Abstract
Objective
To study the prevalence of psychiatric comorbidity based on reference standard diagnostic criteria in patients with human immunodeficiency virus (HIV). Psychiatric illness is common in patients with HIV and has been associated with negative health behaviors and poorer clinical outcomes. Among those persons with psychiatric illness, psychiatric comorbidity (multiple simultaneous diagnoses) is associated with increased psychiatric severity and higher HIV risk behaviors.
Methods
A total of 152 consecutively presenting HIV+ patients at an academic medical center in the southeastern US completed a modified Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) that assessed mood, anxiety, and substance use disorders in the past year and past month.
Results
Fifty percent and 33% of patients had a past-year and past-month diagnosis, respectively. The most common diagnoses were mood disorders (32% past year/21% past month) followed by anxiety (21%/17%) and substance use disorders (22%/11%). Half of those with past-year disorders and 40% of those with past-month disorders met the criteria for multiple diagnoses. Of those with a mood disorder in the past month, 53% also had an anxiety or substance use disorder; of those with an anxiety disorder, 62% also had a mood or substance use disorder; and of those with a substance use disorder, 63% also had a mood or anxiety disorder. Psychiatric comorbidity was associated with younger age, White non-Hispanic race/ethnicity, and greater HIV symptomatology.
Conclusions
Comorbidity of mood, anxiety, and substance use disorders was the exception rather than the rule in this sample. Potential co-occurring disorders should be considered for HIV+ patients presenting with a psychiatric diagnosis.
Keywords: psychiatric comorbidity, prevalence, HIV, depression, anxiety, substance use
Introduction
Co-occurring mental illness has direct clinical relevance for patients infected with human immunodeficiency virus (HIV). Mental illness worsens functional impairment and quality of life (1) and is associated with a more rapid and harder-to-treat progression of HIV disease (2–4). These worsened outcomes are believed to be related to both behavioral mechanisms (noncompliance with HIV medications) (5) as well as direct biological measures (6,7). Given this risk, many medical professionals have used the identification of a psychiatric illness coexisting with HIV infection as a red flag to target for more aggressive and comprehensive clinical management (8–10).
Co-occurring mental illness may play a key role in the southeastern US—the region with a fast-rising HIV epidemic (11). In our earlier work in an infectious disease clinic setting with a patient population reflective of the HIV+ population in the Southeast, we assessed a consecutive sample of 152 patients with a reference standard psychiatric diagnostic interview (12). We documented past-year prevalence for any substance use disorder and any mood/anxiety disorder of 20% and 41%, respectively, consistent with prior reports on increased rates of psychiatric morbidity in HIV+ populations (13,14). Each of these psychiatric disorders, on their own, has been associated with decreased access to highly active antiretroviral therapy (HAART) and poor antiretroviral adherence (15–17).
Yet, for many individuals with mental illness, having more than one psychiatric diagnosis concurrently is common, and this psychiatric comorbidity is strongly related to greater psychiatric severity (18) and higher rates of suicide attempts (19). Psychiatric comorbidity is further linked to worse medical outcomes (20), likely in part through the mechanism of nonadherence to medication treatment (21). Psychiatric comorbidity is particularly worrisome for patients with HIV because of the influence of psychiatric illness on risk behaviors, medication adherence, and clinical course and the fact that psychiatric comorbidity makes each of the component psychiatric illnesses more difficult to treat. Psychiatric comorbidity in patients with HIV can portend worse psychiatric and HIV outcomes as well as increased risk behaviors for secondary transmission of HIV (22,23). Of particular concern for patients with HIV is the association of unmanaged psychiatric illness with nonadherence to HAART (5) and the subsequent development of antiretroviral-resistant HIV (24).
Despite this clinical relevance, we could find no reference standard-based report on the prevalence of psychiatric comorbidity in the HIV+ population apart from one report restricted to past-year psychiatric diagnoses in patients with HIV who received methadone treatment (25). Accordingly, we report here on psychiatric comorbidity, both in the past month and the past year, as established by reference standard Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) diagnoses, in a consecutive population of HIV+ patients presenting for HIV care at an infectious disease clinic in an academic medical center in the southeastern US.
Methods
Study Population
The study population comprised 152 HIV+ patients consecutively recruited at an infectious disease clinic in an academic medical center in the southeastern US as part of a validation study of a psychiatric screening instrument (12). All patients were considered eligible for recruitment who kept a scheduled appointment between August 2003 and June 2004, were HIV+, either were new or had not previously completed the screening instrument, were at least 18 years old, were English-speaking, and were mentally competent to provide informed consent as assessed by their medical provider. After complete description of the study to the subjects, written informed consent was obtained. All study procedures were approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Measures
Participants completed the Structured Clinical Interview for DSM-IV (DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) (SCID) (26) administered by a trained psychiatry research interviewer with 5 years of experience in psychiatry research and 3 years of experience in the administration of SCID. Patients completed the interview either in person or by telephone. Previous research has indicated that the SCID, administered in person or by telephone, has comparable test characteristics (27–30). The interviewer assigned final diagnoses after the interview in consultation with a psychiatrist (B.N.G.). Diagnoses were assigned as being present or absent within the past year and within the past month (current). Only diagnoses included in the Substance Use, Mood, and Anxiety sections of the SCID were considered because these disorders were targeted by the screening instrument as the focus of the parent validation study and because they are the most prevalent psychiatric disorders among patients with HIV. Both the interviewer and the psychiatrist were blinded to the results of the previously administered screening instrument that was the focus of the validation study.
During the interview, participants also provided information on sociodemographic characteristics and if they had experienced each of a list of HIV-related symptoms in the past 6 months: persistent headaches, fevers, oral pain, white patches in the mouth, rashes, nausea or loss of appetite, eye trouble, sinus infection, numbness in the extremities, persistent cough, diarrhea, weight loss, and (for women only) vaginal discharge. Trained chart abstractors collected information on current CD4 counts and antiretroviral treatment status from participants' medical records.
Analyses
We calculated past-year and past-month prevalence estimates for specific diagnoses and for measures of comorbidity. We used logistic regression models to evaluate sociodemographic and clinical characteristics as predictors of psychiatric morbidity and comorbidity. In modeling continuous variables (age and CD4 count), we compared a restricted quadratic spline specification with knots at the 10th, 50th, and 90th percentiles to a simple linear specification in bivariate analyses, and retained the former only if indicated by a statistically significant increase in the log likelihood (p < .05). We visually confirmed the appropriateness of the chosen specification with locally weighted scatterplot smoothing (lowess) graphs. To limit the influence of small numbers of observations at extreme values, we coded all values of CD4 >1000 (n = 5) to 1000. We assessed the global fit of each final model using Pearson and Hosmer-Lemeshow goodness-of-fit statistics, and we examined residuals for observations that were overly influential in the estimation of coefficients (31). We calculated the area under the receiver operating characteristic (ROC) curve to evaluate the discriminatory power of each model.
Results
Recruitment
As described previously (12), 185 patients consented to participate, representing 82% of all patients invited to participate and 65% of all eligible patients who kept appointments during the recruitment period. Although there were no written records of reasons for nonparticipation, the study staff reported that the most common reasons for nonparticipation were expressed lack of interest in the study or anticipated difficulty in scheduling the follow-up interview. Lack of available study personnel constituted the only reason eligible patients were not invited to participate.
SCIDs were successfully completed with 152 (82.2%) of the 185 participants. Of the 33 participants who did not complete the SCID, 28 were not successfully recontacted and 5 refused to complete the SCID when recontacted, citing lack of either time or interest. The distribution of age, race, sex, and health insurance status did not differ significantly between participants who completed the SCID and those who were lost to follow-up. Twenty-one (13.8%) SCIDs were conducted in person and 131 (86.2%) were conducted by phone. Those participants interviewed in person and by phone were comparable on all background characteristics and reported similar prevalence of mood and anxiety disorders, but a higher prevalence of substance use disorders was reported among those interviewed in person (24% versus 8%, p = .03). Results did not change appreciably when analyses were restricted to those interviewed by phone or when the interview format was included as a covariate in multivariable models.
Sample Characteristics
Participants were primarily 30 to 50 years old (range = 20–65 years; mean ± standard deviation = 40.5 ± 8.8 years) (data not shown). Approximately one third of the participants were female. Approximately three quarters were African-American, with the remainder primarily Caucasian. Thirty-six percent received public health insurance and 28% were uninsured. Participants were similar to the clinic patient population in gender and insurance status but were younger on average (mean age = 40.4 versus 42.5 years, p = .01) and more likely to be African-American (72% versus 59%; p < .01).
Prevalence
Psychiatric disorders affected half of the participants in the past year and one third in the past month (Table 1). Mood disorders were the most prevalent (32% in the past year, 21% in the past month), followed by anxiety disorders (21% and 17%) and substance use disorders (22% and 11%). Depression (major depressive disorder or depressive disorder not otherwise specified) accounted for nearly all diagnoses of mood disorders. Posttraumatic stress disorder was the most common anxiety disorder. Alcohol and crack/cocaine use accounted for nearly all substance use disorders, as documented in other research on the HIV epidemic in the South. Apart from panic disorder, most diagnoses of anxiety disorders in the past year were also present in the past month, suggesting low incidence and relatively long duration. In contrast, mood and substance use disorders tended to have higher prevalence in the past year than in the past month, indicating either the resolution or remission of between 25% and 50% of cases occurring in the preceding year.
TABLE 1. Prevalence of Past-Year and Past-Month Mood, Anxiety, and Substance Use Disorders and Comorbidity, 152 HIV+ Patients.
| Disordera | Past Year, n (%) | Past Month, n (%) |
|---|---|---|
| Overall | ||
| Any disorder | 74 (50) | 50 (33) |
| Mood disorders | ||
| Any mood disorder | 48 (32) | 32 (21) |
| Major depressive disorder | 24 (16) | 17 (11) |
| Depressive disorder NOS | 16 (11) | 13 (9) |
| Substance-induced mood disorder | 4 (3) | 1 (1) |
| Other mood disorderb | 5 (3) | 1 (1) |
| Anxiety disorders | ||
| Any anxiety disorder | 32 (21) | 26 (17) |
| Posttraumatic stress disorder | 10 (7) | 9 (6) |
| Panic disorder | 8 (5) | 4 (3) |
| Anxiety disorder NOS | 6 (4) | 5 (3) |
| Social phobia | 4 (3) | 4 (3) |
| Specific phobia | 5 (3) | 5 (3) |
| Obsessive-compulsive disorder | 2 (1) | 2 (1) |
| Substance use disorders | ||
| Any substance use disorder | 33 (22) | 16 (11) |
| Alcohol | 17 (11) | 12 (8) |
| Crack/cocaine | 21 (14) | 7 (5) |
| Cannabis | 3 (2) | 1 (1) |
| Comorbidity | ||
| Any mood/anxiety disorder | 62 (41) | 44 (29) |
| Both mood and anxiety disorders | 18 (12) | 14 (9) |
| Substance use and mood/anxiety disorders | 21 (14) | 10 (7) |
| ≥2 disorders | 37 (24) | 20 (13) |
| ≥3 disorders | 10 (7) | 8 (5) |
Diagnosed with the Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition).
Dysthymia, bipolar I or II, adjustment disorder with depressed mood, bereavement, or mood disorder not otherwise specified.
HIV = human immunodeficiency virus; NOS = not otherwise specified.
Comorbidity
A substantial proportion of the participants with psychiatric illness had multiple diagnoses (Table 2). Of the participants who in the past year had at least one diagnosis (cases), half had two or more diagnoses. Forty percent of past-month cases had multiple diagnoses. When attention was restricted to mood and anxiety disorders only, approximately 30% of cases in the past year and the past month had multiple disorders.
TABLE 2. Comorbidity of Past-Year Past-Month Mood, Anxiety, and Substance Use Disorders, 152 HIV+ Patients.
| Number of Disorders | Past Year (%) | Past Month (%) | ||
|---|---|---|---|---|
| Respondentsa | Casesb | Respondentsa | Casesb | |
| Mood, anxiety, and substance use disorders | ||||
| 0 | 51 | – | 67 | – |
| 1 | 24 | 50 | 20 | 60 |
| 2 | 18 | 37 | 8 | 24 |
| ≥3 | 7 | 14 | 5 | 16 |
| Mood and anxiety disorders only | ||||
| 0 | 59 | – | 71 | – |
| 1 | 29 | 71 | 20 | 68 |
| 2 | 9 | 23 | 7 | 25 |
| ≥3 | 3 | 6 | 2 | 7 |
Percent of all respondents (n = 152).
Percent of all respondents with at least one disorder (past year: n = 74; past month: n = 50).
HIV = human immunodeficiency virus.
Having a single mental health diagnosis was the exception (Figure 1). Of the 50 individuals with at least one psychiatric diagnosis, 32 (64%) had a mood disorder, 26 (52%) had an anxiety disorder, and 16 (32%) had a substance use disorder. Of the 32 participants with a mood disorder, fewer than half (15/32 or 47%) had a mood disorder alone, with the remainder having co-occurring anxiety and/or substance use disorders. Similarly, of the 26 participants with an anxiety disorder, fewer than half (10/26 or 38%) had an anxiety disorder alone; of the 16 participants with a substance use disorder, fewer than half (6/16 or 38%) had a substance use disorder alone. The comorbidity between mood and anxiety disorders was the most common. Of those persons with an anxiety disorder, over half (14/26 or 54%) had a mood disorder whereas of those with a mood disorder, nearly half (14/32 or 44%) had a coexisting anxiety disorder. About one third of those with comorbid anxiety and mood disorders also met the criteria for a substance use disorder (5/14 or 36%).
Figure 1.
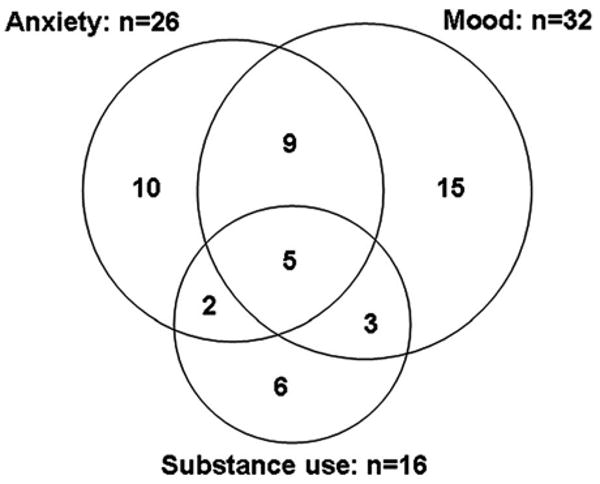
Comorbidity of past-month mood, anxiety, and substance use disorders among 50 HIV+ patients with at least one disorder.
Predictors of Psychiatric Morbidity and Comorbidity
In multivariable logistic regression models predicting current psychiatric morbidity and comorbidity, greater age was predictive of lower prevalence of any psychiatric disorder, any anxiety disorder, and ≥2 and ≥3 disorders (Table 3). Point estimates for the remaining two outcomes (any mood disorder and any substance disorder) were in the same direction but not statistically significant. Point estimates for associations of female gender with outcomes were not statistically significant but the direction of all associations was consistent with expectations (higher prevalence of mood and anxiety disorders; lower prevalence of substance use disorders; and higher prevalence of comorbidity). White non-Hispanic race/ethnicity was predictive of greater risk of mood, anxiety, and comorbid disorders, and point estimates for all other outcomes were in the same direction.
TABLE 3. Predictorsa of Past-Month Psychiatric Morbidity and Comorbidity, 152 HIV+ Patients.
| Characteristic | Any Disorder | Any Mood Disorder | Any Anxiety Disorder | Any Substance Disorder | ≥2 Disorders | ≥3 Disorders |
|---|---|---|---|---|---|---|
| Age, per 10 years | 0.43b (0.25, 0.74) | 0.64 (0.36, 1.16) | 0.31b (0.15, 0.64) | 0.68 (0.32, 1.48) | 0.45b (0.20, 1.00) | 0.29b (0.09, 0.97) |
| Female | 1.09 (0.40, 2.99) | 1.66 (0.51, 5.41) | 2.55 (0.72, 9.04) | 0.46 (0.08, 2.68) | 4.70 (0.92, 24.12) | 5.65 (0.51, 62.28) |
| White non-Hispanic | 2.33 (0.74, 7.29) | 3.94b (1.06, 14.61) | 4.90b (1.14, 21.07) | 2.58 (0.56, 11.91) | 17.21b (2.71, 109.44) | 15.99 (0.98, 260.66) |
| Public/no health insurance | 1.62 (0.65, 4.06) | 1.38 (0.47, 4.04) | 1.97 (0.61, 6.34) | 0.67 (0.16, 2.75) | 1.70 (0.44, 6.59) | 0.58 (0.09, 3.56) |
| >HS education | 0.99 (0.39, 2.49) | 0.49 (0.16, 1.50) | 2.47 (0.81, 7.49) | 0.51 (0.11, 2.34) | 0.55 (0.14, 2.17) | 0.49 (0.07, 3.27) |
| CD4, per 100 cells/mm3 | 1.08 (0.91, 1.27) | 1.18 (0.98, 1.42) | 0.95 (0.77, 1.17) | 0.93 (0.72, 1.20) | 1.12 (0.88, 1.41) | 0.91 (0.63, 1.31) |
| On antiretroviral therapy | 0.29b (0.11, 0.75) | 0.43 (0.15, 1.22) | 0.23b (0.07, 0.75) | 0.16b (0.04, 0.70) | 0.28 (0.07, 1.07) | 0.19 (0.03, 1.29) |
| HIV symptoms endorsed | 1.30b (1.13, 1.49) | 1.36b (1.16, 1.61) | 1.30b (1.09, 1.54) | 1.19 (0.98, 1.44) | 1.36b (1.11, 1.67) | 1.37b (1.03, 1.81) |
| Area under ROC curve | 0.78 | 0.80 | 0.84 | 0.77 | 0.86 | 0.89 |
Figures are odds ratios (95% confidence intervals).
p < .05, two-tailed.
HIV = human immunodeficiency virus; HS = high school; ROC = receiver operating characteristic.
Patients who received antiretroviral therapy were significantly less likely to have any psychiatric disorder, any anxiety disorder, or any substance disorder, with point estimates for other outcomes in the same direction. A greater number of reported HIV-related medical symptoms was positively associated with five out of six outcomes, with the 6th point estimate (any substance use disorder) in the same direction. The relationship between HIV symptomatology and psychiatric morbidity did not change when HIV symptoms potentially caused by mental illness (nausea/loss of appetite and weight loss) were excluded.
Health insurance status, educational attainment, and CD4 count were not significantly associated with any outcome nor was the direction of point estimates consistent across outcomes. The overall models demonstrated good predictive power as measured by the area under the ROC curve, with all areas between 0.77 and 0.89. All models demonstrated satisfactory goodness-of- fit.
Most independent variables showed similar associations with disorders in the past year (data not shown), with the following exceptions: lack of private health insurance was predictive of any psychiatric disorder, and college education was predictive of any anxiety disorder.
Discussion
For those HIV+ patients with a nonpsychotic mental health disorder, having a single mental health diagnosis was the exception rather than the rule. Overall, half of the patients with a reference standard diagnosis of a past-year mental health disorder and 40% of the patients with a past-month diagnosis met the criteria for multiple diagnoses. More than half of the patients with a past-month diagnosis of a mood disorder also had a co-occurring anxiety or substance use disorder; more than half of the patients with an anxiety disorder diagnosis also had a co-occurring mood or substance use disorder; and more than half of the patients with a substance use disorder diagnosis also had a co-occurring mood or anxiety disorder.
The relationship between psychiatric comorbidity and medical illness in general is clinically important because of the association with poorer medication compliance (21) and worse outcomes for both the psychiatric illness (19) and the medical illness (32–34). In HIV+ patients, in particular, the relationship between psychiatric comorbidity and clinical course is especially important because of the potentially life-threatening risk of development of antiretroviral-resistant HIV (24). As demonstrated by our results, the prevalence of comorbid psychiatric illness in patients with HIV is substantial.
The frequency of co-occurring psychiatric disorders in this sample has important implications for medical professionals providing care for HIV+ patients. First, for any patient in whom a single psychiatric disorder is identified, the possibility of additional co-occurring disorders should be strongly considered. Second, comorbidity may affect the psychiatric treatment plan. For example, a patient with major depression and a co-occurring anxiety disorder may require a lower initial antidepressant dose (because of susceptibility to activation side effects) and, in general, would require eventually a higher final antidepressant dose than a patient with major depression alone (35,36). Similarly, substance abuse treatment programs may prove ineffective for individuals with a co-occurring depressive disorder that is not identified and addressed (37). Third, if a patient with an identified psychiatric disorder fails to respond to the current psychiatric treatment plan, the clinician should consider a more thorough psychiatric diagnostic evaluation to assess for additional co-occurring disorders. These conclusions are particularly germane given the reported effectiveness of successful psychiatric treatment in improving HIV medication adherence and clinical outcomes (9,38,39); such benefits may not be realized if the treatment plan does not address co-occurring disorders.
Relative to the general population, this sample of HIV+ patients demonstrated a higher prevalence of psychiatric illness overall but showed a similar pattern of comorbidity among those with at least one diagnosis. The recently completed National Comorbidity Survey Replication (NCS-R) estimated that 34% of the adult English-speaking US population met the criteria for a DSM-IV disorder in the past 12 months compared with 49% in the present sample (18). In both the NCS-R and the present sample, however, 50% of cases had a single disorder and 50% had comorbid disorders.
The high prevalence of mood, anxiety, and substance use disorders observed in the present study is consistent with previous reports of increased rates of psychiatric morbidity in HIV+ individuals. In a national sample of HIV+ individuals engaged in medical care, approximately half reported significant current mental health symptoms, 13% reported symptoms of current drug dependence, and 19% reported heavy drinking (13); approximately one in eight persons were estimated to have mental health symptoms co-occurring with problematic alcohol or drug use (40). US studies based on diagnostic criteria rather than screening instruments have generally found major depressive disorder to be approximately twice as common in HIV+ individuals relative to comparable controls, with prevalence estimates ranging from 4% to 23% (median 8%) (14). In the only identified study to report estimates of psychiatric comorbidity based on diagnostic criteria in HIV+ individuals, Winiarski and colleagues reported that, of 139 HIV+ patients who received methadone treatment, 71% merited at least one additional past-year psychiatric diagnosis besides opioid dependence, and the mean number of past-year psychiatric diagnoses was 3 (range = 1–7) (25). Our finding that 63% (10/16) of patients with a substance use disorder had at least one additional psychiatric diagnosis is consistent with the Winiarski report. Of note, this prior study assessed past-year diagnoses that would be expected to produce higher comorbidity estimates than past-month assessments. Further, the Winiarski study included psychotic disorders and antisocial and borderline personality disorders. We report here only on mood, anxiety, and substance use disorders; thus, our estimates of psychiatric comorbidity are likely to be underestimates of the co-occurrence of all psychiatric disorders.
In this sample, patients with a psychiatric illness were one fourth as likely to be on antiretroviral therapy as patients with no psychiatric illness after controlling for key covariates, such as CD4 count and insurance status. This finding may reflect a tendency on the part of HIV providers either not to initiate antiretroviral therapy in patients with a mental illness or to delay initiation until depression and substance use issues—key barriers to medication adherence—are being successfully addressed (16,17). Alternatively, patients with a psychiatric illness may be more likely to discontinue antiretroviral therapy (41). White non-Hispanics were more likely than patients of other race/ethnicities to have mood, anxiety, and multiple psychiatric disorders; the odds ratio for multiple disorders was strikingly large but also had a very wide confidence interval, reflecting substantial instability around the point estimate due to a small number of patients of other race/ethnicities with multiple disorders.
Although the present study only recruited patients from one infectious disease clinic, the demographic and transmission characteristics of the clinic's patient population mirror the epidemiologic dynamics of HIV/AIDS (acquired immunodeficiency syndrome) in the southeastern US. Specifically, approximately two thirds of patients are African-American with the remainder primarily White; two thirds are male; many come from poor and rural areas; and heterosexual intercourse represents an increasingly important mode of transmission (42,43). The clinic, located in a small urban area, does not serve a specialized (e.g., inner city or primarily injection drug using) patient population but rather serves nearly all persons living with HIV in the immediate vicinity and a large proportion of those within several hours' driving distance. The consecutive sampling design used in the current study yielded a sample largely reflective of the overall clinic population. Consecutive sampling, at the same time avoiding biases inherent in convenience samples, tends to oversample patients with more frequent HIV medical appointments. Patients with psychiatric comorbidity may require more frequent appointments, but mental illness has also been cited as a barrier to accessing care (44); thus, any resulting bias in our estimates of prevalence and comorbidity is uncertain in direction. The present sample had a younger mean age and a higher proportion of African-American patients compared with the clinic as a whole, possibly reflecting either more frequent appointments or greater willingness to participate in these groups.
The South has received increasing attention in recent years as the US region with the fastest growing HIV epidemic. Further, the transition of the HIV epidemic is most pronounced in the South, as HIV increasingly affects women, minorities, the poor, and those from rural areas and is transmitted via heterosexual intercourse (11,42,43,45). Although many explanations of the epidemic's disproportionate impact on the South have been proposed, efforts to turn the tide continue to face considerable challenges (11). Nationally, HIV prevention initiatives are emphasizing secondary prevention (reducing transmission risk behaviors among HIV+ individuals) as a primary strategy in stemming the HIV epidemic (46). Secondary prevention efforts may prove particularly challenging in the context of high prevalence of mental illness, because psychiatric disorders and particularly psychiatric comorbidity predict higher and more intractable risk behavior profiles in persons with HIV (23,47).
Although the SCID is a widely recognized and utilized reference standard instrument for the reliable and valid assignment of DSM-based diagnoses, it is nevertheless likely subject to some measurement error in assessing the unmeasurable latent construct of psychiatric illness. The stringent and detailed criteria required for the assignment of a given diagnosis suggest that the SCID may be relatively more specific than it is sensitive—that is, the SCID may be more likely to miss a case than flag a false-positive. In such a situation, the reported estimates of prevalence and comorbidity would be underestimates. Psychiatric measurement remains a considerable challenge and the focus of extensive research. In the present study, all SCID interviews were conducted by the same interviewer and all diagnoses were reviewed by the same psychiatrist to ensure consistency.
A lower reported prevalence of substance use disorders was observed in those completing the SCID by telephone compared with those interviewed in person. This difference could be due to selection (i.e., substance users being more likely to desire an in-person interview) or differential reporting (e.g., more underreporting of substance use by phone). Before the SCID, all participants completed an initial in-person mental health and substance use screen as part of the parent validation study. Those who ended up selecting an in-person format for the SCID were more likely to have screened positive for probable substance use on the in-person screen than those who ended up electing to complete the SCID by telephone (62% versus 33%; p = .01), whereas the two groups were equally likely to screen positive for probable mental illness (71% versus 68%; p = .75). These results suggest that the difference in observed prevalence of substance use disorders by the SCID interview format is more likely a function of selection than differential reporting.
The findings presented in this study represent the first report of reference standard-based psychiatric comorbidity estimates in a general HIV+ patient population. We find that comorbidity of mood, anxiety, and substance use disorders is the exception rather than the rule, suggesting that consideration of potential co-occurring disorders should be included in the development of a treatment plan for an HIV+ patient presenting with a psychiatric diagnosis.
Acknowledgments
This research was supported, in part, by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), a National Institutes of Health (NIH)-funded program (P30 AI50410), and the NIH-funded UNC General Clinical Research Center (RR-000046). The content of this publication does not necessarily reflect the views or policies of SAMHSA, HRSA, NIH, or DHHS. Dr. Pence was supported, in part, by a Howard Hughes Medical Institute Pre-doctoral Fellowship. Dr. Gaynes was supported, in part, by an NIMH K23 Career Development Award (MH01951-03).
- HAART
highly active antiretroviral therapy
- SCID
Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition)
References
- 1.Sherbourne CD, Hays RD, Fleishman JA, Vitiello B, Magruder KM, Bing EG, McCaffrey D, Burnam A, Longshore D, Eggan F, Bozzette SA, Shapiro MF. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry. 2000;157:248–54. doi: 10.1176/appi.ajp.157.2.248. [DOI] [PubMed] [Google Scholar]
- 2.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV epidemiology research study. JAMA. 2001;285:1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 3.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 4.Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:159–66. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- 5.Gordillo V, del Amo J, Soriano V, González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–9. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 6.Bagasra O, Pomerantz RJ. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis. 1993;168:1157–64. doi: 10.1093/infdis/168.5.1157. [DOI] [PubMed] [Google Scholar]
- 7.Leserman J. The effects of stressful life events, coping, and cortisol on HIV infection. CNS Spectr. 2003;8:25–30. doi: 10.1017/s1092852900023439. [DOI] [PubMed] [Google Scholar]
- 8.Angelino AF, Treisman GJ. Management of psychiatric disorders in patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;33:847–56. doi: 10.1086/322679. [DOI] [PubMed] [Google Scholar]
- 9.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38:432–8. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 10.Bouhnik AD, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, Gastaut JA, Moatti JP, Spire B. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir Ther. 2005;10:53–61. [PubMed] [Google Scholar]
- 11.Aral SO, O'Leary A, Baker C. Sexually transmitted infections and HIV in the southern United States: an overview. Sex Transm Dis. 2006;33:S1–S5. doi: 10.1097/01.olq.0000223249.04456.76. [DOI] [PubMed] [Google Scholar]
- 12.Pence BW, Gaynes BN, Whetten K, Eron JJ, Jr, Ryder RW, Miller WC. Validation of a brief screening instrument for substance abuse and mental illness in HIV-positive patients. J Acquir Immune Defic Syndr. 2005;40:434–44. doi: 10.1097/01.qai.0000177512.30576.9c. [DOI] [PubMed] [Google Scholar]
- 13.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 14.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 15.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–80. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 16.Turner BJ, Fleishman JA, Wenger N, London AS, Burnam MA, Shapiro MF, Bing EG, Stein MD, Longshore D, Bozzette SA. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16:625–33. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairfield KM, Libman H, Davis RB, Eisenberg DM. Delays in protease inhibitor use in clinical practice. J Gen Intern Med. 1999;14:395–401. doi: 10.1046/j.1525-1497.1999.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3:244–54. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherbourne CD, Wells KB, Meredith LS, Jackson CA, Camp P. Comorbid anxiety disorder and the functioning and well-being of chronically ill patients of general medical providers. Arch Gen Psychiatry. 1996;53:889–95. doi: 10.1001/archpsyc.1996.01830100035005. [DOI] [PubMed] [Google Scholar]
- 21.DiMatteo MR, Lepper HS, Croghan TW. depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 22.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Dausey DJ, Desai RA. Psychiatric comorbidity and the prevalence of HIV infection in a sample of patients in treatment for substance abuse. J Nerv Ment Dis. 2003;191:10–7. doi: 10.1097/00005053-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Parienti JJ, Massari V, Descamps D, Vabret A, Bouvet E, Larouze B, Verdon R. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–6. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 25.Winiarski MG, Greene LI, Miller AL, Palmer NB, Salcedo J, Villanueva M. Psychiatric diagnoses in a sample of HIV-infected people of color in methadone treatment. Community Ment Health J. 2005;41:379–91. doi: 10.1007/s10597-005-5076-9. [DOI] [PubMed] [Google Scholar]
- 26.First MH, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders—Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 1990. [Google Scholar]
- 27.Aneshensel CS, Frerichs RR, Clark VA, Yokopenic PA. Telephone versus in-person surveys of community health status. Am J Public Health. 1982;72:1017–21. doi: 10.2105/ajph.72.9.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aneshensel CS, Frerichs RR, Clark VA, Yokopenic PA. Measuring depression in the community: a comparison of telephone and personal interviews. Public Opin Q. 1982;46:110–21. doi: 10.1086/268703. [DOI] [PubMed] [Google Scholar]
- 29.Simon GE, Revicki D, VonKorff M. Telephone assessment of depression severity. J Psychiatr Res. 1993;27:247–52. doi: 10.1016/0022-3956(93)90035-z. [DOI] [PubMed] [Google Scholar]
- 30.Wells KB, Burnam MA, Leake B, Robins LN. Agreement between face-to-face and telephone-administered versions of the depression section of the NIMH diagnostic interview schedule. J Psychiatr Res. 1988;22:207–20. doi: 10.1016/0022-3956(88)90006-4. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 32.Aina Y, Susman JL. Understanding comorbidity with depression and anxiety disorders. J Am Osteopath Assoc. 2006;106:S9–S14. [PubMed] [Google Scholar]
- 33.McLaughlin TP, Khandker RK, Kruzikas DT, Tummala R. Overlap of anxiety and depression in a managed care population: prevalence and association with resource utilization. J Clin Psychiatry. 2006;67:1187–93. doi: 10.4088/jcp.v67n0803. [DOI] [PubMed] [Google Scholar]
- 34.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 35.Lydiard RB. Coexisting depression and anxiety: special diagnostic and treatment issues. J Clin Psychiatry. 1991;52(Suppl):48–54. [PubMed] [Google Scholar]
- 36.Pollack MH. Comorbid anxiety and depression. J Clin Psychiatry. 2005;66(Suppl 8):22–9. [PubMed] [Google Scholar]
- 37.Ostacher MJ. Comorbid alcohol and substance abuse dependence in depression: impact on the outcome of antidepressant treatment. Psychiatr Clin North Am. 2007;30:69–76. doi: 10.1016/j.psc.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Himelhoch S, Moore RD, Treisman G, Gebo KA. Does the presence of a current psychiatric disorder in AIDS patients affect the initiation of antiretroviral treatment and duration of therapy? J Acquir Immune Defic Syndr. 2004;37:1457–63. doi: 10.1097/01.qai.0000136739.01219.6d. [DOI] [PubMed] [Google Scholar]
- 39.Dalessandro M, Conti CM, Gambi F, Falasca K, Doyle R, Conti P, Caciagli F, Fulcheri M, Vecchiet J. Antidepressant therapy can improve adherence to antiretroviral regimens among HIV-infected and depressed patients. J Clin Psychopharmacol. 2007;27:58–61. doi: 10.1097/JCP.0b013e31802f0dd1. [DOI] [PubMed] [Google Scholar]
- 40.Galvan FH, Burnam MA, Bing EG. Co-occurring psychiatric symptoms and drug dependence or heavy drinking among HIV-positive people. J Psychoactive Drugs. 2003;35(Suppl 1):153–60. doi: 10.1080/02791072.2003.10400510. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Margolick JB, Conover CS, Badri S, Riddler SA, Witt MD, Jacobson LP. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–8. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report. 2005. Vol. 17. Atlanta, GA: Centers for Disease Control; 2006. [Google Scholar]
- 43.Reif S, Geonnotti KL, Whetten K. HIV infection and AIDS in the Deep South. Am J Public Health. 2006;96:970–3. doi: 10.2105/AJPH.2005.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Psychological Association. The Report of the Rural Women's Work Group of the Rural Task Force of the American Psychological Association and the American Psychological Association's Committee on Rural Health 2001. Atlanta, GA: Centers for Disease Control; 2006. The Behavioral Health Care Needs of Rural Women. [Google Scholar]
- 45.Karon JM, Fleming PL, Steketee RW, De Cock KM. HIV in the United States at the turn of the century: an epidemic in transition. Am J Public Health. 2001;91:1060–8. doi: 10.2105/ajph.91.7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Institute of Medicine. No Time to Lose: The AIDS Crisis Is Not Over: Getting More from HIV Prevention. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 47.Kelly JA, Murphy DA, Bahr GR, Koob JJ, Morgan MG, Kalichman SC, Stevenson LY, Brasfield TL, Bernstein BM, St Lawrence JS. Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with human immunodeficiency virus (HIV) infection. Health Psychol. 1993;12:215–9. doi: 10.1037//0278-6133.12.3.215. [DOI] [PubMed] [Google Scholar]


