Abstract
Methods for the enantioselective conversion of D-xylose to differentially protected myo-inositol and L-chiro-inositol have been developed. The key transformation is a highly diastereoselective intramolecular SmI2-promoted pinacol coupling. The stereoselectivity was extremely dependent on the conditions, suggesting a change in mechanism. Preliminary mechanistic experiments and possible explanations for this behavior are discussed.
Keywords: Inositol, pinacol, samarium, stereoselective, carbohydrates
Natural phosphorylated and/or glycosylated inositols have been found to play central roles in a variety of cell-signaling pathways in animals,1–9 in desiccation tolerance in plants,10–16 and as protein anchors in all eukaryotes. 17,18 These diverse and, in some cases, medically useful activities, have stimulated great interest in the synthesis of these compounds and their analogues.19–30 In most of the reported syntheses, the preparation of an appropriately protected inositol is prerequisite, but the availability of these key building blocks is limited by lengthy syntheses and a need for the resolution of enantiomers in the case of preparations originating with myo-inositol. The challenges and successes in these approaches have been extensively reviewed.31–33
Our approach to this problem has been to avoid the requirement for resolution of enantiomers by developing enatiospecific syntheses of the important differentially protected inositols from D-xylose. Our preliminary work in this area has been published previously34,35 and we now report the complete details of our study. Of particular importance is the observation that the SmI2-mediated intramolecular pinacol coupling of a pseudo-C2-symmetric dialdehyde 6 proceeds by a different mechanism depending on the conditions, with dramatic effect on the stereoselectivity (Scheme 1).
Scheme 1.
Synthesis of differentially-protected myo-inositols from D-xylose.
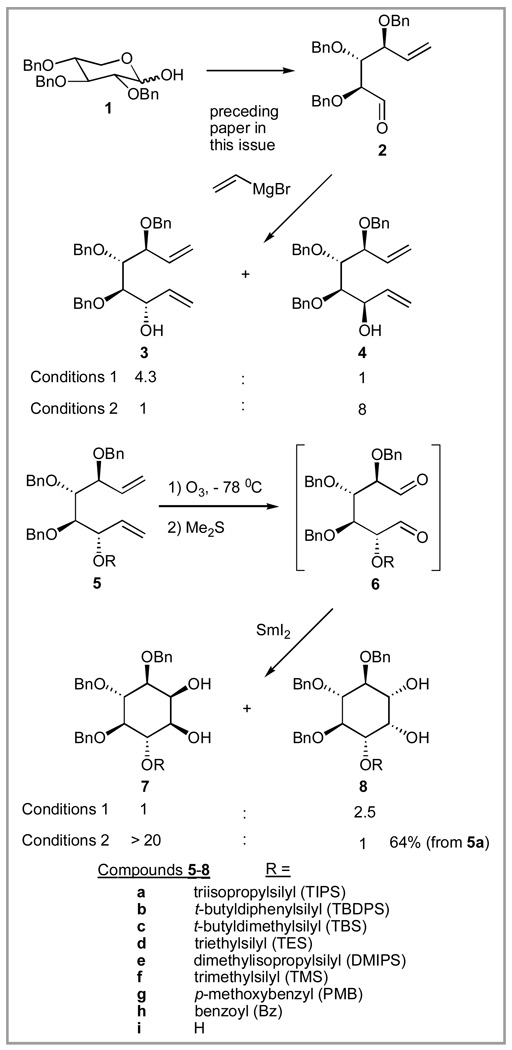
The preceding paper in this issue describes the conversion of 2,3,4-tribenzyl-D-xylose to dienols 3 and 4 with selectivity for either depending on the conditions. However, since 3 and 4 are not easily separable, the mixture was silylated (triisopropylsilyl chloride, DMF, pyridine, AgNO3), and the silylated mixture was readily separated by silica gel chromatography to produce pure 5a (R = TIPS) and 12 (see Scheme 5 for structure). Treatment of 5a with ozone at −78 °C resulted in rapid (<5 min) con sumption of the starting material, producing, after reduction of the ozonide with dimethylsulfide, dialdehyde 6a (R = TIPS). Although the aldehyde could be isolated for 1H NMR characterization, it was somewhat fragile and the subsequent reaction was generally conducted without isolation of 6a. SmI2-promoted pinacol coupling of 6a under standard conditions36 (3 eq SmI2, 3 eq t-BuOH, THF, −78 °C to 20 °C over 5 h) produced a disappointing 1:2.5 ratio of 7a:8a (R = TIPS). The structures of 7a and 8a were established as previously described34 and confirmed by desilylation (Bu4NF, THF), to produce known tribenzyl-myo-inositols 7i37,38 and 8i.39
Scheme 5.
Synthesis of a differentially protected chiro-inositol via SmI2-promoted pinacol coupling.
Since 7a has the correct differential protection pattern for application in the synthesis of most of the known natural myo-inositol glycan (IG) structures,26,40–51 we sought to alter the stereoselectivity in the pinacol reaction to favor 7. Accordingly, we studied the effect of the R group in 6 on the pinacol cyclization, since this group is responsible for the only difference between 7 and 8 (i.e. if R = Bn, then 6 is C2 symmetric and 7 = 8). Each precursor 5b–h was prepared by desilylation of 5a (Bu4NF, THF) to produce pure 3, then resilylation (5b–f), alkylation (5g), or acylation (5h). The results (Table 1) show that the SmI2-mediated pinacol reaction under standard conditions is only modestly sensitive to steric factors in the R group, and that larger R groups favor 8. In the best case (6f, R = TMS) only a modest (1.5:1) selectivity for 7 was realized, clearly unacceptable for an efficient multistep synthesis of IGs.
Table 1.
Stereoselectivity in pinacol cyclization of 6 under standard conditions: 3 eq SmI2, 3 eq t-BuOH, THF, −78 °C to 20 °C.
| Compound | R | Additive | Ratio 7:8 |
|---|---|---|---|
| 6a | TIPS | None | 1:2.5 |
| 6b | TBDPS | None | 1:2.5 |
| 6b | TBDPS | 6 eq TMSCl | 1:2.5 |
| 6b | TBDPS | HMPA (10%) | trans-diol |
| 6c | TBS | None | 1:2 |
| 6d | TES | None | 1:1 |
| 6d | TES | 6 eq TMSCl | 1:1 |
| 6d | TES | HMPA (10%) | trans-diol |
| 6e | DMIPS | None | 1:1 |
| 6f | TMS | None | 1.5:1 |
| 6g | PMB | None | 1:1 |
Addition of HMPA (10% v/v in THF) resulted in drastically reduced production of 7 or 8, with trans-diol becoming the major product. This is consistent with the hypothesis that the predominant formation of cis-diol products in SmI2-promoted pinacol coupling reactions52 is due to chelation of the ketyl oxygens by Sm(III) during the course of the cyclization; strongly chelating HMPA presumably competes with the oxygen atoms for the Sm(III) ion leading to an unchelated transition state predominating.
Addition of chlorotrimethylsilane, known to accelerate SmI2-promoted pinacol reactions,53 had no effect on the stereoselectivity (Table I).
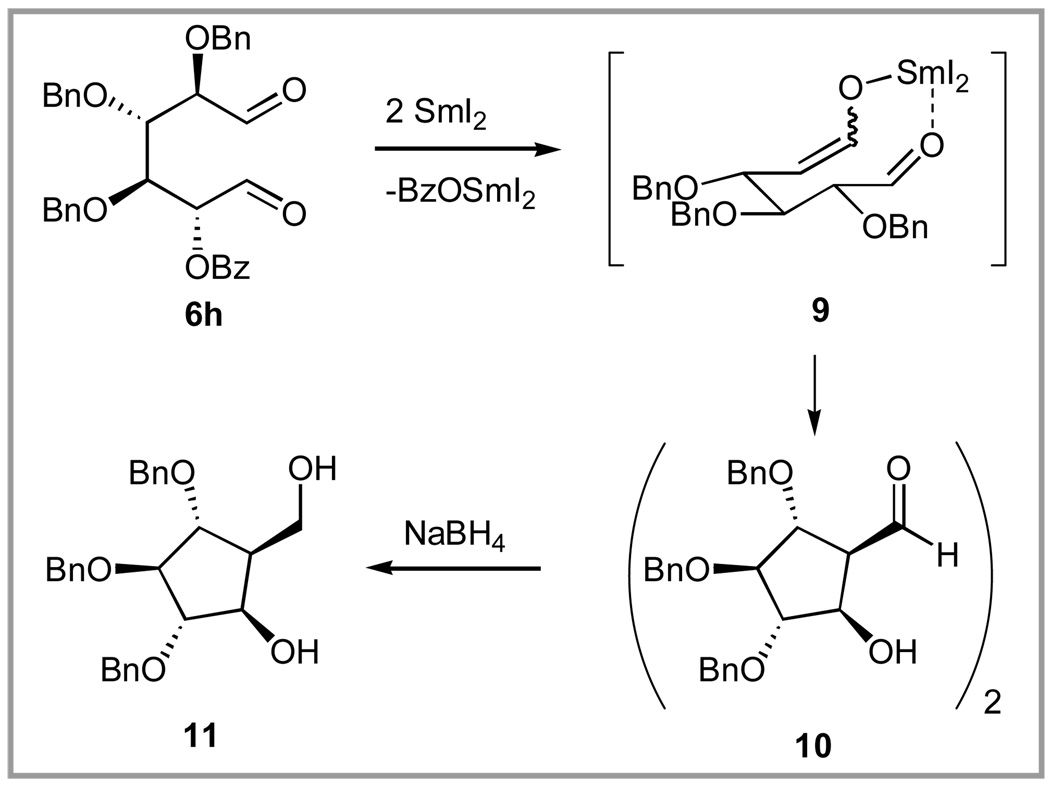
To evaluate the effect of changes in electronic demand of the R group in 6 on the course of the cyclization, we subjected 6g (R = PMB) and 6h (R = Bz) to the reaction conditions. In the case of 6g, no selectivity was observed, but in the case of 6h the reaction took a different course (Scheme 2) producing cyclopentyl aldehyde 10, which was revealed by 1H NMR and MALDI-TOF MS to exist as a dimer. The structure was confirmed by reduction of 10 with NaBH4 to produce known alcohol 11.54
Scheme 2.
Reductive elimination of 6h produced a polyoxygenated cyclopentane.
Surprisingly, a small change in the reaction conditions for the SmI2-promoted pinacol cyclization of 6a had a profound effect on the stereoselectivity. When 6a was treated with 6 eq of SmI2 at −78 °C for 20 min, followed by dropwise addition of sat. aq. NaHCO3 at −78 °C and then warming to 20 °C, diol 7 was obtained in 64% yield (from 5) with only a trace of isomeric 8 being observed.
To explore the sensitivity of the high stereoselectivity under these modified conditions to the R group in 6 we subjected 6a, 6c, 6d, and 6g to the reaction. The stereoselectivity under the modified conditions (Table 2) is more sensitive to the steric bulk in R than under the normal conditions and is in the opposite direction: bulkier substituents favor formation of 7.
Table 2.
Stereoselectivity in pinacol cyclization of 6 under modified conditions: 6 eq SmI2, THF, −78 °C; sat. aq. NaHCO3, −78 °C; warm to 20 °C.
| Compound | R | Ratio 7 : 8 |
|---|---|---|
| 6a | TIPS | >20:1 |
| 6c | TBS | 2.9:1 |
| 6d | TES | 2.7:1 |
| 6g | PMB | 1:1 |
The dramatic change in stereoselectivity in the pinacol coupling reaction is intriguing and suggests a change in mechanism under the modified conditions. To explore the origin of this effect, we conducted a number of experiments designed to identify the important variables in the stereoselectivity (Table 3).
Table 3.
The effect of reaction conditions on the stereoselectivty of SmI2-promoted pinacol cyclization of 6a. Reactions were performed with 6 eq SmI2 in THF under the conditions listed.
| Entry | Reaction Conditions | Quench Conditions | Ratio 7: 8 |
|---|---|---|---|
| 1 | −78 °C, 10 min, then 20 °C, 5h | NaHCO3 at 20 °C | 1:2.5 |
| 2 | −78 °C, 10 min | NaHCO3 at −78 °C | >20:1 |
| 3 | −78 °C, 10 min | H2O at −78 °C | >20:1 |
| 4 | −78 °C, 10 min, then 20 °C, 10 min | O2 (air) | 1:1 |
| 5 | −78 °C, 10 min | NaHCO3 at −78 °C, then O2 (air) at −78 °C | >20:1 |
| 6 | −78 °C, 10 min | O2 (air) at −78 °C | 6a recov. |
| 7 | −78 °C, 10 min | I2 at −78 °C | 6a recov. |
| 8 | −78 °C, 10 min, then 20 °C, 10 min, then −78 °C, 10 min | NaHCO3 at −78 °C | 1:1 |
| 9 | −78 °C, 10 min | 1M NH4OH at −78 °C | >20:1 |
| 10 | −78 °C, 10 min | 2M HCl at −78 °C | >20:1 |
| 11 | −72 °C, 10 min | NaHCO3 at −72 °C | >20:1 |
| 12 | −61 °C, 30 min | NaHCO3 at −72 °C | >20:1 |
| 13 | −50 °C, 10 min | NaHCO3 at −50 °C | >20:1 |
| 14 | −25 °C, 10 min | NaHCO3 at −25 °C | 1:1.5 |
| 15 | −78 °C, 10 min | Bu3SnH, then NaHCO3 at −78 °C | >20:1 |
| 16 | −78 °C, 10 min | CuBr, then NaHCO3 at −78 °C | >20:1 |
| 17 | −78 °C, 10 min | MeOH, then NaHCO3 at −78 °C | >20:1 |
| 18 | −78 °C, 10 min | PhCO2H, then NaHCO3 at −78 °C | >20:1 |
| 19 | −78 °C, 10 min | CF3CO2H, then NaHCO3 at −78 °C | >20:1 |
| 20 | −78 °C, 10 min | Bu3SnH, then O2 (air) at−78 °C | 6a recov. |
| 21 | −78 °C, 10 min | PhSH, then O2 (air) at−78 °C | 6a recov. |
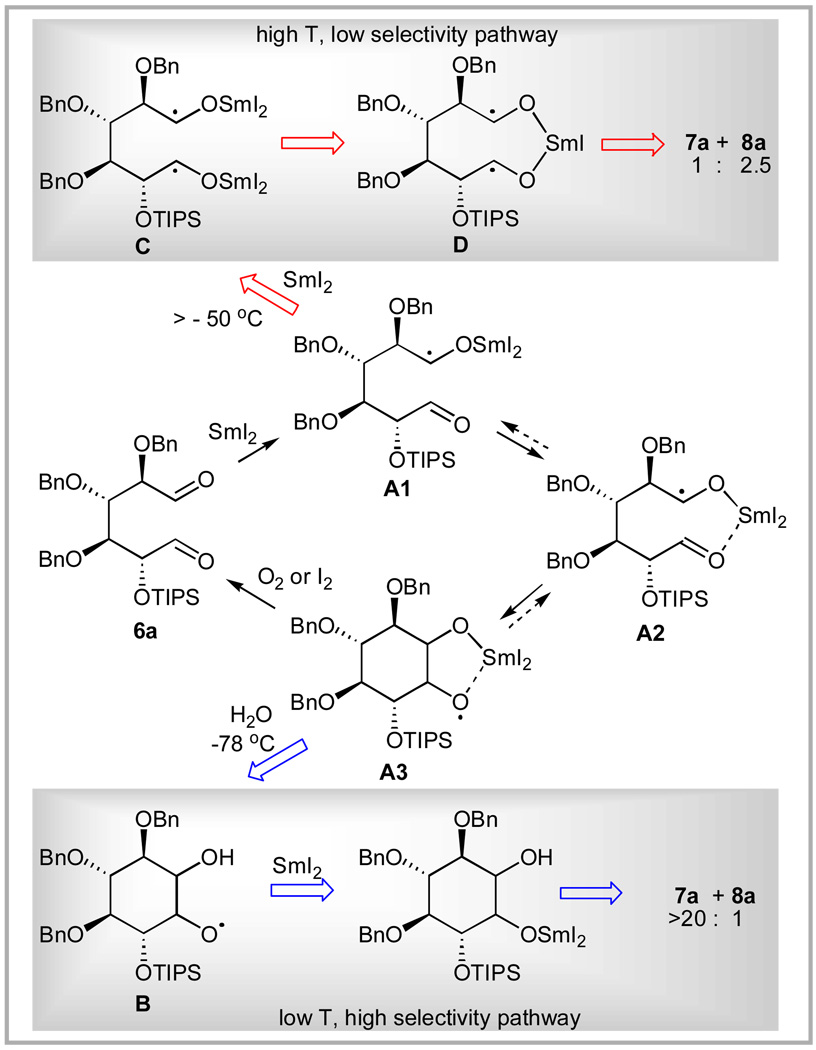
First, we established that the key element in the stereoselectivity is the temperature of addition of water: omission of t-BuOH from the standard conditions (entry 1) or omission of the NaHCO3 from the aqueous quench under modified conditions (entry 3) did not affect the stereoselectivity. On the other hand, warming to −25 °C or above prior to addition of water (entries 1, 4, 8, and 14) always resulted in poor stereoselectivities favoring 8 if any selectivity was seen at all.
When O2 (air) or I2 was admitted to the reaction mixture at −78 °C (entries 6 and 7), dialdehyde 6a was recovered from the reaction. This suggests that the intermediate, let’s call it A, formed from Sm(II) reduction of one or both aldehydes at low temperature is formed reversibly and can be readily reoxidized to aldehyde. However, if aq. NaHCO3 is added at low temperature and then O2 (air), is admitted at −78 °C (entry 5), the reaction proceeds to diol product with high selectivity for 7. By contrast, warming to 20 °C without aq. NaHCO3 addition and then recooling and admitting O2 (air) results in diol products 7 and 8 without stereoselectivity (entry 8) showing that the act of warming also commits the reaction to products.
These results suggest that water reacts with the reversibly formed low temperature intermediate A to produce a more reactive intermediate B that proceeds to product at low temperature and with high stereoselectivity, while warming without water ultimately results in reaction of A to form products, but with poor stereoselectivity.
We performed a series of experiments to see if we could trap intermediates A or B by addition of hydrogen atom donors (entries 15, 20, and 21) or acids and bases of various types (entries 9, 10, 17–19), but without success. We also evaluated the temperature dependence of the stereoselectivity (entries 11–14) and found that the high selectivity for 7 is maintained up to −50 °C, and then disappears by −25 °C suggesting that the reaction of A to products occurs in this temperature range in the absence of water.
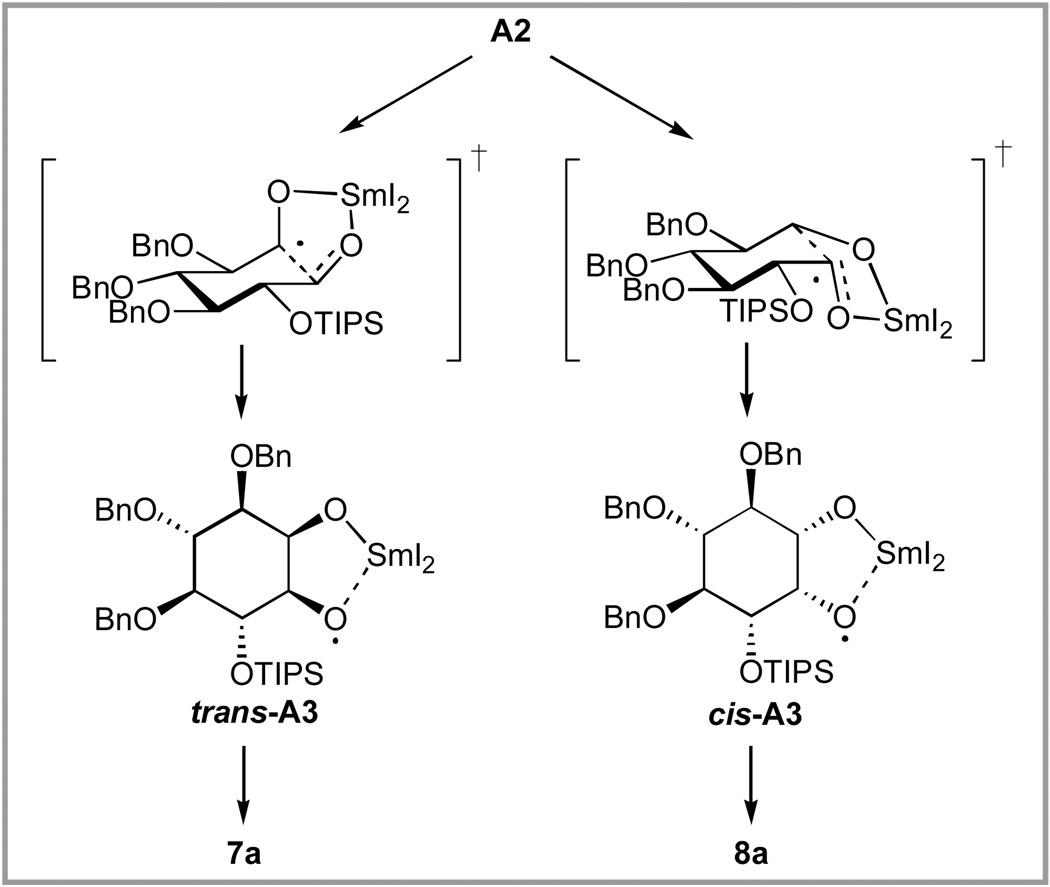
The identities of A and B remain unknown, but a hypothesis that is consistent with our data and literature precedent is shown in Scheme 3. According to this proposal, reduction of 6a at −78 °C results in ketyl A1 that rapidly proceeds via A2 to cyclized radical anion A3. This cyclization is expected to proceed preferentially via a transition state in which the incipient alkoxyradical is in the equatorial position (Scheme 4), leading to the A3 isomer in which the samarium-chelated oxygens are trans to the OTIPS group (trans-A3). A similar stereoselectivity has been reported in other 6-exo-trig radical cyclizations.55
Scheme 3.
A hypothesis for the origin of the observed change in stereoselectivity in the SmI2-promoted cyclization of 6a as a function of temperature of quenching.
Scheme 4.
Stereoselective formation of 7a.
Since SmI2 reduction of aldehydes is known to be an inner sphere process requiring bonding of the Sm(II) center to the electron acceptor,56 A3 is stable at −78 °C, because the reducible oxygen atom is flanked by the bulky Sm(III) and TIPS centers, precluding further coordination. Intermediate A3 would be expected to reoxidize to 6a when treated with O2 or I2, as observed. If water is admitted to the reaction at low temperature, the samarium alkoxyl bond hydrolyzes leading to intermediate B that is now accessible to an irreversible inner sphere electron transfer by a second Sm(II) to produce products retaining the stereochemical integrity of A3, thus leading to 7a with high selectivity.
By contrast, when the reaction mixture is warmed above −50 °C, the rate of the reverse sequence (i.e. A3→A2→A1) would increase significantly. Reversibility in the addition of ketyls to carbonyls has been previously reported. 57–59 The uncoordinated aldehyde in A1 would then be accessible to be reduced by Sm(II) to produce diketyl C, which upon ligand exchange to produce D would cyclize very rapidly. Because of the high reactivity of D, the selectivity is lower, producing a 1:2.5 ratio of 7a to 8a.
Furthermore, the weak dependence of the stereoselectivity in the cyclization on the size of the R-group in 6 (Table 1) under the higher temperature conditions is consistent with the high reactivity of D, whereas the much stronger dependence of the selectivity on R under the modified conditions (Table 2) is consistent with the change in steric influence of R on the energies of the transition states shown in Scheme 4 as OTIPS is replaced with smaller groups.
A second mechanistic possibility that cannot be ruled out from our data is that 6a does not react with SmI2 at −78 °C at any appreciable rate. When water is added, the SmI2 becomes a stronger reducing agent,60 accelerating the reduction of 6a, with the resulting ketyl proceeding on to product via any of several pathways. In the absence of water, reaction only occurs when the temperature exceeds −50 °C and the rate of reduction becomes appreciable. According to this hypothesis, the difference in stereoselectivity is due to the large difference in the temperature at which the cyclization occurs, with the lower temperature cyclization producing high selectivity for 7a. This proposal is consistent with our inability to trap any intermediates at −78 °C, but the large magnitude of the change in stereoselectivity is surprising. This may reflect a different reduction mechanism with the more reactive H2O-SmI2 species, perhaps to an outer sphere process. Clearly, additional mechanistic studies are required to further clarify the origin of this remarkable change in stereoselectivity.
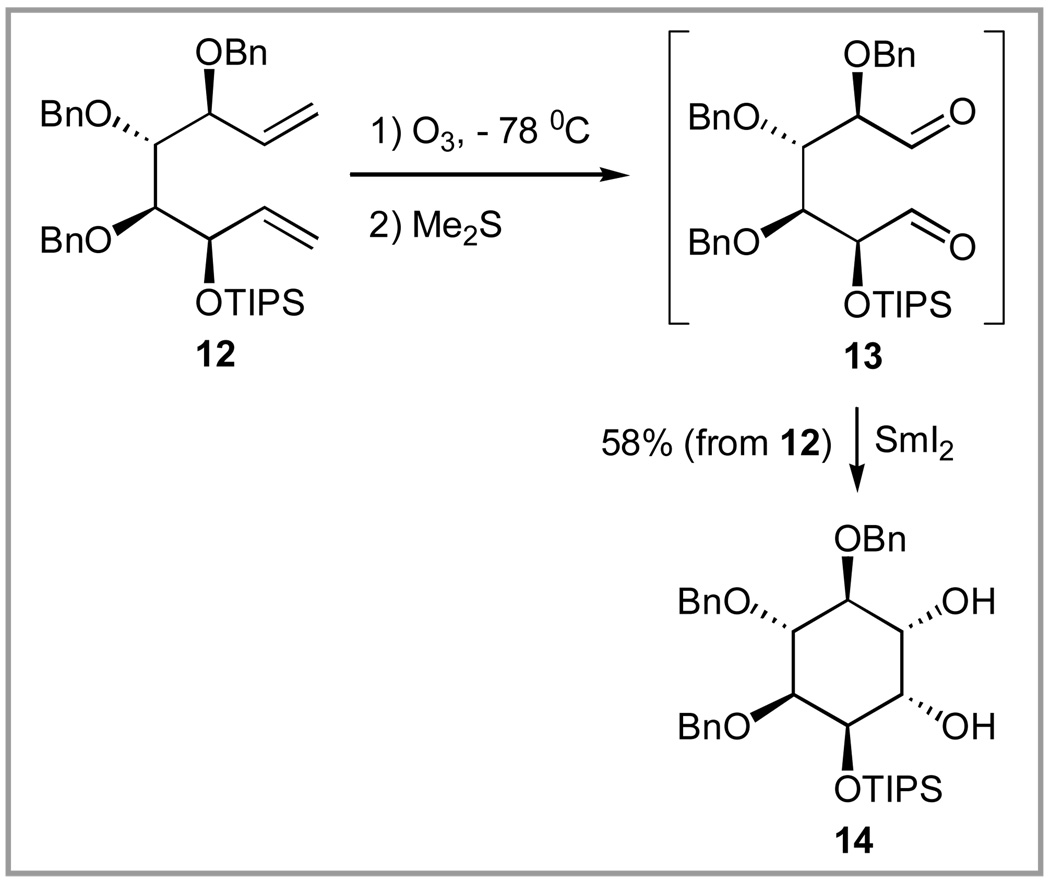
The conversion of the other diene isomer 12 to a cyclitol was much simpler because a single isomer was obtained, as expected based on the literature precedent36 (Scheme 5). Accordingly, ozonolysis of 12 produced dialdehyde 13, that was only moderately stable so was carried on without purification. Pinacol cyclization under standard conditions36 (3 eq SmI2, 3 eq t-BuOH, THF, −78 °C to 20 °C over 5 h) produced desired L-chiro-inositol 14 in 58% overall yield. The structure of 14 was confirmed by conversion to pentabenzyl-(-)-quebrachitol as peviously described.35
In conclusion, highly efficient syntheses of differentially protected myo- and L-chiro-inositols have been developed. An intriguing change in mechanism affecting stereoselectivity has been observed in the SmI2-promoted pinacol reaction warranting further study. It should also be noted that since both enantiomers of aldehyde 2 are readily available from the very inexpensive D-xylose precursor,61 the procedure described here also provides a straightforward preparation of the D-chiro-inositol skeleton.
Unless otherwise noted all commercially obtained reagents were used without purification. THF was distilled from sodium benzophenone ketyl prior to use. Dichloromethane was distilled from calcium chloride. Reactions were carried out under an argon atmosphere in oven-dried glassware using standard syringe, cannula and septa techniques. Ozonolysis was performed with a flow of O3 in dry O2 produced by a Welsbach ozonator operating at 0.05 CFM. Reactions were monitored by TLC (Silica Gel 60 F254, 250 µm) and visualized with UV light and/or heating with a p-anisaldehyde stain (2.5% p-anisaldehyde, 3.5% sulfuric acid, 1% acetic acid, 93% ethanol) or a Haines-Isherwood stain (1 g (NH4)6Mo7O24·4H2O, 10 mL of 1M HCl, 3 mL of HClO4, 90 mL H2O). Flash chromatography was performed on silica gel (32–63 µm). Optical rotations were measured with an Autopol III automatic polarimeter. 1H and 13C NMR spectra were recorded on a Bruker Avance 300 MHz, JEOL 300 MHz or a Varian Inova 400 MHz spectrometer.
3(S)-Triisopropylsilyloxy-4(S),5(R),6(S)-tribenzyloxy-1,7-octadiene (5a)
To the crude mixture of 3 and 4 (3:1, 2.4 g, 5.4 mmol) obtained as described in the preceding paper, in DMF (12 mL) and pyridine (1.2 mL) was added AgNO3 (3.7 g, 22 mmol) followed by triisopropylsilyl chloride (2.3 mL, 10.8 mmol) at 20 °C. The reaction mixture was stirred for 2 h at 20 °C, after which it was diluted with ether (40 mL) and water (40 mL). The aqueous layer was separated and extracted with additional ether (3 × 30 mL). The combined organic extracts were washed with brine, dried (MgSO4), and evaporated. The residue was chromatographed (hexane:ether, 97:3) to give 12 (0.69 g, 18% over 3 steps) and 5a (2.07 g, 54% over 3 steps). For 5a:[α]25D: +9.7 (c 0.2, CHCl3). 1H NMR (CDCl3): δ = 7.35-7.23 (m, 15H), 6.13-6.01 (m, 1H), 5.86-5.74 (m, 1H), 5.27-5.05 (m, 4H), 4.79-4.58 (m, 5H), 4.40-4.35 (m, 2H), 4.09 (ψt, J = 7.7 Hz, 1H), 3.79 (dd, J = 3.6, 6.3 Hz, 1H), 3.65 (dd, J = 3.6, 5.2 Hz, 1H), 0.75-1.60 (m, 21H). 13C NMR (CDCl3): δ = 139.2, 138.9, 138.6, 136.1, 128.3, 128.2, 128.17, 128.13, 127.9, 127.5, 127.4, 127.3, 118.8, 115.2, 81.9, 80.2, 78.1, 74.5, 74.1, 73.8, 70.6, 18.2, 12.6. HRMS (ESI): m/z calcd for C38H52NaO4Si (M+Na)+ 623.3533; found: 623.3517.
Procedure for desilylation
To 5a or 12 (40 mg, 0.067 mmol) in THF (0.5 mL) was added TBAF (0.32 mL of 1.0 M solution in THF, 0.32 mmol) at 0 °C. The reaction mixture was then warmed to room temperature and stirred for 40 min. The solvent was evaporated and the residue was chromatographed (hexane-ethyl acetate, 6 : 1) to give pure 3 (24 mg, 82%) or 4 (26 mg, 87%).
3(S),4(R),5(R)-Tribenzyloxy-6(S)-hydroxy-1,7-octadiene (3 and 5i)
[α]25D: +9.7 (c 0.4, CHCl3). 1H NMR (CDCl3): δ = 7.32-7.25 (m, 15H), 6.02-5.82 (m, 2H), 5.37-5.14 (m, 4H), 4.77-4.61 (m, 5H), 4.36 (d, J = 11.9 Hz, 1H), 4.10 (m, 2H), 3.74 (m, 2H), 2.63 (d, J = 7.4 Hz, 1H). 13C NMR (CDCl3): δ = 138.3, 138.2, 137.9, 135.3, 128.6, 128.5, 128.2, 128.0, 127.9, 127.8, 119.3, 116.3, 81.8, 81.4, 80.2, 74.8, 72.8, 72.0, 70.8. HRMS (ESI): m/z calcd for C29H32NaO4 (M+Na)+ 467.2198; found: 467.2201.
3(S),4(R),5(R)-Tribenzyloxy-6(S)-hydroxy-1,7-octadiene (4)
[α]25D: +24.8 (c 1.8, CHCl3). 1H NMR (CDCl3): δ = 7.36-7.25 (m, 15H), 5.98-5.82 (m, 2H), 5.41-5.22 (m, 4H), 4.90 (d, J = 11.4 Hz, 1H), 4.76-4.56 (m, 4H), 4.43 (m, 2H), 4.24 (ψt, J = 6.0 Hz, 1H), 3.78 (dd, J = 3.9, 6.0 Hz, 1H), 3.62 (ψt, J = 4.4 Hz, 1H), 3.20 (d, J= 6.6 Hz, 1H). 13C NMR (CDCl3): δ = 138.7, 138.5, 138.4, 138.0, 135.4, 128.5, 128.4, 128.1, 127.9, 127.8, 119.1, 115.6, 82.1, 82.0, 80.0, 75.3, 75.1, 72.0, 70.5. HRMS (ESI): m/z calcd for C29H32NaO4 (M+Na)+ 467.2198; found: 467.2190.
2(R)-Triisopropylsilyloxy-3(S),4(S),5(R)-tribenzyloxy-1,6-hexadial (6a)
A solution of 5a (1.0 g, 1.6 mmol) in pyridine (0.5 mL), CH2Cl2 (4.0 mL), and MeOH (20 mL) was cooled to −78 °C and subjected to a flow of O3 in dry O2 (0.05 CFM). When TLC indicated complete disappearance (about 5 min) of 5a (hexane: EtOAc, 9:1, Rf (5a)=0.55), Me2S (5 mL) was added. The reaction was allowed to warm to 20 °C and kept for 1 h, after which it was evaporated. The residue was dissolved in ether (40 mL), washed with aqueous 1M NH4Cl (20 mL), water (20 mL), brine, dried (MgSO4), and evaporated to produce 990 mg of crude 6a. This residue was coevaporated with heptane (2 × 10 mL) and toluene (2 × 10 mL) and then used directly in the next reaction. 1H NMR for crude 6a (300 MHz, CDCl3): δ 9.80 (s, 1H), 9.78 (s, 1H), 7.40-7.15 (m, 15H), 4.81 (d, 1H, J= 13.0 Hz), 4.63 (d, 1H, J=13.2), 4.52 (d, 1H, J=13.0 Hz), 4.42 (d, 1H, J=13.0 Hz), 4.38 (d, 1H, J=13.2 Hz), 4.29 (d, 1H, J=13.0 Hz), 4.09 (d, 1H, J=7.3 Hz), 4.05-3.94 (m, 2H), 3.75 (d, 1H, J=7.3 Hz), 1.04 (m, 21H).
3,4,5-Tri-O-benzyl-6-O-triisopropylsilyl-D-myo-inositol (7a) and 4,5,6-tri-O-benzyl-3-O-triisopropylsilyl-D-myo-inositol (8a)
To 100 mL of 0.1M solution of SmI2 in THF was added dropwise a solution of the above crude dialdehyde 6a (990 mg) in 50 mL of THF at −78 °C. The mixture was stirred at −78 °C for 20 min, then aqueous saturated NaHCO3 (40 mL) was added dropwise via syringe. The cold bath was removed and after 10 min the flask was opened to the atmosphere and allowed to warm up. Water (100 mL) was added and the white slurry was extracted with ethyl acetate (3 × 150 mL). The organic layer was washed with 10% aqueous Na2S2O3 (100 mL), brine and dried (MgSO4). The product was purified by column chromatography (CH2Cl2-benzene-ethyl acetate, 50 : 50 : 2) to give 632 mg of pure 7a (64% yield over 2 steps) followed by 30 mg of pure 8a. For 7a: [α]D 25: −18.2° (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3): δ 7.35-7.15 (m, 15H), 4.95 (d, 1H, J=11.4 Hz), 4.83 (d, 1H, J=10.7 Hz), 4.74-4.64 (m, 4H), 4.17 (m, 1H), 4.11 (ψt, 1H, J=8.8 Hz), 3.94 (ψt, 1H, J=8.8 Hz), 3.53 (dd, 1H, J=8.8, 2.9 Hz), 3.42 (m, 1H), 3.33 (ψt, 1H, J=8.8 Hz), 2.54 (br s, 1H), 2.48 (br d, 1H). 13C NMR (CDCl3): δ = 138.9, 138.4, 137.7, 128.4, 128.2, 128.0, 127.8, 126.8, 79.0, 78.7, 78.5, 78.1, 77.9, 76.1, 75.7, 75.4, 18.2, 18.1, 13.1. FAB HRMS (NBA/NaI): m/z calcd for C36H50NaO6Si (M+Na)+ 629.3276; found: 629.3272. For 8a: [α]D25: −20.4° (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3): δ 7.37-7.15 (m, 15H), 4.95 (d, 1H, J=12 Hz), 4.88 (d, 1H, J=12 Hz), 4.84 (d, 1H, J=12 Hz), 4.82 (s, 2H), 4.78 (d, 1H, J=12 Hz), 4.10 (m, 1H), 3.88-3.75 (m, 3H), 3.53 (br d, 1H, J=10 Hz), 3.46 (ψt, 1H, J=10 Hz), 2.55 (br s, 1H), 2.41 (br s, 1H). 13C NMR (CDCl3): δ = 138.9, 138.6, 138.4, 128.5, 128.3, 128.1, 127.9, 127.7, 127.0, 79.1, 78.8, 78.5, 78.2, 77.9, 76.1, 75.8, 75.5, 18.1, 12.7. HRMS (ESI): m/z calcd for C36H51O6Si (M+H)+ 607.3449; found: 607.3463.
3,4,5-Tri-O-benzyl-D-myo-inositol (7i)
To a solution of 7a (4.0 mg) in 0.5 mL of THF was added 0.1 mL of 1M Bu4NF in THF (containing 5% water) and the reaction was stirred at 20 °C for 3 h, then evaporated. The residue was dissolved in CH2Cl2 (10 mL), washed with water (10 mL), dried (MgSO4), and evaporated. The residue was purified by preprative TLC (10% MeOH:CH2Cl2) to produce pure 7i (1.5 mg, 51%), whose 1H NMR spectrum was identical to that reported previously.38
4,5,6-Tri-O-benzyl-D-myo-inositol (8i)
To a solution of 8a (8 mg, 13.2 µmol) in 0.5 mL of THF was added 0.2 mL of 1M Bu4NF in THF (containing 5% water) and the reaction was stirred at 20 °C for 15 h, then evaporated. The residue was dissolved in CH2Cl2 (15 mL), washed with water (15 mL), dried (MgSO4), and evaporated. The residue was purified by chromatography (10% MeOH in CH2Cl2) to produce pure 8i (4.9 mg, 83%) whose 1H NMR spectrum was identical to that reported previously.39
3(R)-Triisopropylsilyloxy-4(S),5(R),6(S)-tribenzyloxy-1,7-octadiene (12)
To the crude mixture of 3 and 4 (1:8.5, 0.72 g, 1.6 mmol) obtained as described in the previous paper, in DMF (3.8 mL) and pyridine (0.38mL) was added AgNO3 (1.1 g, 6.5 mmol) followed by triisopropylsilyl chloride (0.7 mL, 3.3 mmol) at 20 °C. The reaction mixture was stirred for 2 h at 20 °C, after which it was diluted with ether (10 mL) and water (10 mL). The aqueous layer was separated and extracted with additional ether (3 × 10 mL). The combined organic extracts were washed with brine, dried (MgSO4), and evaporated. The residue was chromatographed (hexane:ether, 97:3) to give 12 (0.62 g, 57% over 3 steps) and 5 (0.072 g, 7% over 3 steps). For 12: [α]25D: +18.2 (c 0.2, CHCl3). 1H NMR (CDCl3): δ = 7.35-7.24 (m, 15H), 6.15-6.04 (m, 1H), 5.96-5.85 (m, 1H), 5.29-5.15 (m, 4H), 4.88 (d, J = 11.0 Hz, 1H), 4.80-4.67 (m, 4H), 4.62-4.56 (m, 2H), 4.08 (dd, J = 6.1, 7.2 Hz, 1H), 3.84 (dd, J = 2.5, 6.1 Hz, 1H), 3.59 (ψt, J = 5.6 Hz, 1H), 1.50-0.80 (m, 21H). 13C NMR (CDCl3): δ = 139.4, 138.9, 138.6, 138.5, 135.8, 128.5, 128.3, 128.27, 128.2, 128.1, 128.0, 127.6, 127.3, 118.7, 116.0, 84.2, 82.0, 81.4, 77.4, 75.9, 75.0, 70.8, 18.3, 12.5. HRMS (ESI): m/z calcd for C38H52NaO4Si (M+Na)+ 623.3533; found: 623.3522.
2(S)-Triisopropylsilyloxy-3(S),4(S),5(R)-tribenzyloxy-1,6-hexadial (13)
A solution of 12 (65 mg, 0.11 mmol) in pyridine (20 µL), CH2Cl2 (2 mL) was cooled to −78 °C and subjected to a flow of O3 in dry O2 (0.05 CFM). When TLC indicated complete disappearance (about 5 min) of 5a (hexane:EtOAc, 9:1, Rf (12)=0.6), Me2S (0.2 mL) was added. The reaction was allowed to warm to 20 °C and kept for 1 h, after which it was treated with water (1.5 mL) and the layers separated. The aqueous phase was reextracted with CH2Cl2 (2 × 2 ML), dried (MgSO4), and evaporated to produce crude 13. This residue was used directly in the next reaction. 1H NMR for crude 13 (300 MHz, CDCl3): δ 9.68 (s, 1H), 9.63 (s, 1H), 7.40-7.15 (m, 15H), 4.75 (d, 2H, J= 12 Hz), 4.58 (s, 2H), 4.51 (d, 1H, J=12.0 Hz), 4.48 (d, 1H, J=12.0 Hz), 4.25 (s, 1H), 4.12-4.05 (m, 2H), 3.95 (ψt, 1H, J=5 Hz), 1.04 (m, 21H).
1-O-Triisopropylsilyl-2,3,4-tri-O-benzyl-L-chiro-inositol (14)
The crude dialdehyde 13 from above was diluted with t-butanol (30 µL, 0.3 mmol) in THF (6 mL) and added dropwise to a cold (− 78 °C) solution of SmI2 (0.6 mmol) in THF (6 mL). The reaction mixture was stirred at −78 °C for 3 h and then overnight at 20 °C. Sat. NaHCO3 (6 mL) was added and the white slurry was extracted with ethyl acetate (2 × 10 mL). The organic layer was washed with 10% Na2S2O3, sat. NaCl, and dried (MgSO4). Evaporation of the solvent and flash chromatography (hexane-ethyl acetate, 8:2) afforded 14 (38 mg, 58%) followed by an unidentified isomeric diol (3 mg). For 14: [α]D25: −39.0° (c 1.0, CHCl3). 1H NMR (300 MHz, CDCl3): δ 7.38-7.26 (m, 15H), 5.01 (d, 1H, J=11.5 Hz), 4.97 (d, 1H, J=10.8 Hz), 4.81 (d, 1H, J=10.8 Hz), 4.77 (d, 1H, J=11.4 Hz), 4.70 (d, 1H, J=11.5 Hz), 4.64 (d, 1H, J=11.4 Hz), 4.31 (dd, 1H, J=3.8, 2.7 Hz), 4.02-3.89 (m, 3H), 3.80 (dd, 1H, J=9.8, 2.7 Hz), 3.63 (ψt, 1H, J=9.3 Hz), 2.3 (br s, 1H), 1.65 (br s, 1H), 1.02 (m, 21H). 13C NMR (CDCl3): δ = 138.45, 138.42, 138.3, 128.4, 128.1, 128.0, 127.81, 127.77, 127.73, 127.69, 127.4, 127.2, 127.1, 81.8, 81.0, 80.1, 75.2, 75.1, 73.5, 71.7, 70.9, 70.8, 17.9, 17.8, 12.2. FAB HRMS (NBA/NaI) m/e 629.3278, M + Na+. Calcd for C36H50O6Si 629.3276.
Acknowledgment
A. K. would like to acknowledge the financial support from the U.S. National Institutes of Health (RR-16480 and CA-99957) under the BRIN/INBRE and AREA programs. M.D. acknowledges financial support from the National Institutes of Health (DK-44589 and GM-84819). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Alexander Kornienko, Email: akornien@nmt.edu.
Marc d’Alarcao, Email: mdalarcao@science.sjsu.edu.
References
- 1.Potter BVL, Lampe D. Angew. Chem.-Int. Edit. Engl. 1995;34:1933. [Google Scholar]
- 2.Berridge MJ, Irvine RF. Nature. 1989;341:197. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 3.Varela-Nieto I, Leon Y, Caro HN. Comp. Biochem. Physiol., B: Comp. Biochem. 1996;115:223. doi: 10.1016/0305-0491(96)00087-9. [DOI] [PubMed] [Google Scholar]
- 4.Jones DR, Varela-Nieto I. Mol. Med. 1999;5:505. [PMC free article] [PubMed] [Google Scholar]
- 5.Stralfors P. Bioessays. 1997;19:327. doi: 10.1002/bies.950190410. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ. Annu. Rev. Biochem. 1987;56:159. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanowicz P, Pujol JP. M S-Med. Sci. 2001;17:577. [Google Scholar]
- 8.Shashkin PN, Wasner HK, Ortmeyer HK, Hansen BC. Diabetes. Metab. Res. Rev. 2001;17:273. doi: 10.1002/dmrr.218. [DOI] [PubMed] [Google Scholar]
- 9.McConville MJ, Menon AK. Mol. Membr. Biol. 2000;17:1. doi: 10.1080/096876800294443. [DOI] [PubMed] [Google Scholar]
- 10.Obendorf RL, Horbowicz M, Dickerman AM, Brenac P, Smith ME. Crop Sci. 1998;38:78. [Google Scholar]
- 11.Obendorf RL. Seed Sci. Res. 1997;7:63. [Google Scholar]
- 12.Szczecinski P, Gryff-Keller A, Horbowicz M, Lahuta LB. J. Agric. Food Chem. 2000;48:2717. doi: 10.1021/jf000182g. [DOI] [PubMed] [Google Scholar]
- 13.Ma JM, Horbowicz M, Obendorf RL. Seed Sci. Res. 2005;15:329. [Google Scholar]
- 14.Horbowicz M, Brenac P, Obendorf RL. Planta. 1998;205:1. doi: 10.1007/s004250050290. [DOI] [PubMed] [Google Scholar]
- 15.Horbowicz M, Brenac P, Obendorf RL. Planta. 1998;205:1. doi: 10.1007/s004250050290. [DOI] [PubMed] [Google Scholar]
- 16.Horbowicz M, Obendorf RL. Crop Sci. 2005;45:1264. [Google Scholar]
- 17.Ferguson MAJ. J. Cell. Sci. 1999;112:2799. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Mayor S. Cell. Mol. Life Sci. 2001;58:1969. doi: 10.1007/PL00000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plourde R, d'Alarcao M, Saltiel AR. J. Org. Chem. 1992;57:2606. [Google Scholar]
- 20.Jaramillo C, Chiara JL, Martin-Lomas M. J. Org. Chem. 1994;59:3135. [Google Scholar]
- 21.Khiar N, Martin-Lomas M. Strategies for the Synthesis of Inositol Phosphoglycan Second Messengers. In: Chapleur Y, editor. Carbohydrate Mimics: Concepts and Methodology. Weinheim: Wiley-VCH Publishers; 1998. p. 443. [Google Scholar]
- 22.Lopez-Prados J, Martin-Lomas M. J. Carbohydr. Chem. 2005;24:393. [Google Scholar]
- 23.Frick W, Bauer A, Bauer J, Wied S, Muller G. Biochemistry. 1998;37:13421. doi: 10.1021/bi9806201. [DOI] [PubMed] [Google Scholar]
- 24.Jaworek CH, Iacobucci S, Calias P, d'Alarcao M. Carbohydr. Res. 2001;331:375. doi: 10.1016/s0008-6215(01)00047-7. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty N, d'Alarcao M. Bioorg. Med. Chem. Lett. 2005;13:6732. doi: 10.1016/j.bmc.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Xue J, Guo ZW. Bioorg. Med. Chem. Lett. 2002;12:2015. doi: 10.1016/s0960-894x(02)00301-3. [DOI] [PubMed] [Google Scholar]
- 27.Mayer TG, Weingart R, Munstermann F, Kawada T, Kurzchalia T, Schmidt RR. Eur. J. Org. Chem. 1999:2563. [Google Scholar]
- 28.Murakata C, Ogawa T. Tetrahedron Lett. 1991;32:671. [Google Scholar]
- 29.Gigg R, Gigg J. Synthesis of Glycosylphoshatidylinositol. In: Large D, Warren CD, editors. Glycolipids and Related Compounds. New York: Marcel Dekker; 1997. p. 327. [Google Scholar]
- 30.Prestwich GD, Dorman G, Elliott JT, Marecak DM, Chaudhary A. Photochem. Photobiol. 1997;65:222. doi: 10.1111/j.1751-1097.1997.tb08548.x. [DOI] [PubMed] [Google Scholar]
- 31.Billington DC. Chem. Soc. Rev. 1989;18:83. [Google Scholar]
- 32.Gultekin MS, Celik M, Balci M. Curr. Org. Chem. 2004;8:1159. [Google Scholar]
- 33.Sureshan KM, Shashidhar MS, Praveen T, Das T. Chem. Rev. 2003;103:4477. doi: 10.1021/cr0200724. [DOI] [PubMed] [Google Scholar]
- 34.Kornienko A, Turner DI, Jaworek CH, d'Alarcao M. Tetrahedron: Asymmetry. 1998;9:2783. [Google Scholar]
- 35.Kornienko A, d'Alarcao M. Tetrahedron Lett. 1997;38:6497. [Google Scholar]
- 36.Chiara JL, Cabri W, Hanessian S. Tetrahedron Lett. 1991;32:1125. [Google Scholar]
- 37.Desai T, Fernandez-Mayoralas A, Gigg J, Gigg R, Payne S. Carbohydr. Res. 1990;205:105. doi: 10.1016/0008-6215(90)80132-m. [DOI] [PubMed] [Google Scholar]
- 38.Chung SK, Yu SH, Chang YT. J. Carbohydr Chem. 1998;17:385. [Google Scholar]
- 39.Gilbert IH, Holmes AB, Pestchanker MJ, Young RC. Carbohydr. Res. 1992;234:117. [Google Scholar]
- 40.Hederos M, Konradsson P. J. Am. Chem. Soc. 2006;128:3414. doi: 10.1021/ja057339b. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Prados J, Cuevas F, Reichardt NC, de Paz JL, Morales EQ, Martin-Lomas M. Org. Biomol. Chem. 2005;3:764. doi: 10.1039/b418041k. [DOI] [PubMed] [Google Scholar]
- 42.Kwon YU, Soucy RL, Snyder DA, Seeberger PH. Chem. Eur. J. 2005;11:2493. doi: 10.1002/chem.200400934. [DOI] [PubMed] [Google Scholar]
- 43.Smith TK, Crossman A, Brimacombe JS, Ferguson MAJ. EMBO J. 2004;23:4701. doi: 10.1038/sj.emboj.7600456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu J, Jayaprakash KN, Schlueter U, Fraser-Reid B. J. Am. Chem. Soc. 2004;126:7540. doi: 10.1021/ja038807p. [DOI] [PubMed] [Google Scholar]
- 45.Lu J, Jayaprakash KN, Fraser-Reid B. Tetrahedron Lett. 2004;45:879. [Google Scholar]
- 46.Xue J, Guo ZW. J. Am. Chem. Soc. 2003;125:16334. doi: 10.1021/ja0382157. [DOI] [PubMed] [Google Scholar]
- 47.Reichardt NC, Martin-Lomas M. Angew. Chem., Int. Ed. Engl. 2003;42:4674. doi: 10.1002/anie.200351950. [DOI] [PubMed] [Google Scholar]
- 48.Tailler D, Ferrieres V, Pekari K, Schmidt RR. Tetrahedron Lett. 1999;40:679. [Google Scholar]
- 49.Mayer TG, Schmidt RR. Eur. J. Org. Chem. 1999:1153. [Google Scholar]
- 50.Baeschlin DK, Chaperon AR, Charbonneau V, Green LG, Lay SV, Lucking U, Walther E. Angew. Chem.-Int. Edit. 1998;37:3423. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3423::AID-ANIE3423>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 51.Murakata C, Ogawa T. Carbohydr. Res. 1992;235:95. doi: 10.1016/0008-6215(92)80081-b. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann HMR, Munnich I, Nowitzki O, Stucke H, Williams DJ. Tetrahedron. 1996;52:11783. [Google Scholar]
- 53.Honda T, Katoh M. Chem. Commun. 1997:369. [Google Scholar]
- 54.Chenede A, Pothier P, Sollogoub M, Fairbanks AJ, Sinay P. J. Chem. Soc. Chem. Commun. 1995:1373. [Google Scholar]
- 55.Hiramatsu N, Takahashi N, Noyori R, Mori Y. Tetrahedron. 2005;61:8589. [Google Scholar]
- 56.Miller RS, Sealy JM, Shabangi M, Kuhlman ML, Fuchs JR, Flowers RA. J. Am. Chem. Soc. 2000;122:7718. [Google Scholar]
- 57.Clerici A, Porta O. Tetrahedron. 1983;39:1239. [Google Scholar]
- 58.Clerici A, Porta O. J. Org. Chem. 1987;52:5099. [Google Scholar]
- 59.Clerici A, Porta O, Riva M. Tetrahedron Lett. 1981;22:1043. [Google Scholar]
- 60.Hasegawa E, Curran DP. J. Org. Chem. 1993;58:5008. [Google Scholar]
- 61.Kireev AS, Breithaupt AT, Collins W, Nadein ON, Kornienko A. J. Org. Chem. 2005;70:742. doi: 10.1021/jo048459p. [DOI] [PubMed] [Google Scholar]