latency (F2,8 = 8.01, P < 0.05) (Fig. 4). This indicates that the increase in unrewarded responding was most likely due to an effect within the mPFC.
Fig. 3.
The effects of mPFC inactivation on performance of the DS task. Injection of higher (50 and 100 ng) doses of M/B reduced the DS response ratio (A). Bilateral M/B injection into the mPFC increased DS response latency (B), NS response ratio (C) and uncued active lever responses (D). *P < 0.01, **P < 0.001 and ***P < 0.0001 compared with saline. †P < 0.05 compared with 50 or 100 ng of M/B.
Fig. 4.
Microinjection of M/B into the MC. MC inactivation had no effect on DS or NS response ratios (A, C) and active lever uncued responses (D), while there was a significant increase in DS response latency (B). #P < 0.05 and *P < 0.01 compared with saline.
To determine whether an increase in motivation for sucrose reward could explain the increased unrewarded responding, we asked whether M/B infused into the mPFC caused an increase in lever-pressing during the uncued FR1 task. M/B (25 and 50 ng) infused into the mPFC had no effects on the number of active lever responses on this task (F2,14 = 0.49, P = 0.63; Fig. 5B). Because an increase in lever pressing may not have been observed due to a ceiling effect, we increased the animals’ motivation for sucrose by providing them with only 3 g of food (instead of the usual 13 g) on the day before the test session. When they were more food restricted, animals responded with more lever presses than when they were moderately restricted (t = −3.86, P < 0.01; Fig. 5C). These results suggest that the activity of the mPFC plays an essential role in responding to learned reward-predictive cues as well as in the inhibitory control of behaviors inappropriate to the learned task.
Dissociable contributions of dmPFC and vmPFC to reward-seeking behaviors
Inactivation of the mPFC both reduced responding to the reward-predictive DS and increased the probability of unrewarded behaviors, such as responding to the NS and uncued responding on the active lever. We previously reported a reduction in DS responding after injection of dopamine receptor antagonists into the NAc, and an increase in unrewarded responding after general inactivation of the NAc (Yun et al., 2004a). Recent evidence suggests that the ventral mPFC, which projects more strongly to the NAc shell than to the core, exerts a suppressive effect on reward-seeking behavior, whereas the dorsal mPFC, which projects more strongly to the NAc core, exerts an activating effect (Passetti et al., 2002; Risterucci et al., 2003; Peters et al., 2008a,b). Therefore, we compared the effects of dmPFC and vmPFC inactivation on performance of the DS task.
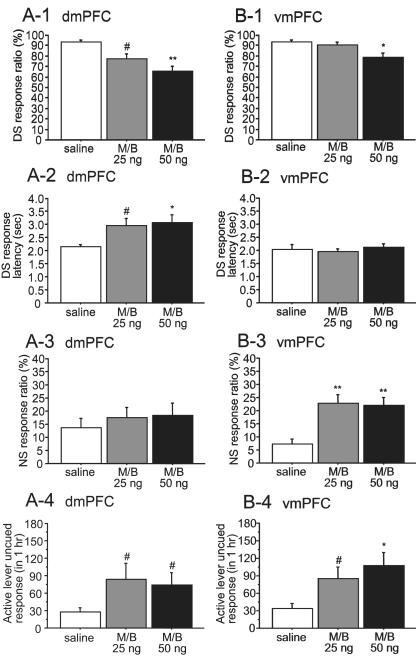
DS responding was impaired by dmPFC inactivation at doses of 25 and 50 ng (F2,14 = 11.27, P < 0.01; Fig. 6A-1), whereas only the 50 ng dose of M/B injected into the vmPFC reduced the DS response ratio (F2,14 = 9.50, P < 0.01; Fig. 6B-1), and to a smaller degree than dmPFC inactivation. Furthermore, M/B injection into the dmPFC induced an increase in DS response latency (F2,14 = 5.34, P < 0.05; Fig. 6A-2), whereas vmPFC inactivation had no effect on this measure (F2,14 = 0.35, P = 0.71; Fig. 6B-2). On the other hand, inactivation of the vmPFC, but not the dmPFC, induced a significant increase in NS response ratio (vmPFC, F2,14 = 13.67, P < 0.001; dmPFC, F2,14 = 1.97, P = 0.18) (Fig. 6A-3, 6B-3). Both dmPFC and vmPFC inactivation augmented uncued responding on the active lever (dmPFC, F2,14 = 4.75, P < 0.05; vmPFC, F2,14 = 8.70, P < 0.01) (Fig. 6A-4, 6B-4). Thus, dmPFC but not vmPFC inactivation significantly reduced responding to reward-predictive cues. In contrast, vmPFC but not dmPFC inactivation increased responding to the unrewarded cue. Inactivation of either site enhanced uncued responding on the active lever.
Fig. 6.
Distinct effects of dmPFC and vmPFC inactivation on DS behaviors. DS responding was impaired by dmPFC inactivation using 25 and 50 ng M/B (A-1), whereas only the 50 ng M/B dose injected into the vmPFC reduced the DS response ratio (B-1). M/B injection into the dmPFC increased DS response latency (A-2), whereas vmPFC inactivation had no effect on the latency (B-2). On the other hand, inactivation of the vmPFC but not the dmPFC induced a significant increase in the NS response ratio (A-3, B-3). Both dmPFC and vmPFC inactivation augmented uncued responses on the active lever (A-4, B-4). #P < 0.05, *P < 0.01 and **P < 0.001 compared with saline.
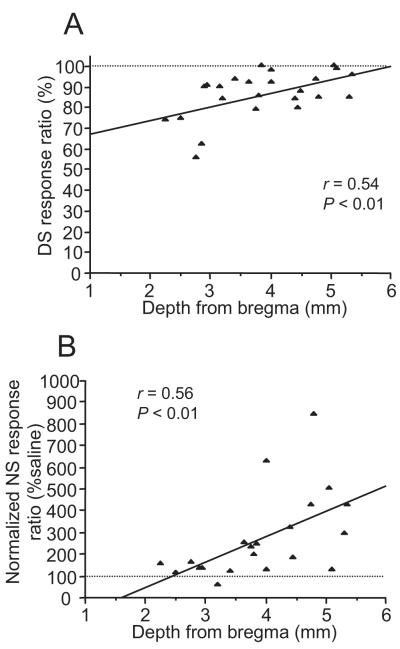
To further confirm this conclusion, we analyzed the effects of individual injections of 25 ng M/B into the mPFC as a function of the depth of the injection site as determined by histological reconstruction. The DS and NS response ratios were significantly correlated with the depth of injection sites (DS: r = 0.54, P < 0.01, n = 24; NS: r = 0.56, P < 0.01, n = 21) (Fig. 7), whereas uncued responding showed no significant correlation with the injection sites (r = −0.007, P = 0.98, n = 22). The DS response ratio was reduced at more dorsal injection sites (Fig. 7A), while the NS response ratio (normalized to saline) increased at more ventral sites (Fig. 7B). These findings support the conclusion that the mPFC has dissociable functions in reward-seeking behaviors, with the dmPFC promoting cue-evoked responding and the vmPFC inhibiting responding to discrete unrewarded sensory cues, and both sites inhibiting uncued responding.
Fig. 7.
Relationship between inactivation sites in the mPFC and performance of the DS task. There was a significant correlation between the depth of injection sites and the effect of inactivation on the DS (n = 24) (A) or normalized NS ratio (n = 21) (B). The number of injection sites is derived from 16 animals for mPFC and dmPFC/vmPFC injections.
Histological verification of injection sites
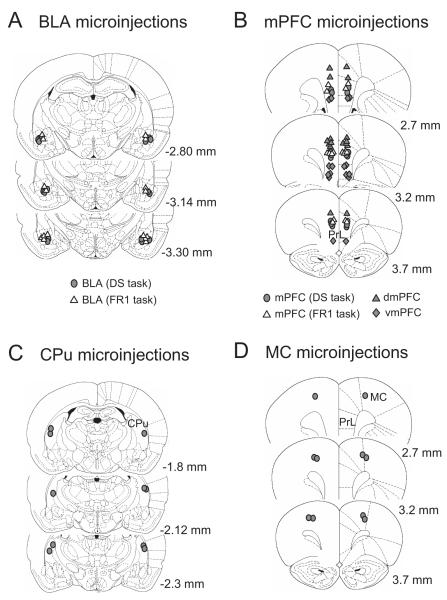
The tips of microinjection cannulae were verified to be in the BLA, mPFC (including dmPFC and vmPFC), CPu and MC of animals trained on the DS task (Fig. 8). The injection sites for mPFC inactivation were distributed in the middle part of the prelimbic cortex. The locations of the dmPFC inactivations were in the anterior cingulate cortex and boundary between the cingulate and prelimbic cortex, while the injections sites for the vmPFC were mainly in the infralimbic cortex. Microinjection sites of animals trained on the uncued FR1 task were within the same area of the BLA and mPFC (middle part of the prelimbic cortex) as in the DS task-trained animals.
Fig. 8.
Microinjection sites of the BLA (A), mPFC (B), CPu (C) and MC (D) in animals trained on the DS task (filled symbols) or uncued FR1 task (open triangles). The locations of the dmPFC inactivation were in the anterior cingulate cortex and boundary between the anterior cingulate and prelimbic cortex. mPFC injection sites were within the prelimbic cortex, while the locations for vmPFC inactivation were mainly in the infralimbic cortex. Numbers on each coronal section indicate distance from bregma according to Paxinos and Watson (1998).
Discussion
To obtain reward in the DS task, animals must perform an operant response during DS presentation. During the long (30 sec) intervals between cue presentations, and during NS presentation, reward is not available. Previously, we showed that dopamine acting in the NAc is required for animals to respond during the DS (Yun et al., 2004a,b). However, NAc neurons also inhibit behaviors that animals have learned to suppress in the context of a reward seeking task, as demonstrated by an increase in the probability of unrewarded actions after general inactivation of the NAc (Yun et al., 2004a). Here, we show that, similar to dopaminergic input from the VTA, both the BLA and dmPFC are required for responding to the DS. In contrast, inactivation of the vmPFC resulted in disinhibition of unrewarded behaviors, similar to the effects of general inactivation of the NAc. Since the BLA, dmPFC and vmPFC all project to the NAc, our results suggest that NAc neurons that promote reward-seeking in response to cues are driven by BLA and dmPFC neurons, whereas NAc neurons that inhibit responding to unrewarded cues are driven by inputs from the vmPFC. Furthermore, our results suggest that inputs from both dmPFC and vmPFC to NAc neurons inhibit uncued responding in this task.
Inactivation of the BLA
Inactivation of the BLA reduced both the DS response ratio and uncued responding in the FR1 task. The latter effect suggests that BLA inactivation either reduces motivation for reward or produces a motor impairment. However, we did not observe significant reduction in FR1 task responding at the 12.5 ng dose of M/B, and decreases in DS response ratio at each dose (12.5 ng, 43%; 25 ng, 64%; 50 ng, 85% relative to saline treatment) were greater than the reduction in responding on the FR1 task caused by equivalent doses (12.5 ng, 9%; 25 ng, 26%; 50 ng, 40%). Therefore, DS responding is more sensitive to BLA inactivation than FR1 responding, and this is likely due to differences in task structure. In particular, in the DS task, animals must respond to an explicit cue presented at relatively long (~30 sec) intervals, whereas no explicit cues are presented in the FR1 task and reward is always available in exchange for a lever press. Thus, BLA inactivation, particularly at low doses of M/B, relatively specifically impairs some aspect of reward-seeking triggered by discrete reward predictive cues.
Since the amygdala consists of multiple subdivisions such as the BLA, lateral and central nucleus, and these nuclei are small and adjacent to each other, it is possible that M/B injected into the BLA diffused to adjacent nuclei and affected behavioral performance. In particular, the central nucleus of the amygdala is required for performance of Pavlovian approach behavior, which is outwardly similar to the DS task: when a cue is presented, the animal locomotes towards an operandum, responds on it, and consumes reward (Parkinson et al., 2000). However, in Pavlovian approach behavior, the cue is usually the operandum itself, whereas the auditory DS sets the occasion for approach to the lever. In the former case, the cue may activate a general motivational process subserved by the central nucleus, whereas in the latter, the cue may activate an association between the DS and the specific reward it predicts. The ability to act on such an association requires the BLA rather than the central nucleus (Balleine and Killcross, 2006). Furthermore, lesions of the BLA that spare the central nucleus impair performance of a DS task for cocaine reward (Yun and Fields, 2003), suggesting that the effects of our injections were specific to the BLA. Nevertheless, our results do not rule out a contribution of the central nucleus to performance of the DS task for sucrose reward.
Although uncued responding in the DS task was not significantly reduced by BLA inactivation with low (12.5 and 25 ng) doses of M/B, the data show a trend towards a reduction in responding (Fig. 1D) especially when compared with the effects of the same doses of M/B on the uncued FR1 task (Fig. 5A). Because uncued responding in both the DS and FR1 task is not controlled by explicitly presented cues, it is paradoxical that uncued responding in the DS task should be more sensitive to BLA inactivation than uncued responding in the FR1 task. A potential resolution lies in the similarity of the effects of BLA inactivation and injection of dopamine receptor antagonists into the NAc. Specifically, the DS and NS response ratio, as well as active lever uncued responses in the DS task, decreased after injection of dopamine receptor antagonists into the NAc, whereas FR1 task performance was unaffected (Yun et al., 2004a). Recently, we showed that infusion of dopamine antagonists into the NAc reduced not only cue responding, but all responding that is initiated when the animal is not engaged in task-related reward-seeking behavior, such as pressing the lever or entering the reward receptacle (Nicola and Fields, 2007). These findings explain why uncued responding in the DS task, in which reward is only intermittently available and animals therefore become disengaged from task performance, is reduced by NAc dopamine antagonists, whereas uncued responding in the FR1 task, in which reward is always available and animals therefore rarely become disengaged, is unaffected. If the BLA’s glutamatergic projection to the NAc is required for NAc dopamine to promote responding from the disengaged state, one would expect the behavioral effects of BLA inactivation to parallel those of NAc dopamine antagonist injection.
This hypothesis is supported by evidence of anatomical and functional interaction of BLA and VTA afferents in the NAc. For instance, although dopamine attenuates excitation of NAc neurons evoked by stimulation of amygdalar afferents (Yim and Mogenson, 1982, 1983, 1986; Charara and Grace, 2003), dopamine increases the relative amplitude of strong, as compared to weak, glutamate-induced excitation in NAc neurons (Kiyatkin and Rebec, 1996; Nicola et al., 2000; Horvitz, 2002; Hjelmstad, 2004; Nicola et al., 2004a). Indeed, excitation of NAc neurons by DSs depends on the VTA; these excitations are also dependent on the BLA (Ambroggi et al., 2007). Furthermore, the glutamatergic terminals of BLA neurons projecting to the NAc synapse in close apposition to VTA-derived dopaminergic varicosities (Brog et al., 1993; Johnson et al., 1994; Phillips et al., 2003). These synapses are a possible site where the BLA glutamatergic projection can act presynaptically to increase dopamine efflux in the NAc (Floresco et al., 1998). These mutually facilitatory interactions between dopaminergic and glutamatergic afferents to the NAc may explain the similarity of the effects of NAc dopamine receptor antagonism and BLA inactivation on performance of the DS task.
Inactivation of the mPFC
In contrast to BLA inactivation, inactivation of the mPFC increased both the NS response ratio and responding in the absence of cues in the DS task, although, at higher doses, mPFC inactivation also decreased the DS response ratio and increased the DS response latency. Since mPFC inactivation had no effect on responding in the uncued FR1 task, the effects on the DS task are unlikely to be due to changes in motivation. The seemingly opposing effects of mPFC inactivation (decreasing DS responding while increasing responding to unrewarded cues) are likely due to the different roles of the dmPFC and vmPFC. Specifically, vmPFC inactivation increased the NS response ratio, whereas dmPFC inactivation reduced DS response ratio and increased DS response latency. A growing body of evidence delineates distinct functions of the dmPFC and vmPFC in several tasks (Passetti et al., 2002; Heidbreder and Groenewegen, 2003; Vidal-Gonzalez et al., 2006). For instance, Risterucci et al. (2003) show that lesions of the prelimbic and infralimbic cortex but not the anterior cingulate cortex produce increased premature responding and disrupted motor readiness in a reaction time task, in which subjects have to release a lever at the onset of a cue light. Furthermore, in a study using a five-choice task (Passetti et al., 2002), where animals have to choose one correct aperture out of 5 that is guided by light stimulus at the rear, an anterior cingulate lesion strongly reduced correct responding, increased correct response latency, and increased frequency of omissions compared to prelimbic-infralimbic cortex lesion. In contrast, perseverative responses were induced by prelimbic-infralimbic cortex but not anterior cingulate lesions. Finally, a recent study found that dmPFC, BLA or NAc core inactivation tended to reduce spontaneous recovery of cocaine-seeking behavior in abstinent animals, whereas vmPFC or NAc shell inactivation tended to increase this behavior (Peters et al., 2008a,b). Thus, previous findings are consistent with a role for the dmPFC in activation of reward-seeking behavior, particularly in response to cues, whereas the dmPFC exerts an inhibitory effect on behavior that accounts for the disinhibition of unrewarded responding when vmPFC is inactivated.
Since cue responding is impaired by both dopamine antagonists in NAc and general dmPFC inactivation, it is possible that the glutamatergic projection from the dmPFC is linked to dopaminergic neurotransmission within the NAc. Indeed, excitatory NAc neuronal responses to the DS, which require an intact projection from the VTA (Yun et al., 2004b), also depend on the mPFC (Ishikawa et al., 2008). These finding are supported by anatomical and electrophysiological evidence that mPFC projections interact with dopaminergic afferents within the NAc. Specifically, single NAc neurons receive convergent inputs from the mPFC and dopaminergic terminals (Sesack and Pickel, 1992). This structural relationship could underlie dopamine’s modulatory effects on the excitatory drive from mPFC neurons (Brady and O’Donnell, 2004). Such mPFC and VTA interaction within the NAc could promote the cue-evoked firing of NAc neurons, which in turn could facilitate the reward-seeking response to cues in the DS task.
Role of BLA and mPFC neuronal activity in reward-seeking behavior
Inactivation of the BLA and dmPFC resulted in effects similar to injection of dopamine receptor antagonists in the NAc: reduction in responding during DS presentation (Yun 2004a,b). In contrast, inactivation of the vmPFC had effects similar to NAc inactivation: an increase in responding to unrewarded cues (Yun 2004a). These functional differences could be due to the distinct connections of the dmPFC and vmPFC with the core and shell of the NAc. Anatomical studies show that the dmPFC mainly projects to the NAc core, while the vmPFC selectively innervates the NAc shell (Brog et al., 1993; Zahm, 2000). Indeed, responding from a state of task disengagement (which is likely to occur often during the DS task because reward availability is sparse) is more dependent on dopamine release in the NAc core than in the shell (Nicola and Fields, 2007). Thus, current evidence is consistent with the hypothesis that the vmPFC projection to the shell serves to inhibit reward-seeking behavior, whereas the BLA and dmPFC projections to the core serve to dopamine-dependently promote reward seeking. This conclusion is further supported by recent evidence that vmPFC and NAc shell inactivations disinhibit cocaine-seeking behavior, whereas BLA and NAc core inactivations reduce it (Peters et al., 2008a,b).
Also consistent with this hypothesis, BLA and dmPFC neurons respond phasically to reward-predictive cues in several behavioral tasks (Muramoto et al., 1993; Schoenbaum et al., 1998, 1999; Jodo et al., 2000). Phasic, cue-evoked excitation of NAc neurons appears to be required for animals to respond appropriately to reward-predictive cues (Nicola et al., 2004b; Yun et al., 2004b; Ambroggi et al., 2007; Ishikawa et al., 2008), and this firing could be due to the phasic responses of BLA and dmPFC neurons to cues. Therefore, we predict that phasic firing of neurons in the BLA and dmPFC also occurs in response to DSs, and that inactivation of the BLA and dmPFC reduces DS responding because these phasic responses are abolished. On the other hand, disinhibition of unrewarded responding might be due to attenuation of tonic firing (occurring in the absence of discrete cues or behavioral events) of vmPFC neurons in the context of the task, because these behavioral changes occur continuously and are not triggered by external stimuli. Future work should focus on determining how neurons in the BLA and mPFC influence the firing of NAc core and shell neurons to excite and inhibit reward-seeking behavior.
Acknowledgements
The authors are grateful to Dr. V. Kharazia for histology work.
Funding State of California/University of California, San Francisco (Alcohol and Substance Abuse Research Program to H.L.F.); National Institutes of Health (DA019473 to S.M.N.); Wheeler Center for the Neurobiology of Addiction.
Comprehensive list of abbreviations
- BLA
basolateral nucleus of the amygdala
- CPu
caudate putamen
- dmPFC
dorsal medial prefrontal cortex
- DS
discriminative stimulus
- FR
fixed ratio
- M/B
muscimol and baclofen
- MC
motor cortex
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- NS
non-rewarded stimulus
- vmPFC
ventral medial prefrontal cortex
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambroggi F, Ishikawa A, Seroussi A, Fields HL, Nicola SM. Evidence that dopamine enhance nucleus accumbens responses to incentive cues by gating an excitatory input from the basolateral amygdala. Soc Neurosci Abstr. 2007 [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, O’Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28:1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci. 2006;23:1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behav Neurosci. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO. Dopamine excites nucleus accumbens neurons through the differential modulation of glutamate and GABA release. J Neurosci. 2004;24:8621–8628. doi: 10.1523/JNEUROSCI.3280-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contrasting contributions of the prefrontal cortex and amygdala to cue-evoked reward seeking behavior. Soc Neurosci Abstr. 2007 [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;18:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Suzuki Y, Kayama Y. Selective responsiveness of medial prefrontal cortex neurons to the meaningful stimulus with a low probability of occurrence in rats. Brain Res. 2000;856:68–74. doi: 10.1016/s0006-8993(99)02386-0. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61:851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitation of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivation of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology. 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Fields HL. Nucleus accumbens core dopamine is required to transition from task disengagement to task engagement. Soc Neurosci Abstr. 2007 [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Hopf F Woodward, Hjelmstad GO. Contrast enhancement: a physiological effect of striatal dopamine? Cell Tissue Res. 2004a;318:93–106. doi: 10.1007/s00441-004-0929-z. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004b;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of the medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. (2000) [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology. 2008b;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–1508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Salazar RF, White W, Lacroix L, Feldon J, White IM. NMDA lesions in the medial prefrontal cortex impair the ability to inhibit responses during reversal of a simple spatial discrimination. Behav Brain Res. 2004;152:413–424. doi: 10.1016/j.bbr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Peoples LL. Firing patterns of accumbal neurons during a pavlovian-conditioned approach task. J Neurophysiol. 2006;96:652–660. doi: 10.1152/jn.00068.2006. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of nucleus accumbens neurons to amygdala stimulation and its modification by dopamine. Brain Res. 1982;239:401–415. doi: 10.1016/0006-8993(82)90518-2. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of ventral pallidal neurons to amygdala stimulation and its modulation by dopamine projections to nucleus accumbens. J Neurophysiol. 1983;50:148–161. doi: 10.1152/jn.1983.50.1.148. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Mesolimbic dopamine projection modulates amygdala-evoked EPSP in nucleus accumbens neurons: an in vivo study. Brain Res. 1986;369:347–352. doi: 10.1016/0006-8993(86)90548-2. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004a;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004b;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]