Abstract
To elucidate the effects of steady-state methadone exposure on responding to cocaine conditioned stimuli and on cocaine-induced alterations in central opioid, hypocretin/orexin, and D2 receptor systems, male Sprague-Dawley rats received intravenous infusions of 1 mg/kg/inf cocaine paired with an audiovisual stimulus over three days of conditioning. Then, mini pumps releasing vehicle or 30 mg/kg/day methadone were implanted (SC), and lever pressing for the stimulus was assessed in the absence of cocaine and after a cocaine prime (20 mg/kg, IP). It was found that rats treated with vehicle, but not methadone, responded for the cocaine conditioned stimulus and displayed elevated mu-opioid receptor mRNA expression in the nucleus accumbens core and basolateral amygdala, reduced hypocretin/orexin mRNA in the lateral hypothalamus, and reduced D2 receptor mRNA in the caudate-putamen. This is the first demonstration that steady-state methadone administered after cocaine exposure blocks cocaine-induced behavioral and neural adaptations.
Keywords: Methadone, cocaine, mu-opioid receptor, hypocretin/orexin, dopamine receptor
Introduction
Our laboratories have been exploring whether steady-state methadone (SSM) exposure can alter behaviors motivated by cocaine, and whether these alterations co-occur with alterations in gene expressions in regions of the brain known to play a role in addictive behaviors. In one study, we found that SSM exposure blocked cocaine seeking as assessed by the conditioned place preference procedure, as well as cocaine-induced up-regulation of mu-opioid receptors (MOP-r) mRNA in the nucleus accumbens core (Leri et al. 2006). The effect of cocaine on MOP-r mRNA was consistent with several other reports of cocaine-induced alterations of the endogenous opioid system (Azaryan et al. 1998; Cohen et al. 1991; Hurd et al. 1992; Izenwasser et al. 1996; Spangler et al. 1993; Turchan et al. 2002; Unterwald 2001). Furthermore, because the changes in MOP-r mRNA expression were selectively found in neural areas involved in the regulation of incentive motivation and addictive behaviors (Di Chiara 1995; Koob 1992; Wise 1987), our findings provided further evidence for a role of cocaine-induced modification of MOP-r function in the development and maintenance of cocaine seeking (Gorelick et al. 2005; Kreek 1996; Schroeder et al. 2007; Zubieta et al. 1996).
However, in our aforementioned study, cocaine was administered during SSM exposure, and therefore methadone could have interfered with some direct pharmacological action of cocaine. In addition, although the test of place preference was administered during SSM exposure, MOP-r mRNA was measured only 10 days after withdrawal from methadone. Finally, because we did not measure mRNA expression of the endogenous ligands of the MOP-r, we did not clarify whether changes in MOP-r could have reflected alterations in pre-synaptic opioid-mediated neural transmission.
The first objective of the present study was to address these limitations. Hence, SSM was administered after the period of cocaine exposure, and mRNA expressions of MOP-r, pro-opiomelanocortin (POMC), prepro-enkephalin (ppEnk), and prepro-dynorphin (ppDyn) in mesolimbic and nigrostriatal regions was investigated right after the behavioral test given during SSM exposure. Furthermore, to specifically investigate the effect of SSM on the expression of responses to cocaine conditioned stimuli, we employed a behavioral method based on a conditioned reinforcement procedure (Davis and Smith 1987) recently adapted to study cocaine seeking in rats (Goddard and Leri 2006). This method is conceptually similar to place conditioning in that rats associate environmental stimuli with the effects of cocaine administered by the experimenter and then, in a drug-free state, emit an unlearned response motivated by these stimuli (Calcagnetti and Schechter 1993). Its advantage over other conditioned reinforcement procedures (Di Ciano and Everitt 2004) or typical models of cue-induced drug seeking based on intravenous self-administration (Capriles et al. 2003; Kruzich et al. 2001; Shalev et al. 2002) is that rats never learn a response for the primary reinforcer and hence the neural changes measured during acquisition/performance of this task cannot reflect the formation of stimulus-response habits. Another advantage over traditional self-administration procedures is that rats receive drug infusions passively and, therefore, all animals in a given group receive identical amounts of drug. This is particularly important because the scope of our present research is to compare gene expression in groups of rats exposed to cocaine alone or in combination with methadone. Finally, this model has advantages over place conditioning in that it is more sensitive to variations in cocaine dosages (Bardo et al. 1995; Goddard and Leri 2006) and rats can be exposed to larger quantities of drug over short periods of time.
The second objective of this study was to quantify, in the same animals, mRNA expression of two other genes implicated in addictive behaviors. The first is the hypocretin/orexin (Hcrt) gene in the lateral hypothalamus (de Lecea et al. 1998; Sakurai et al. 1998) which is co-localized with dynorphin (Chou et al. 2001) and MOP-r (Georgescu et al. 2003), and which is known to play a role in the regulation of goal-directed behaviors (DiLeone et al. 2003; Harris et al. 2005; Narita et al. 2006; Scammell and Saper 2005; Sutcliffe and de Lecea 2002) including cocaine seeking (Boutrel et al. 2005). The second is the dopamine D2 receptor (DR2) gene in the dorsal striatum. DR2 agonists are known to precipitate cocaine seeking (De Vries et al. 2002; Self et al. 1996) and low DR2 availability in the ventral and dorsal striatum predict vulnerability to cocaine reinforcement (Dalley et al. 2007; Nader and Czoty 2005). Furthermore, in the dorsal striatum, DR2 is co-localized with the MOP-r (Ambrose et al. 2004), and reduced availability of DR2 binding in this region is associated with cocaine addiction and cocaine cravings (Volkow et al. 2006; 2007). The effect of SSM on mRNA expression of these two genes in rats exposed to cocaine has never been investigated.
Additional quantifications targeted the hypothalamic-pituitary-adrenal (HPA) hormonal levels, which are altered in drug abusers and normalized by methadone maintenance (Kreek 1992; Kreek et al. 2002).
Experimental procedures
Subjects
Subjects were adult male Sprague-Dawley rats (Charles River, QC) weighing 225-250g at the beginning of all experiments. They were singly housed and maintained on a reverse light/dark cycle (8:00 lights off; 20:00 lights on) with free access to food and water except during behavioral testing. All experiments were approved by the Animal Care Committee of the University of Guelph and were carried out in accordance with the recommendations of the Canadian Council on Animal Care.
Surgery & Apparatuses
Osmotic mini pumps
Chronic exposure to SSM was achieved by implanting osmotic mini pumps SC (Alzet model 2ML2, 0.5 μl per hour for 14 days, Durect Corporation, Cupertino, CA). Isoflurane was used to anaesthetize the rats and a small incision between the scapulae was made in the skin. Subcutaneous connective tissues were spread apart using a haemostat to make a small pocket for the pump. Osmotic pumps were placed into the pocket with flow moderator directed away from the incision. Wound clips kept the incision closed.
Intravenous catheterization
Rats were surgically implanted with intravenous silastic catheters (Dow Corning, Midland, MI) in the right jugular vein, under general anesthesia induced by a combination of sodium pentobarbital (18.5 mg/kg IP, MTC Pharmaceutical, Cambridge, ON), morphine (5 mg/kg SC, Ontario Veterinary College, Guelph, ON) and diazepam (1 mg/kg SC, Sabex Inc., Boucherville, QC). Rats were given atropine sulfate (4.5 mg/kg SC, Ontario Veterinary College, Guelph, ON) just before surgery and Depocillin (300,000 IU, 0.1 ml/rat IM, Intervet Canada, Whitby, ON) immediately following surgery. The catheter was secured to the vein with silk sutures and was passed subcutaneously to the top of the skull where it exited into a connector (a modified 22 gauge cannula; Plastics One, Roanoke, VA) mounted to the skull with jeweler's screws and dental cement. A plastic blocker was placed over the opening of the connector when not in use. Catheters were flushed daily with saline and every second day with 0.1 ml of a saline-heparin solution (0.2 mg/ml Hepalean 1.000 IU, Organon, Toronto, ON).
Activity chambers
Twelve, custom made (University of Guelph, ON), activity chambers were used to measure locomotion activity. The boxes were located in the center of the laboratory room. Each chamber (30 × 40 × 26 cm) was made of dark gray PVC and was covered by black wire mesh to allow video tracking of the rats during testing. The tracking software employed was EthoVision (version 3, Noldus Information Technology, The Netherlands).
Operant chambers
Twenty-six Plexiglas operant chambers (model ENV-008CT, Med Associates, Georgia, VT) were each enclosed in larger sound-attenuating plywood chambers (model ENV-018M, Med Associates). Each chamber had a house light (28 V), and two levers, one retractable and one stationary, located 10 cm apart and 8 cm above the floor. Presses on the retractable lever (active lever) activated a white light (28 V) and a 65 dB buzzer located 3 and 8 cm above the lever, respectively. The stationary lever served to control for non-specific lever responding; pressing this lever had no consequence (inactive lever), but all presses were recorded. Infusion pumps (Razel Scientific Instruments, Stamford, CT) for the delivery of drug solutions during the period of conditioning were positioned outside the sound-attenuating chamber.
Solution hybridization RNase protection-TCA precipitation, immunoreactivity and gas liquid chromatography
The frontal cortex (FC), caudate-putamen (CP), core subdivision of the nucleus accumbens (NAcC), shell subdivision of the nucleus accumbens (NAcS), central amygdala (CeA), basolateral amygdala (BLA), ventral tegmental area (VTA), medial hypothalamus (MH), lateral hypothalamus (LH), and anterior pituitary (AP) were dissected on ice, homogenized in guanidinium thiocyanate buffer and extracted with acidic phenol and chloroform. The mRNA levels for POMC, ppEnk, ppDyn, MOP-r, Hcrt and DR2 were quantified in specific regions on the basis of known levels of constitutive expression as well as previous results showing expression changes as a result of exposure to cocaine (Zhou et al. 2002; 2008). The solution hybridization RNase protection-TCA precipitation protocol has been described in detail in earlier reports (Branch et al. 1992; Zhou et al. 2006). A 2100 bp fragment from the rat MOP-r cDNA, a 860 bp fragment from the rat DR2 cDNA, a 538 bp fragment from the rat POMC cDNA, a 935 bp fragment from the rat ppEnk cDNA, or a 1700 bp fragment from the rat ppDyn cDNA, was cloned into the polylinker region of the pSP64 plasmid (Promega, Madison, WI) in both the sense and antisense orientations. A 531 bp fragment from rat Hcrt cDNA was cloned into the polylinker region of pBC SK+ (Stratagene, La Jolla, CA). To determine the total attomoles of each mRNA in each extract, the amounts calculated from the standard curves were multiplied by 5.71 for MOP-r, 2.91 for DR2, 2.04 for POMC, 1.58 for ppEnk, 1.4 for ppDyn, or 1.3 for Hcrt to correct for the difference in length between the sense transcript (2100, 860, 538, 935, 1700 or 531 bases for the MOP-r, DR2, POMC, ppEnk, ppDyn or Hcrt, respectively) and the full-length mRNA (12, 2.5, 1.1, 1.5, 2.4, or 0.7 k base for the MOP-r, DR2, POMC, ppEnk, ppDyn or Hcrt).
Trunk blood was collected immediately after decapitation, and plasma was separated. Levels of corticosterone (CORT) and adrenocorticotrophic hormone (ACTH) immunoreactivity were assayed using kits from MP Biomedicals (Costa Mesa, CA) and DiaSorin Inc. (Stillwater, MN). All CORT and ACTH values were determined in duplicate in a single assay. Plasma methadone levels were determined by gas liquid chromatography at Bendiner & Schlesinger Inc. Cutoff point for plasma detective limit was 300 ng/ml (Borg et al. 1999).
Behavioral testing
Behavioral testing began after intravenous surgery and consisted of six phases. Table 1 represents these phases as well as the experimental groups, sample size, and drug treatments.
Table 1.
Groups, sample size, experimental phase and corresponding drug treatment. Darkened rows highlight groups important for the behavioral assessment of steady-state methadone (SSM) exposure.
| Groups | Sample Size | Test of baseline responding for a novel audiovisual stimulus 1 session (3h) | Pavlovian conditioning 1 (2h) and 2 (4h) sessions | Implantation of osmotic mini-pumps and subsequent tests of responding for the cocaine conditioned stimulus 5 sessions (3h) | Reinstatement tests (2 3h sessions) of responding for the cocaine conditioned stimulus following: | |
|---|---|---|---|---|---|---|
| Prime I | Prime II | |||||
| VVV | 8 | No injections/drugs | Vehicle | Vehicle | Vehicle | Vehicle |
| VMV | 8 | Vehicle | Methadone | Vehicle | Vehicle | |
| CMC | 8 | Cocaine | Methadone | Vehicle | Cocaine | |
| CVC | 10 | Cocaine | Vehicle | Vehicle | Cocaine | |
| CVV | 7 | Cocaine | Vehicle | Vehicle | Vehicle | |
| VVC | 8 | Vehicle | Vehicle | Vehicle | Cocaine | |
Phase 1: Test of baseline level of responding for a novel audiovisual stimulus
Rats were placed in the operant chambers, and following a delay of 5 min, a 3h session started with the activation of a house light, and 10 sec later, the entry of an active lever and the activation of a light-buzzer compound stimulus for 45 sec. Subsequent presses on this lever led to the activation of the light-buzzer compound stimulus for 10 sec. This baseline session was necessary because our pilot studies highlighted the existence of large individual differences in spontaneous tendency to respond for a novel light-buzzer compound stimulus. Therefore, individual scores on this variable were used to assign rats to different drug treatments (see Table 1).
Phase 2: Pavlovian conditioning
On three Pavlovian conditioning sessions (1 × 2h and 2 × 4h) given over 3 consecutive days, rats received passive intravenous infusions of 1 mg/kg/inf cocaine (or vehicle) accompanied by the presentation of the light-buzzer compound stimulus. The duration of the first session was shorter in order to reduce possible aversive effects of intense cocaine exposure in cocaine-naïve rats. During all conditioning sessions, the stimulus was activated 5 sec before, and during the 10 sec intravenous infusion (300 μ l). Rats received one stimulus-infusion pairing every 4 min. During this phase, the active lever was not introduced in the chamber.
Phase 3: Implantation of osmotic mini-pumps and test of locomotion
Two days following the last conditioning session, rats were implanted with 30 mg/kg/day methadone (or vehicle)-filled osmotic mini pumps and, after four days of recovery from surgery and adaptation to methadone, animals received a 2-h long test of locomotor activity in order to evaluate possible methadone-induced motor impairments.
Phase 4: Tests of responding reinforced by the cocaine conditioned stimulus
The following day, lever pressing for the cocaine conditioned buzzer-light stimulus was reassessed by introducing the active lever in the chamber and allowing the rats to freely press it in the absence of cocaine. In other words, contingencies present in Phases 1 and 4 were identical. Each test session lasted 3 hours, and rats received a total of 5 test sessions over 5 consecutive days. Because during this phase cocaine was not infused, each of these test sessions also served to extinguish the conditioned reinforcing property of the cocaine conditioned stimulus.
Phase 5: Reinstatement
Just prior to the second-last test of responding for the cocaine conditioned stimulus, all rats received an injection of vehicle (Prime I) and, the following day just prior to the last test session (3h), they received a priming injection of 20 mg/kg (IP) cocaine (or vehicle; Prime II - see Table 1). All rats were sacrificed immediately after the conclusion of Prime II reinstatement session. At the time of euthanasia, animals were still exposed on methadone (or vehicle).
In sum, as illustrated in Table 1, this study included 6 groups of rats. The three groups most critical to the study of the effect of methadone on responding reinforced by the cocaine conditioned cues (darkened rows in Table 1) received: vehicle during conditioning, vehicle pumps and vehicle on Prime II (VVV group; n=8); cocaine during conditioning, vehicle pumps, and cocaine on Prime II (CVC group; n=10); and cocaine during conditioning, methadone pumps, and cocaine on Prime II (CMC group; n=8). Three additional groups were created to interpret the gene expression data. Animals in these groups underwent the same phases of behavioral testing, but received different combinations of cocaine conditioning/methadone/cocaine priming treatments. Thus, the methadone only group (VMV; n=8) was exposed to vehicle during conditioning, methadone pumps and vehicle on Prime II; the cocaine conditioning only group (CVV; n=7) received cocaine during conditioning, vehicle-pumps, and vehicle on Prime II; and, finally, the cocaine prime only group (VVC; n=8) received vehicle during conditioning, vehicle pumps, and cocaine on Prime II.
Drugs and doses
Cocaine HCL (Dumex, Toronto, On) and methadone HCL (Pharmascience, Montreal, Qc) were dissolved in physiological 0.9% saline. The doses of cocaine employed during Pavlovian conditioning (1 mg/kg/inf) and priming (20 mg/kg) were selected on the basis of a previous study that employed an identical methodology which demonstrated substantial conditioned responding and reinstatement (Goddard and Leri 2006). Total quantity of cocaine exposure achieved through conditioning with 1 mg/kg/inf is similar to levels achieved in rats that actively self-administered the same dose (Leri et al. 2004). Thirty mg/kg/day methadone was used because this methadone dose has been previously shown to block cocaine induced reinstatement of cocaine seeking (Leri et al. 2004), and to block formation and expression of cocaine place conditioning (Leri et al. 2006) without producing general locomotor and other behavioral deficits (Leri et al. 2007).
Statistical analyses
A two-way mixed design ANOVA (factors: Test Session and Group) was used to compare operant responding emitted at the different phases of behavioral testing. Separate one-way ANOVAs were used to compare mRNA expression levels in the different groups for each gene. The Bonferroni correction was applied to the p value when multiple ANOVAs were performed on the same gene measured in various brain regions (MOP-r, POMC, ppEnk, ppDyn). Alternative statistical approaches are reported in the Results sections. Individual measurements deviating more than 2 standard deviations from the group mean were removed from the analyses (plasma hormonal levels: ACTH = 3, CORT = 1 values removed; mRNA levels: LH Hcrt = 3, MH POMC = 3, MH MOP-r = 2, NAcC POMC = 2, NAcC ppEnk = 3, NAcC MOP-r = 1, CP ppDyn = 1, CP DR2 = 4, BLA MOP-r = 1, CE ppDyn = 3, CE MOP-r = 1, FC ppEnk = 1, FC MOP-r = 1, values removed). In case of significant interactions or significant main effects, multiple comparisons were performed using the Newman Keul's method to identify individual mean differences (α = 0.05). In general, the specific values of negative findings are not reported. All statistical analyses were performed using SigmaStat (version 3.0 for Windows, SPSS Inc).
Results
Behavior
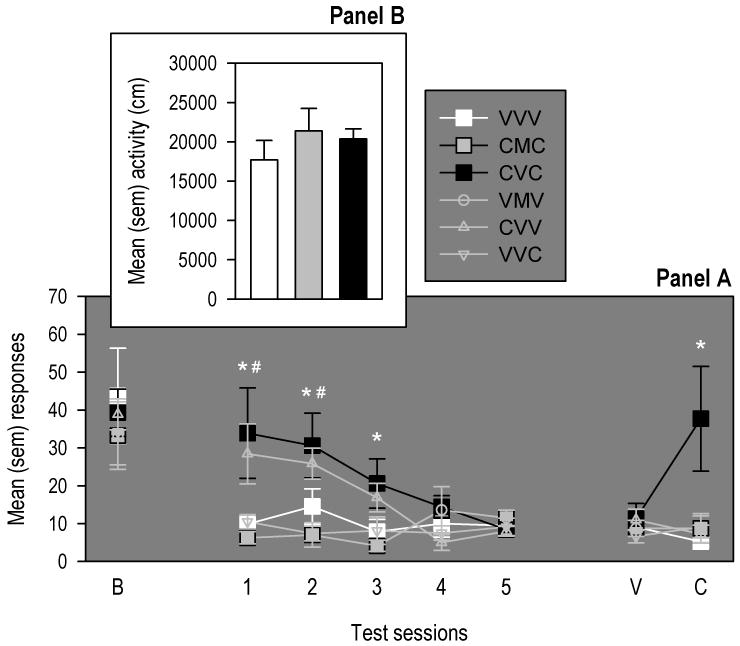
Panel A of Figure 1 represents responses on the active lever across all test sessions in all groups, but only the comparison between the VVV, CVC and the CMC groups is of empirical interest (darkened rows in Table 1). The ANOVA revealed a significant interaction between Test session and Group [F(35,301)=1.95, p<0.005], as well as significant main effects of Test session [F(7,301)=28.17, p<0.001] and Group [F(5,43)=3.05, p<0.05]. Multiple comparisons indicated that, in comparison to drug-naïve animals (VVV group), rats conditioned with cocaine and implanted with vehicle-filled pumps (CVC group) displayed significantly higher levels of responding maintained by the cocaine conditioned stimulus. Furthermore, in these rats, the priming injection of cocaine was effective in reinstating responding after extinction induced by repeated testing. In contrast, rats conditioned with cocaine and implanted with methadone-filled pumps (CMC group) showed no maintenance of responding for the cocaine conditioned stimulus, and showed no reinstatement following the cocaine prime. Importantly, this lack of operant responding was not the result of motor sedation as rats implanted with methadone pumps displayed normal locomotor activity during the test of locomotion administered before the beginning of operant testing (see panel B of Figure 1; one-factor independent group ANOVA: [F(2,23)=0.71, p=0.50]). No notable Test session or Group effects were observed on responding on the inactive lever responding, which was generally low.
Figure 1.
Panel A: mean (SEM) responses made on the active lever at each test of responding for the activation of an audiovisual compound stimulus before (i.e., Baseline; B) and after (Tests 1-5 Primes I & II) Pavlovian conditioning of this stimulus with IV infusions of cocaine (1 mg/kg/infusion). On Prime I session, all rats were injected with vehicle (V). On Prime II session, some rats (CVC, CMC and VVC) were injected with a 20 mg/kg cocaine prime (C). The * indicates a significant difference between VVV and CVC groups. The # indicates a significant difference between VVV and CVV groups. Panel B: mean (SEM) horizontal distance (cm) travelled by drug-naïve rats (VVV group) and cocaine conditioned/primed groups implanted with 30 mg/kg/day methadone-(CMC group) and vehicle-(CVC) filled mini-pumps. This test of locomotion activity was administered before the period of conditioning.
Plasma hormonal levels
No significant group differences were found in CORT or ACTH levels (all groups compared; Table 2).
Table 2.
Groups, treatments, and results of the radioimmunoassay, gas liquid chromatography (for plasma methadone) and solution hybridization RNase TCA precipitation assay. The values represent the means and SEM. mRNA measured in attomole/μ g total RNA. The values omitted from darkened rows are represented graphically in appropriate figure. N/A = not assayed.
| Group | VVV | VMV | CMC | CVC | CVV | VVC | |
|---|---|---|---|---|---|---|---|
| Pavlovian conditioning | Vehicle | Cocaine | Cocaine | Vehicle | |||
| Osmotic mini pumps | Vehicle | Methadone | Methadone | Vehicle | Vehicle | ||
| Prime II | Vehicle | Cocaine | Vehicle | Cocaine | |||
| Plasma Methadone (ng/ml) | N/A | 451 (14) | 491 (10) | N/A | N/A | N/A | |
| Plasma | ACTH (pg/ml) | 461 (56) | 419 (52) | 434 (56) | 382 (49) | 467 (43) | 450 (52) |
| CORT (ng/ml) | 343 (56) | 394 (65) | 416 (122) | 238 (34) | 300 (51) | 462 (96) | |
|
Lateral Hypothalamus (LH) Medial Hypothalamus (MH) |
Hcrt mRNA | Figure 2 | |||||
| POMC mRNA | 15.2 (1.7) | 16.0 (1.8) | 16.3 (2.2) | 15.7 (2.5) | 17.0 (0.9) | 14.2 (2.1) | |
| MOP-r mRNA | 0.20 (0.02) | 0.20 (0.02) | 0.20 (0.03) | 0.18 (0.03) | 0.20 (0.02) | 0.19 (0.03) | |
| Anterior Pituitary (AP) | POMC mRNA | 410 (18) | 430 (25) | 383 (14) | 425 (18) | 394 (28) | 391 (29) |
| Nucleus Accumbens Core (NAcC) | POMC mRNA | 1.23 (0.15) | 1.23 (0.18) | 1.35 (0.14) | 0.94 (0.18) | 1.25 (0.07) | 1.87 (0.53) |
| ppEnk mRNA | 59.0 (9.2) | 62.6 (7.5) | 64.5 (7.3) | 73.6 (11.4) | 62.8 (4.7) | 67.0 (11.0) | |
| MOP-r mRNA | Figure 3, Panel A | ||||||
| Nucleus Accumbens Shell (NAcS) | MOP-r mRNA | 0.40 (0.03) | 0.41 (0.06) | 0.41 (0.06) | 0.42 (0.03) | 0.42 (0.02) | 0.44 (0.04) |
| Caudate-Putamen (CP) | ppDyn mRNA | Figure 4 | |||||
| MOP-r mRNA | 0.12 (0.01) | 0.13 (0.01) | 0.13 (0.01) | 0.18 (0.03) | 0.13 (0.01) | 0.14 (0.02) | |
| DR2 mRNA | Figure 5 | ||||||
| Basolateral Amygdala (BLA) | MOP-r mRNA | Figure 3, Panel B | |||||
| Central Amygdala (CE) | ppDyn mRNA | 6.31 (0.63) | 5.21 (1.17) | 7.14 (0.42) | 6.57 (0.52) | 7.02 (1.03) | 5.38 (0.57) |
| MOP-r mRNA | 1.54 (0.10) | 1.50 (0.09) | 1.47 (0.14) | 1.50 (0.13) | 1.50 (0.06) | 1.49 (0.08) | |
| Ventral Tegmental Area (VTA) | MOP-r mRNA | 0.37 (0.02) | 0.40 (0.07) | 0.42 (0.04) | 0.39 (0.02) | 0.38 (0.01) | 0.40 (0.03) |
| Frontal Cortex (FC) | ppDyn mRNA | 0.89 (0.07) | 0.96 (0.07) | 0.98 (0.06) | 1.01 (0.07) | 0.91 (0.06) | 0.89 (0.15) |
| ppEnk mRNA | 32.5 (3.6) | 31.7 (6.0) | 35.9 (5.1) | 24.9 (1.2) | 30.7 (2.7) | 31.8 (2.4) | |
| MOP-r mRNA | Figure 3, Panel C | ||||||
Plasma methadone levels
No differences in plasma methadone levels were found between the VMV and the CMC groups (see Table 2).
mRNA expression levels
Statistical analyses revealed that mRNA expression levels of most genes measured in the various regions of the brain did not differ significantly among experimental groups (see Table 2). These genes/regions included: POMC in the MH, AP, and NAcC; ppEnk in NAcC and FC; MOP-r in MH, NAcS, CP, CA, and VTA; and ppDyn in the CA and FC.
However, as illustrated in Figure 2, expression of Hcrt mRNA in the LH was significantly reduced in animals conditioned and primed with cocaine (CVC group), but not in rats similarly exposed to cocaine and implanted with methadone pumps (CMC group; [F(5,40) = 2.68, p<0.05]). Further, multiple comparisons revealed that hypocretin/orexin mRNA in this region was not altered in rats that received cocaine conditioning only (CVV group) or cocaine priming only (VVC group).
Figure 2.
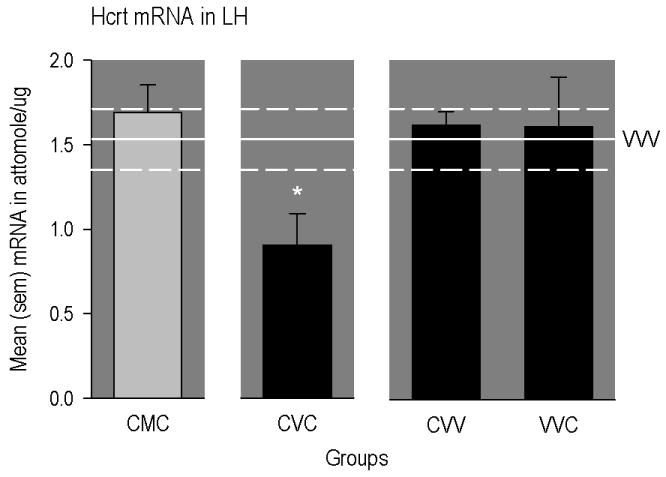
Mean (SEM) concentrations of different mRNAs in different regions of the rat brain. The horizontal line represents mean levels measured in drug-naïve rats (VVV group). The upper and lower dashed lines represent positive and negative SEMs. The * indicates a significant difference from VVV group. CMC group = conditioned with cocaine, implanted with methadone-filled mini-pumps; primed with cocaine. CVC group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with cocaine. CVV group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with vehicle. VVC group = conditioned with vehicle, implanted with vehicle-filled mini-pumps; primed with cocaine.
Further, as illustrated in Panel A of Figure 3, expression of MOP-r mRNA in the NAcC was significantly elevated in animals conditioned and primed with cocaine (CVC group), but not in rats conditioned and primed with cocaine and implanted with methadone pumps (CMC group; [F(2,42) = 8.25, p<0.006; significant after Bonferroni correction]). Multiple comparisons further revealed that MOP-r mRNA in this region was not altered in rats that received cocaine conditioning only (CVV group) or cocaine priming only (VVC group).
Figure 3.
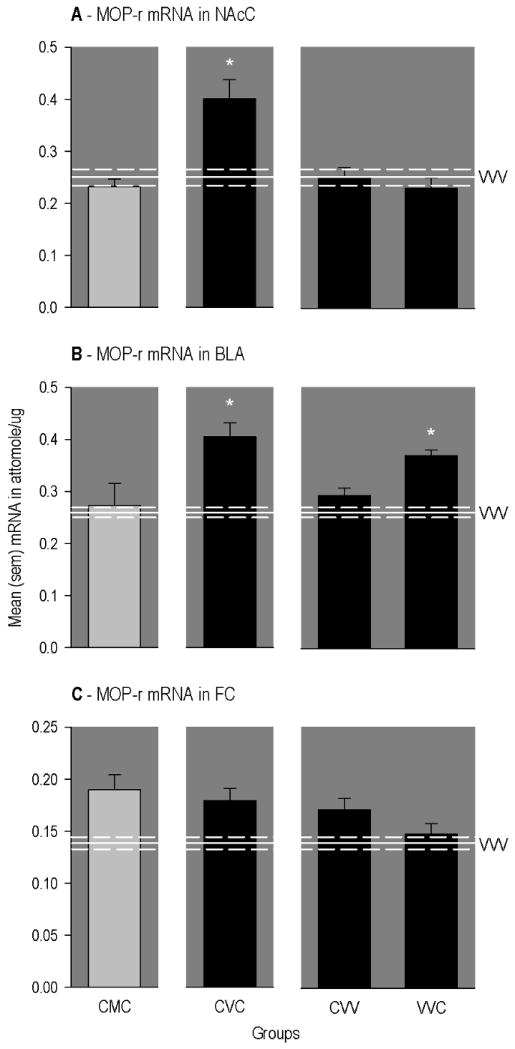
Mean (SEM) concentrations of different mRNAs in different regions of the rat brain. The horizontal line represents mean levels measured in drug-naïve rats (VVV group). The upper and lower dashed lines represent positive and negative SEMs. The * indicates a significant difference from VVV group. CMC group = conditioned with cocaine, implanted with methadone-filled mini-pumps; primed with cocaine. CVC group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with cocaine. CVV group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with vehicle. VVC group = conditioned with vehicle, implanted with vehicle-filled mini-pumps; primed with cocaine.
A somewhat different pattern of group differences was found for expression of MOP-r mRNA in the BLA. That is, as illustrated in Panel B of Figure 3, expression level was significantly elevated in animals conditioned and primed with cocaine (CVC group), but not in rats similarly exposed to cocaine and implanted with methadone pumps (CMC group; [F(5, 42) = 6.28, p<0.006; significant after Bonferroni correction]). However, MOP-r mRNA in this region was also significantly elevated in rats that received cocaine priming only (VVC group).
In the case of MOP-r mRNA in the FC (Panel C of Figure 3), expression levels were higher in rats conditioned and primed with cocaine whether maintained on vehicle (CVC group) or methadone (CMC group; [F(5,41) = 4.24, p<0.005; not significant after Bonferroni correction]). Also, multiple comparisons indicated that MOP-r mRNA was elevated in rats that received cocaine conditioning only (CVV group), but not cocaine priming only (VVC group).
In the CP, expression of ppDyn mRNA (Figure 4) was elevated in rats conditioned and primed with cocaine whether maintained on vehicle (CVC group) or methadone (CMC group; [F(5,41) = 4.24, p<0.016; significant after Bonferroni correction]). Also ppDyn mRNA in this region was significantly elevated in rats that received cocaine priming only (VVC group), but not cocaine conditioning only (CVV group).
Figure 4.
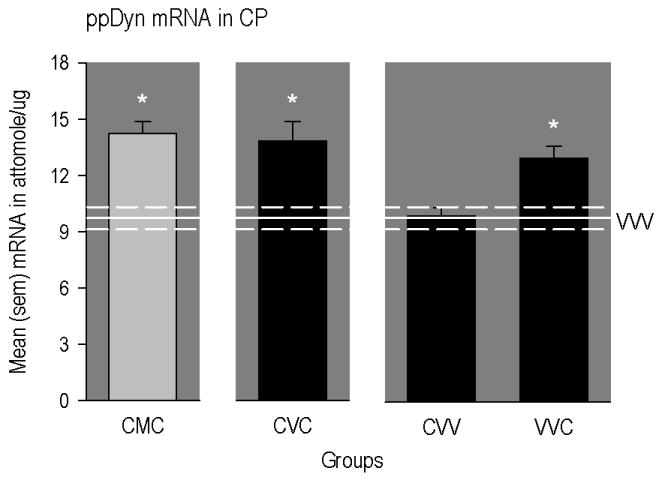
Mean (SEM) concentrations of different mRNAs in different regions of the rat brain. The horizontal line represents mean levels measured in drug-naïve rats (VVV group). The upper and lower dashed lines represent positive and negative SEMs. The * indicates a significant difference from VVV group. CMC group = conditioned with cocaine, implanted with methadone-filled mini-pumps; primed with cocaine. CVC group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with cocaine. CVV group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with vehicle. VVC group = conditioned with vehicle, implanted with vehicle-filled mini-pumps; primed with cocaine.
Finally, expression of DR2 mRNA in the CP (Figure 5) was significantly reduced in animals conditioned and primed with cocaine (CVC group), but not in rats similarly exposed to cocaine and implanted with methadone pumps (CMC group; [F(5,39) = 6.11, p<0.001]). Further, multiple comparisons indicated that DR2 mRNA was also significantly reduced in rats that received cocaine conditioning only (CVV group), but not cocaine priming only (VVC group).
Figure 5.
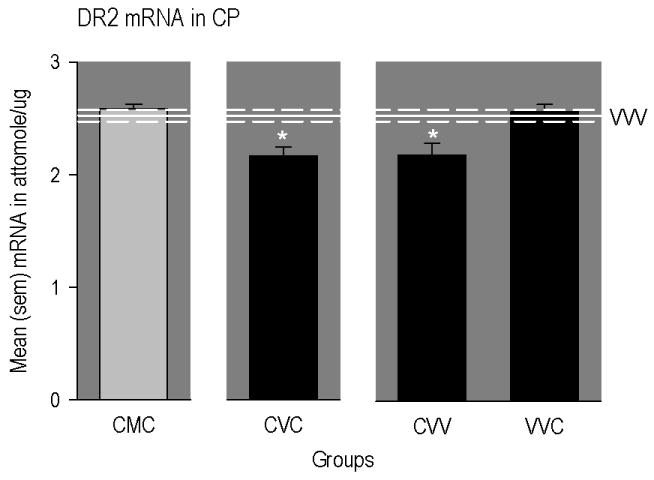
Mean (SEM) concentrations of different mRNAs in different regions of the rat brain. The horizontal line represents mean levels measured in drug-naïve rats (VVV group). The upper and lower dashed lines represent positive and negative SEMs. The * indicates a significant difference from VVV group. CMC group = conditioned with cocaine, implanted with methadone-filled mini-pumps; primed with cocaine. CVC group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with cocaine. CVV group = conditioned with cocaine, implanted with vehicle-filled mini-pumps; primed with vehicle. VVC group = conditioned with vehicle, implanted with vehicle-filled mini-pumps; primed with cocaine.
Discussion
In this study, rats exposed to cocaine during conditioning and, subsequently, to steady-state methadone, did not display significant operant responding reinforced by a cocaine conditioned stimulus, and cocaine priming had no effect on their behavior. In addition, rats exposed to cocaine and SSM did not display alterations in MOP-r, Hcrt and DR2 mRNA expression in regions of the brain known to play a role in addictive behaviors. To our knowledge, this is the first demonstration that SSM administered after cocaine exposure blocks cocaine-induced alterations in behavior and gene expression in the rat brain.
Our present results are consistent with findings from other studies on the application of a conditioned reinforcement procedure to study cocaine seeking in rats (Goddard and Leri 2006) and effects of cocaine conditioning on mRNA expressions in the rat brain (Leri et al. 2006). In fact, animals trained to associate an audiovisual stimulus with intravenous infusions of cocaine displayed significant levels of responding reinforced solely by this conditioned stimulus during extinction tests, as well as during a test of reinstatement induced by a priming injection of cocaine (CVC group). Also, in comparison to drug-naïve animals (VVV group), these rats displayed site-specific mRNA expression differences in the endogenous opioid system, hypocretin/orexin system, and D2 receptors. More specifically, in CVC rats, we observed a down-regulation of Hcrt mRNA expression in the LH, up-regulations of MOP-r mRNA expression in the NAcC, BLA and FC (but not significant after Bonferroni correction), up-regulation of ppDyn mRNA in the CP, and down-regulation of DR2 mRNA in the CP.
SSM blocked the expression of responding reinforced by the cocaine conditioned stimulus, both during the tests in extinction conditions and after the priming injection of cocaine (CMC group). This effect of SSM could not have been completely caused by direct pharmacologic interactions between methadone and cocaine because the two drugs were administered at different times; that is, methadone pumps were implanted after the period of cocaine conditioning. Only during priming was cocaine administered to animals on SSM, but even in this case a pharmacokinetic interference between SSM and cocaine is doubtful. In fact, acute cocaine administration is known to up-regulate ppDyn mRNA expression in the CP (Zhou et al. 2002; Ziolkowska et al. 2006) and this alteration was found in animals sacrificed 3 hours after the cocaine prime, whether exposed to SSM or not (CMC and CVC groups). Similarly, an interference of SSM on cognitive abilities required to display cocaine seeking is unlikely as rats exposed to 30 mg/kg/day have been found to express foot-shock induced reinstatement (Leri et al. 2004).
The effect of SSM on operant responding maintained by the cocaine conditioned cue is also not explainable by generalized motor deficits because SSM exposed rats displayed normal levels of locomotion when tested in activity chambers prior to the beginning of operant testing. This may appear at odds with the findings that methadone decreases operant responding in monkeys (Negus and Mello 2004) and produces sedation in humans (Walsh et al. 1995). However, in our laboratory rats, motor sedation induced by doses equal or above 30 mg/kg/day SSM administered by SC mini pumps dissipate within a few hours from implantation of pumps. Furthermore, 2-4 days following mini pump surgery, animals on SSM display dose-dependent elevations, not decreases, in locomotion activity (Leri et al. 2004) and normal lever pressing behavior (Leri et al. 2007). Finally, we have reported that 30 mg/kg/day SSM administered via mini pump does not reduce behaviors motivated by food, and does not reduce basal dopamine concentrations in the NAc (Leri et al. 2007).
Accordingly, the results of the present study indicate that 30 mg/kg/day SSM reduced the conditioned motivational value of the cocaine conditioned stimulus, and therefore reduced its ability to serve as a reinforcer and maintain operant behavior. This is consistent with the observation that 30 mg/kg/day SSM also reduced approach to a cocaine conditioned environment, as well as responding on a cocaine conditioned lever during extinction and after cocaine priming (Leri et al. 2004; 2006).
The results of our gene expression data provide novel evidence that can be helpful in understanding this behavioral effect of SSM. In fact, rats implanted with methadone-filled pumps after cocaine conditioning (CMC group) displayed levels of Hcrt mRNA in the LH, MOP-r mRNA in the NAcC and BLA, and DR2 mRNA in the CP that were within control levels (VVV group). Importantly, because the delivery of cocaine infusions during conditioning was controlled by a computer, the lack of alterations in gene expression levels observed in CMC rats cannot be attributed to lower quantity, or different regimen, of cocaine exposure. Interestingly, SSM did not reduce cocaine-induced elevation of MOP-r mRNA expression in the FC (but not significant after Bonferroni correction) and, by itself (VMV group), did not alter any of the plasma hormonal levels and gene expressions measured in this study.
Nevertheless, caution should be used when interpreting the relationships between SSM, behavior and mRNA expression because of at least two reasons. First, we did not measure protein levels, and thus our findings are limited to protein biosynthesis and/or release. Second, the effects of cocaine on mRNA expression were quite diverse. That is, when levels of gene expression in drug-naïve rats (VVV group) was compared to levels measured in rats conditioned and primed with cocaine (CVC group), conditioned only (CVV group) or primed only (VVC group), three patterns of results emerged. First, changes in Hcrt mRNA expression in the LH and MOP-r mRNA expression in the NAcC were observed only in cocaine conditioned animals primed with cocaine (CVC group); animals that received either treatment alone (CVV or VVC groups) showed unaltered expression levels. This observation has two implications. First, it suggests that cocaine priming produced rapid (i.e., within 3 hours) down-regulation of Hcrt and up-regulation of MOP-r mRNA expressions in animals that responded for the cocaine conditioned stimulus. Second, it indicates that the changes in mRNA expression are likely linked to cocaine exposure during conditioning rather than to learning occurring during conditioning. Further, changes in MOP-r mRNA expression in the BLA and ppDyn in the CP were observed in animals primed with cocaine that did not display behavioral evidence of conditioning because they were never conditioned (VVC group). This implies that the rapid expression of these genes was the result of an acute action of cocaine which was not influenced by past cocaine exposure or cocaine conditioning, and which was probably not related to the activation of behavior by cocaine conditioned stimuli. Finally, the decrease in DR2 mRNA expression in the CP was observed in rats that received cocaine conditioning, whether primed with cocaine (CVC group) or not (CVV group). Because these rats displayed significant operant responding during the extinction tests, and because decrease in D2 receptor binding/function in the striatum of animals and humans is not limited to cocaine exposure or cocaine addiction (Fehr et al. 2008;Heinz et al. 2005;Sun et al. 2003;Zijlstra et al. 2008), it is likely that reduced DR2 mRNA in the CP resulted from conditioning and that it played a role in the expression of behavior motivated by the cocaine conditioned stimulus.
Although our gene expression analysis was limited to mRNA levels, and tissue was collected after the behavioral tests, there is experimental evidence suggesting that the behavior observed in rats exposed to SSM could indeed reflect unaltered expression of MOP-r, and/or hypocretin/orexin, and/or DR2 genes in the regions investigated. In fact, the medium-size spiny neurons in the NAcC that project to the dorsolateral ventral pallidum (Heimer et al. 1991; Zahm and Brog 1992) express MOP-r (Herkenham and Pert 1981; Mansour et al. 1995; Napier and Mitrovic 1999). These neurons are important components of a “motive” circuit (Mogenson et al. 1980) regulating the expression of motor responses to motivationally significant stimuli (Kalivas et al. 1999), including cocaine conditioned stimuli (Hollander and Carelli 2007). Hypocretin/orexin neurons in the LH, possibly because of reciprocal anatomical connections with the NAc and the VTA (Alberto et al. 2006; Scammell and Saper 2005; Yoshida et al. 2006), are known to play a role in the regulation of goal-directed behaviors (DiLeone et al. 2003; Harris et al. 2005; Narita et al. 2006; Sutcliffe and de 2002) including cocaine seeking and sensitization (Borgland et al. 2006; Boutrel et al. 2005). The decrease in LH Hcrt mRNA levels observed in rats conditioned and primed with cocaine (CVC group) is consistent with a recent observation of similar decreases in rats that expressed a cocaine place preference (Zhou et al. 2008). Decreased brain reward function after cocaine administration, as reflected by increases in intracranial self-stimulation thresholds (ICSS), is considered central to cocaine addiction (Koob 2008). Central infusion of hypocrein-1 (orexin A) has been found to elevate ICSS thresholds in the LH, indicating a decrease in excitability of brain reward systems (Boutrel et al. 2005). Therefore, decreased hypocretin/orexin gene expression (and therefore possibly decreased biosynthesis and release) observed in our cocaine conditioning studies may be associated with hypocretin/orexin inhibition resulting from hyper-activated brain reward systems. Finally, neurons in the CP that project to the ventral pallidum express predominantly D2 receptors (Robertson and Jian 1995), and reduced availability of DR2 binding in this region has been consistently associated to cocaine addiction and cocaine cravings (Volkow et al. 2006; 2007). In this study, we measured plasma levels of prolactin in groups of rats not exposed to methadone measured by radioimmunoreactivity and found significantly lower levels in rats conditioned and primed with cocaine in comparison to VVV rats (data not shown). Because levels of prolactin in plasma are normally inhibited by the activation of DR2 by dopamine released in the hypothalamic-hypophyseal portal system (Leebaw et al. 1978), it is possible that the down-regulation in DR2 mRNA observed in the CP of cocaine conditioned rats represented a homeostatic adaptation to a hyper-active/hyper-responsive dopaminergic system.
It is unclear is how these findings in rats speak to effectiveness of SSM maintenance in reducing cocaine addiction in humans, as clinical studies on this subject have yielded inconsistent findings (Bux et al. 1995; Dunteman et al. 1992; Foltin et al. 1995; Foltin and Fischman 1996; Kosten et al. 1986; Maremmani et al. 2007; Peles et al. 2006). Also unclear is how SSM prevented the expression of neural adaptations associated with cocaine exposure and cocaine conditioning. Because we found no significant alterations in measures of mRNA expression of endogenous ligands of the MOP-r, we suspect that the changes observed did not reflect alterations in pre-synaptic opioid-mediated neural transmission. Therefore, steady-state methadone exposure may have blocked other pre/post-synaptic mechanisms of neuronal plasticity (Kauer and Malenka 2007; Nestler 2001; Nestler 2002) either because of its agonist action at MOP-r (Nugent et al. 2007), or because of its non-competitive antagonist action at glutamate NMDA receptors (Davis and Inturrisi 1999; Ebert et al. 1995; Ebert et al. 1998; Gorman et al. 1997). A role for altered glutaminergic transmission in the striatum is likely as steady state administration of buprenorphine, a partial MOP-r agonist, has been found to increase levels of glutamate in the nucleus accumbens and to block expression of sensitization to the locomotor-activating effects of cocaine (Placenza et al. 2008). Finally, our study does not clarify whether the effect of steady-state methadone on responses to cocaine associated cues is attributable to its pharmacodynamic or to its pharmacokinetic. However, the observations that rats display larger cocaine place preference after bolus injections of methadone (Bilsky et al. 1992) and that monkeys prefer methadone–cocaine combinations over cocaine alone (Wang et al. 2001) suggest that methadone pharmacokinetic is a critical determinant of its behavioral effect.
Nevertheless, our study clearly demonstrates that exposure to steady-state methadone in rats blocks the development/expression of responding to cocaine conditioned cues, as well as cocaine-induced alterations of Hcrt mRNA in the LH, MOP-r mRNA in the NAcC and BLA, and DR2 mRNA in the CP. We propose that additional work should be carried out to explore the molecular mechanisms mediating these effects of SSM because, to our knowledge, this is the first evidence of blockade of cocaine-induced neural adaptations by a drug treatment administered after cocaine exposure, and by a substance that is already clinically available in many countries of the world (World Health Organization 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberto CO, Trask RB, Quinlan ME, Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J Neurosci. 2006;(39):10043–10050. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ambrose LM, Unterwald EM, Van Bockstaele EJ. Ultrastructural evidence for co-localization of dopamine D2 and micro-opioid receptors in the rat dorsolateral striatum. Anat Rec A Discov Mol Cell Evol Biol. 2004;(1):583–591. doi: 10.1002/ar.a.20054. [DOI] [PubMed] [Google Scholar]
- Azaryan AV, Clock BJ, Rosenberger JG, Cox BM. Transient upregulation of mu opioid receptor mRNA levels in nucleus accumbens during chronic cocaine administration. Can J Physiol Pharmacol. 1998;(3):278–283. [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta- analysis. Neurosci Biobehav Rev. 1995;(1):39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Montegut MJ, Delong CL, Reid LD. Opioidergic modulation of cocaine conditioned place preferences. Life Sci. 1992;(14):L85–L90. doi: 10.1016/0024-3205(92)90105-x. [DOI] [PubMed] [Google Scholar]
- Borg L, Broe DM, Ho A, Kreek MJ. Cocaine abuse sharply reduced in an effective methadone maintenance program. J Addict Dis. 1999;(4):63–75. doi: 10.1300/J069v18n04_06. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de LL. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;(52):19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res Mol Brain Res. 1992;(3):231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug Alcohol Depend. 1995;(1):29–35. doi: 10.1016/0376-8716(94)01058-s. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Extinction of cocaine-induced place approach in rats: a validation of the “biased” conditioning procedure. Brain Res Bull. 1993;(5-6):695–700. doi: 10.1016/0361-9230(93)90102-h. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;(1-2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BM, Nguyen TV, Hyman SE. NIDA Res Monogr. 1991. Cocaine-induced changes in gene expression in rat brain; pp. 175–181. [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Inturrisi CE. d-Methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharmacol Exp Ther. 1999;(2):1048–1053. [PubMed] [Google Scholar]
- Davis WM, Smith SG. Conditioned reinforcement as a measure of the rewarding properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 199–210. [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;(1):18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;(2):95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004 1:202–203. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;(6):759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Dunteman GH, Condelli WS, Fairbank JA. Predicting cocaine use among methadone patients: analysis of findings from a national study. Hosp Community Psychiatry. 1992;(6):608–611. doi: 10.1176/ps.43.6.608. [DOI] [PubMed] [Google Scholar]
- Ebert B, Andersen S, Krogsgaard-Larsen P. Ketobemidone, methadone and pethidine are non-competitive N-methyl-D-aspartate (NMDA) antagonists in the rat cortex and spinal cord. Neurosci Lett. 1995;(3):165–168. doi: 10.1016/0304-3940(95)11364-3. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol. 1998;(5):553–559. doi: 10.1016/s0006-2952(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;(4):507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Christiansen I, Levin FR, Fischman MW. Effects of single and multiple intravenous cocaine injections in humans maintained on methadone. J Pharmacol Exp Ther. 1995;(1):38–47. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of methadone or buprenorphine maintenance on the subjective and reinforcing effects of intravenous cocaine in humans. J Pharmacol Exp Ther. 1996;(3):1153–1164. [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;(8):3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard B, Leri F. Reinstatement of conditioned reinforcing properties of cocaine-conditioned stimuli. Pharmacol Biochem Behav. 2006;(4):540–546. doi: 10.1016/j.pbb.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;(12):1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;(1):5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;(8):1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981;(5814):415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;(13):3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res Mol Brain Res. 1992;(1-2):165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Heller B, Cox BM. Continuous cocaine administration enhances mu- but not delta-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. Eur J Pharmacol. 1996;(1-2):187–191. doi: 10.1016/0014-2999(95)00828-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann N Y Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;(5):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Gawin FH, Rounsaville BJ, Kleber HD. Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse. 1986;(1-2):1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205–230. [PubMed] [Google Scholar]
- Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J Addict Dis. 1996;(4):73–96. doi: 10.1300/J069v15n04_05. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;(9):710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci. 2001;(5):1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Leebaw WF, Lee LA, Woolf PD. Dopamine affects basal and augmented pituitary hormone secretion. J Clin Endocrinol Metab. 1978;(3):480–487. doi: 10.1210/jcem-47-3-480. [DOI] [PubMed] [Google Scholar]
- Leri F, Sorge RE, Cummins E, Woehrling D, Pfaus JG, Stewart J. High-Dose Methadone Maintenance in Rats: Effects on Cocaine Self-Administration and Behavioral Side Effects. Neuropsychopharmacology. 2007;32:2290–300. doi: 10.1038/sj.npp.1301357. [DOI] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE, Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;(7):1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Leri F, Zhou Y, Goddard B, Cummins E, Kreek MJ. Effects of high-dose methadone maintenance on cocaine place conditioning, cocaine self-administration, and mu-opioid receptor mRNA expression in the rat brain. Neuropsychopharmacology. 2006;31:1462–1474. doi: 10.1038/sj.npp.1300927. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;(4):283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Mellini A, Pacini M, Marini G, Lovrecic M, Perugi G, Shinderman M. Alcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment program. J Addict Dis. 2007;(1):61–70. doi: 10.1300/J069v26n01_08. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;(2-3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;(8):1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Napier TC, Mitrovic I. Opioid modulation of ventral pallidal inputs. Ann N Y Acad Sci. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;(2):398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressive-ratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend. 2004;(3):297–309. doi: 10.1016/j.drugalcdep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;(3):637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;(7139):1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Peles E, Kreek MJ, Kellogg S, Adelson M. High methadone dose significantly reduces cocaine use in methadone maintenance treatment (MMT) patients. J Addict Dis. 2006;(1):43–50. doi: 10.1300/J069v25n01_07. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Rajabi H, Stewart J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology. 2008 Jul 5; doi: 10.1007/s00213-008-1210-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;(4):1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Saper CB. Orexin, drugs and motivated behaviors. Nat Neurosci. 2005;(10):1286–1288. doi: 10.1038/nn1005-1286. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology. 2007;(2):265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;(5255):1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;(1):1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;(4):323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Sun W, Ginovart N, Ko F, Seeman P, Kapur S. In vivo evidence for dopamine-mediated internalization of D2-receptors after amphetamine: differential findings with [3H]raclopride versus [3H]spiperone. Mol Pharmacol. 2003;(2):456–462. doi: 10.1124/mol.63.2.456. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de LL. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;(5):339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Turchan J, Maj M, Przewlocka B, Przewlocki R. Effect of cocaine and amphetamine on biosynthesis of proenkephalin and prodynorphin in some regions of the rat limbic system. Pol J Pharmacol. 2002;(4):367–372. [PubMed] [Google Scholar]
- Unterwald EM. Regulation of opioid receptors by cocaine. Ann N Y Acad Sci. 2001;937:74–92. doi: 10.1111/j.1749-6632.2001.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;(1):361–372. [PubMed] [Google Scholar]
- Wang NS, Brown VL, Grabowski J, Meisch RA. Reinforcement by orally delivered methadone, cocaine, and methadone-cocaine combinations in rhesus monkeys: are the combinations better reinforcers? Psychopharmacology. 2001;(1):63–72. doi: 10.1007/s002130100731. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacological Therapy. 1987;(1-2):227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The methadone fix. Bulletin of the World Health Organization. 2008;(3):161–240. [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;(5):845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;(4):751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;(1):137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–12234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Yuferov VP, Sora I, Ho A, Uhl GR, Kreek MJ. Effects of acute “binge” cocaine on preprodynorphin, preproenkephalin, proopiomelanocortin, and corticotropin-releasing hormone receptor mRNA levels in the striatum and hypothalamic-pituitary-adrenal axis of mu-opioid receptor knockout mice. Synapse. 2002;(4):220–229. doi: 10.1002/syn.10101. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den BW, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;(4):262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Ziolkowska B, Stefanski R, Mierzejewski P, Zapart G, Kostowski W, Przewlocki R. Contingency does not contribute to the effects of cocaine self-administration on prodynorphin and proenkephalin gene expression in the rat forebrain. Brain Res. 2006;1069:1–9. doi: 10.1016/j.brainres.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;(11):1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]