Abstract
Considerable epidemiological evidence indicates that dietary consumption of moderate levels of polyphenols decreases both the incidence of cardiovascular disease and the mortality associated with myocardial infarction. Molecular mechanisms of this cardiovascular protection remain uncertain but can involve changes in rates of nitric oxide (NO) generation by endothelial nitric oxide synthase (eNOS). We examined the vascular responses to quercetin using a combination of biochemical and vessel function criteria. Quercetin treatment for 30 min enhanced relaxation of rat aortic ring segments. Moreover, the addition of L-NAME (100 μM) or charybdotoxin (ChTx) blocked quercetin-mediated vasorelaxation thus demonstrating the effect was partially dependent on NOS and endothelium-derived hyperpolarizing factor (EDHF). Additionally, bovine aortic endothelial cells (BAEC) treated with quercetin showed a rapid increase of intracellular Ca2+ concentrations as well as a dose- and time-dependent stimulation of eNOS phosphorylation with a concomitant increase in NO production. These results demonstrate that quercetin-mediated stimulation of eNOS phosphorylation increases NO bioavailability in endothelial cells and can thus play a role in the vascular protective effects associated with improved endothelial cell function.
Keywords: nitric oxide synthase, endothelium, cardioprotection, eNOS, heart, catalase, endothelium-derived hyperpolarizing factor, phosphorylation, aortic ring segments, H2O2, fura-2, mircofluorometric Ca2+ imaging
Introduction
The endothelium plays an important role in maintaining vascular homeostasis. Oxidative stress has been implicated in numerous vascular diseases. Cardiovascular risk factors, such as hypertension, diabetes and smoking, alter the redox state in the vessel and can lead to endothelial dysfunction resulting in atherosclerosis. Endothelial dysfunction and the loss of nitric oxide (NO) production are early manifestations and hallmarks of vascular disorders. The increase in oxidative stress, due to endothelial dysfunction and lack of NO bioavailability, alters several physiological functions such as leukocyte adhesion, platelet aggregation, and blood flow in the endothelium [1].
Consumption of flavonoids found in fruits, vegetables, and beverages such as tea and wine are inversely correlated with mortality from coronary heart disease [2–4]. The Rotterdam study demonstrated an inverse relationship between flavonoid intake and occurrence of myocardial infarction [5]. The cardiovascular benefits of flavonoid-rich foods have been attributed to their antioxidant effects (inhibiting the oxidation of low density lipoproteins (LDL) [6]), NO-mediated vasodilatory effects, reducing platelet aggregation [7], and activating Nrf2-induced phase II detoxification through antioxidant response element (ARE)-mediated gene expression [8–11].
Epidemiological studies have suggested an inverse correlation between the incidence and mortality rates from coronary heart disease and light to moderate alcohol consumption (1–2 drinks/day), in particular red wine. Several studies have shown the beneficial effects of moderate alcohol consumption have been attributed to the alcohol content [12–14]. Special attention has focused on the French Paradox - a lower incidence of cardiovascular mortality among the Mediterranean population in association with red wine consumption despite having a diet high in saturated fat intake. Moreover, studies demonstrate the beneficial effects of red wine on the vasculature are attributed to the high polyphenol content found in red wines [11, 15, 16]. In particular, red wine polyphenols (RWPs) as well as individual polyphenolic compounds found in red wine, such as quercetin, resveratrol, and catechin, attenuated the progression of atherosclerotic lesions [14, 17, 18]. Pioneering ex vivo studies suggested that flavonoids, in particular red wine polyphenols, may favorably affect endothelial function by stimulating endothelium-dependent relaxation through NO/NOS-dependent pathway [19–21]. Moreover, these effects were partially attenuated with PI3K inhibitors (Wortmannin and LY294002) [22]. These data indicate that dietary polyphenols may play a role in the cardiovascular protection by improving endothelial cell function.
Quercetin is the most prevalent flavonoid in the human diet and abundantly found in apples, onions, berries, and red wines. A recent study demonstrated that low to moderate oral supplementation of quercetin (50, 100, or 150 mg/day) for two weeks increased plasma quercetin concentrations dose-dependently in healthy individuals. The highest dose of 150 mg/day increased plasma quercetin concentrations over 570% with a median concentration of 0.38 μM and a maximal concentration observed at 1.3 μM [23]. Quercetin is part of the flavone family which contains a double bond in the central aromatic ring. It is a polyphenol that has been shown to engage in redox cycling reactions in cellular systems thereby endowing both antioxidant and prooxidant (by generating H2O2) properties [24, 25]. The effects of quercetin on vessel function are not clear and specifically whether the proposed generation of H2O2 can modulate eNOS activity and vessel relaxation. This study provides new insight in the ability of quercetin to improve endothelial function by inducing vasorelaxation through an eNOS phosphorylation process blocked by an increase in catalase activity, Ca2+-mediated eNOS-dependent and -independent pathways. These results demonstrate that quercetin-mediated stimulation of eNOS phosphorylation increases NO bioavailability in endothelial cells and can thus play a role in the vascular protective effects associated with improved endothelial cell function.
Experimental procedures
Biochemicals
All chemicals were obtained from Sigma (St. Louis, MO), unless specified. Quercetin was obtained from Acros Organics (Morris Plains, NJ). Monoclonal eNOS and polyclonal phospho-eNOS (Ser1177) antibodies were obtained from Transduction Laboratories (Lexington, KY) and Cell Signaling Technology (Beverly, MA), respectively. The secondary antibody was obtained from Amersham-Pharmacia Biotech (Piscataway, NJ). Ad.catalase and Ad.empty were obtained from the Gene Transfer Vector Core (University of Iowa; Iowa City, IA).
Animals
Ten-week-old Sprague-Dawley rats ranging from 300–350g body weight were obtained from Charles River Laboratories, Inc (Wilmington, MA) and were maintained at constant humidity (60 ± 5%), temperature (24 ± 1°C), and light cycle (6AM to 6 PM). All rats were fed a standard rat pellet diet (Ralston Purina Diet) ad libitum. All animal protocols were approved by the Animal Review Board of the University of Alabama at Birmingham. All animals were anesthetized prior to surgical procedures with ketamine and Rompun (10 and 1.5 mg/100 g body weight, respectively).
Quercetin preparation
Quercetin was made fresh daily, dissolved in DMSO (> 24 mg/mL), and the exact concentration was obtained using UV spectroscopy (extinction coefficient; €μM = 0.026; Abs, 377 nm).
Vessel reactivity studies
The assessment of vascular function was conducted as previously described [26]. In brief, the aorta was excised, cleaned of fat and excess tissue, cut into individual ring segments (2–3 mm width), and suspended from a force-displacement transducer in a water-jacketed tissue bath containing bicarbonate-buffered, Krebs-Henseleit buffer (all of the following in mM concentrations-118 NaCl, 4.6 KCl, 27.2 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, 1.75 CaCl2, 0.03 Na2EDTA, and 11.1 glucose). The buffer was maintained at 37 and aerated with 95% O2/5% CO2. Isometric tension was then measured in isolated aortic ring segments (a passive load of 2g was applied to all ring segments and maintained throughout the experiment). At the beginning of each experiment, ring segments were depolarized with 70 mM potassium chloride (KCl) to determine maximal contractile capacity of the vessel. Rings were then thoroughly washed and allowed to equilibrate.
In subsequent experiments, ring segments were pretreated with or without L-NAME for 30 min followed by quercetin or vehicle (DMSO) treatment for 30 min. Ring segments were submaximally contracted (40% of KCl response) with phenylephrine (PE), ~3×10−8 to 10−7 M. When tension development reached a plateau, acetylcholine (ACh), 10−9 to 3×10−6 M was added cumulatively to the bath to stimulate endothelium-dependent relaxation via calcium-dependent NO formation in response to ligand receptor interactions. In other experiments, ring segments were treated (as indicated above) and contractile responses were tested by the cumulative addition of PE. Dose response profiles under different experimental conditions were analyzed and tested for differences in relaxation and contraction parameters. In a subset of studies, sodium nitroprusside (SNP, 5 μM) was added to elicit residual EC-independent relaxation and ensure the ability of the rings to respond to NO. In some experiments, vessels were denuded to confirm endothelial-dependence of the enhanced response to Ach.
For hyperpolarization experiments, ring segments were depolarized with 70 mM KCl twice to achieve maximal contractile capacity of the vessels. Rings were then thoroughly washed and allowed to equilibrate before the addition of 50 nM charybdotoxin (ChTx) for 30 min. Immediately following ChTx, the rings were contracted with another dose of 70 mM KCl (achieved maximal contractile capacity) and then 5 μM quercetin or equivalent DMSO was added to the vessel bath for 30 min in intact and denuded vessels. At the end of the experiments, acetylcholine (Ach) dose responses were performed to ensure maximal relaxation. Data was collected for all experiments and downloaded to an IBM PC for later analysis using Workbench for Windows (v.3) software.
cGMP analysis
Rat thoracic aortas were excised, cleaned, cut into ring segments, weighed, placed in Krebs buffer and bubbled continuously with 95% O2/5% CO2. The ring segments were incubated with 5 μM quercetin for 60 min, immediately flash frozen in liquid nitrogen, and stored at −80°C until analysis. The cGMP values were determined using the cGMP enzyme immunoassay assay from Cayman Chemical Company (Ann Arbor, MI) according to the manufacturer’s specifications. Additionally, aortic ring segments were also incubated with 25 μM BAPTA/AM for 30 min prior to the addition of quercetin. Diethylenetriamine/NO (DETA/NO), a nitric oxide donor was used as a positive control. Protein concentrations were used for normalization and determined by the BCA protein assay kit (Pierce, Rockford, IL). Data were expressed as pmol/mg protein.
Cell Culture
Bovine aortic endothelial cells (BAECs) were isolated from descending thoracic aortas and maintained in Medium 199 (Cellgro; Herndon, VA) containing 5% fetal bovine serum (Hyclone; Logan, UT), 5% fetal calf serum (Hyclone), 10 μM thymidine (Gibco; Carlsbad, CA), and Antibiotic Antimycotic Solution (Cellgro). Cells were monitored visually for typical cobblestone morphology indicative of endothelial cells by staining for von Willebrand factor expression and were not passaged for more than eight cycles.
Western blotting
Confluent BAECs were treated with quercetin for 15–120 min. After treatment, cells were rinsed twice with ice cold phosphate buffer saline (PBS) and homogenized in lysis buffer consisting of 1% Triton X-100, 10 mM NaF, 1 mM vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin, 10 mg/ml leupeptin in Tris-buffer saline at pH 7.5. The protein concentration was determined by using BCA protein assay kit. Protein was denatured by boiling, separated on a 7.5 % SDS-PAGE gel, and transferred to nitrocellulose. Membranes were then incubated overnight in blocking buffer containing anti-phospho-eNOS (Ser1177) at a dilution of 1:1000, washed with TTBS, incubated with horseradish peroxidase-conjugated antibody and washed extensively. Immunoreactive bands were visualized using chemiluminescence (Pierce).
Assessment of NO production
NO was detected by chemiluminescence following the reduction of NO2− with KI and CuSO4, according to manufacturer’s instructions (Sievers). BAECs were grown to confluency in six-well plates in 10% fetal bovine serum medium. The medium was removed and cells were washed twice with HBSS at 37°C prior to incubation with 0.75 ml of HBSS containing 25 μM L-arginine plus the addition of quercetin or vehicle (DMSO) for 30–120 min. Following treatment, the HBSS plus treatment was collected from each well and centrifuged at 1,000 × g for 10 min. The resulting supernatant was then injected (50 μl via Hamilton syringe) into the Sievers nitric oxide analyzer [27]. Each treatment was performed in triplicate and normalized to cell protein content. A serial dilution of nitrite standards were prepared for each experiment using a freshly prepared NaNO2 solution. Background levels of NO2− concentrations were determined using samples of dH2O and HBSS containing L-arginine.
Adenovirus gene transfer
Adenoviruses were purchased from the University of Iowa Gene Transfer Vector Core Facility. AdCMVCatalase (AdCat), AdCMVLacZ (AdLacZ), Adempty were manufactured at the University of Iowa’s Vector Core Facility by inserting catalase and LacZ genes into the E1 region of an Ad5 E1/partial E3-deleted replication-deficient adenoviral vector [28]. Briefly, BAECs were infected with the AdCat at the indicated multiplicity of infection (MOI) for 24 h after which the infection medium was removed and replaced with fresh medium for an additional 24 h. AdLacZ or Adempty (25, 50 MOI) were used as a control for adenovirus infection.
Catalase enzymatic activity
Catalase activity was determined as described previously [29]. Briefly, 200 μg of total cell lysate was added to 30 mM H2O2 in 50 mM potassium phosphate buffer (pH 7.8), and the disappearance of H2O2 was measured at 240 nm for 60 sec at 8 sec intervals.
Calcium measurements
Microfluorometric imaging of intracellular free Ca2+ ([Ca2+]i) was performed on BAECs cultured on glass coverslips until confluence. Briefly, BAECs were rinsed with HBSS twice and then loaded with 5μM fura-2/AM (Molecular Probes; Carlsbad, CA) for 15 min. After three washes with HBSS, coverslips were placed in a imaging chamber mounted on a fixed-stage upright microscope (Zeiss Axioskop FS; Oberkochen, Germany) and continuously perfused (2 mL/min) with HBSS at room temperature. Fura-2 was alternatively excited at 360 and 380 nm using a monochromator (Polychrome-IV, TILL Photonics; Munich, Germany), and its emission (>510 nm) filtered and detected with a frame-transfer cooled CCD camera (Quantix-57, Photometrics; Tucson, AZ). After BAECs were equilibrated (~10 min), time-lapse series of fura-2 image pairs were acquired every 2 sec before and after bath application of DMSO (vehicle), 2.5 or 5 μM quercetin. Background-subtracted fluorescence intensity measurements were obtained within regions-of-interest (ROIs) defined over BAEC cell bodies. The average ratio of 360nm and 380nm fluorescence within each ROI was used as an estimate of intracellular Ca2+ concentration, as these two parameters are directly proportional to each other [30]. Synchronized image acquisition and monochromator control was performed using custom-written software (TIWorkBench, written by T.I.) on a G4 Macintosh computer (Apple). Each imaging session had 10–20 cells per experiment, and results were derived from at least seven independent experiments.
Results
Quercetin reduces vasoconstrictor sensitivity in rat aortic ring segments by an NO-dependent mechanism
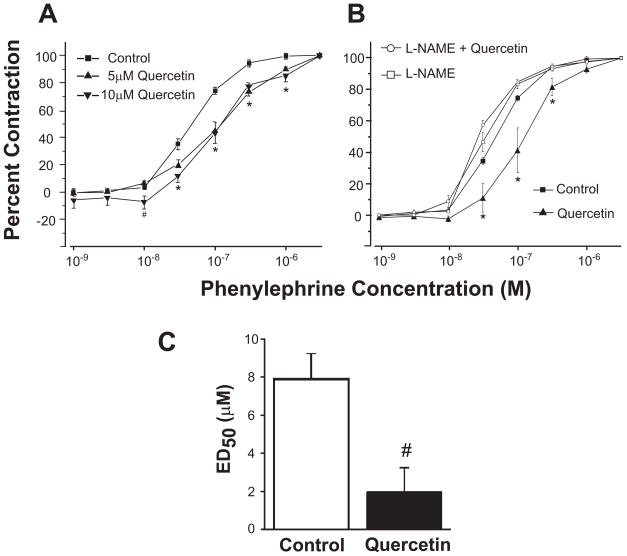
Quercetin (5 and 10 μM for 30 min) attenuated phenylephrine (PE)-induced contraction in rat aortic vessels compared to vehicle control (DMSO). Line graphs represent concentration-response curves of aortic ring segments to PE following the incubation of aortic ring segments with 5 and 10 μM quercetin for 30 min (Fig 1A). There was no distinguishable difference in contractile response between 5 and 10 μM quercetin at 30 min, between untreated and vehicle treated (DMSO), and longer treatments (up to 120 min) did not alter the response significantly (not shown). Therefore the aortic ring segments were incubated with 5 μM quercetin for 30 min in all subsequent experiments. In Figure 1B, the contractile response of a normal rat aorta (filled squares) was altered by pretreating the vessels with NOS inhibitor, L-NAME, the contractile curve shifted to the left indicating the basal NOS activity in the rat aorta (open squares). Quercetin treatment (filled triangle) for 30 minutes shifted the contractile response to the right indicating more PE was needed to produce the same contractile response compared to the vehicle control or L-NAME treated vessels. The quercetin response was completely blocked by pretreating the ring segments with L-NAME (100 μM) for 30 min (open circles). Quercetin treatment for 30 min had a significant vasorelaxant effect in response to acetylcholine (Ach). Aortic ring segments treated with quercetin or DMSO for 30 min showed a dose-dependent relaxation in response to acetylcholine (Ach) with an ED50 of 1.97±1.2 or 7.96±1.4, respectively (Fig 1C).
Figure 1. Quercetin attenuates phenylephrine (PE)-induced contraction in rat aortic vessels.
Line graphs represent concentration-response curves of aortic ring segments to PE. (A) Contractile response of rat aortic ring segments incubated with vehicle control (DMSO), 5 and 10 μM quercetin for 30 min. (B) Aortic rings were incubated with 100 μM L-NAME for 15 minutes followed by DMSO or quercetin treatment for 30 minutes. Aortic ring segments were incubated with DMSO or 5 μM quercetin for 30 min followed by an Ach dose response (3×10−9–3×10−5 M). Results are presented as mean ± SEM, n=8–14. #, p<0.05, significant difference compared to control; *, p<0.05, significant difference compared to all groups (control, L-NAME, and L-NAME and quercetin).
Quercetin stimulates eNOS phosphorylation at Ser1179
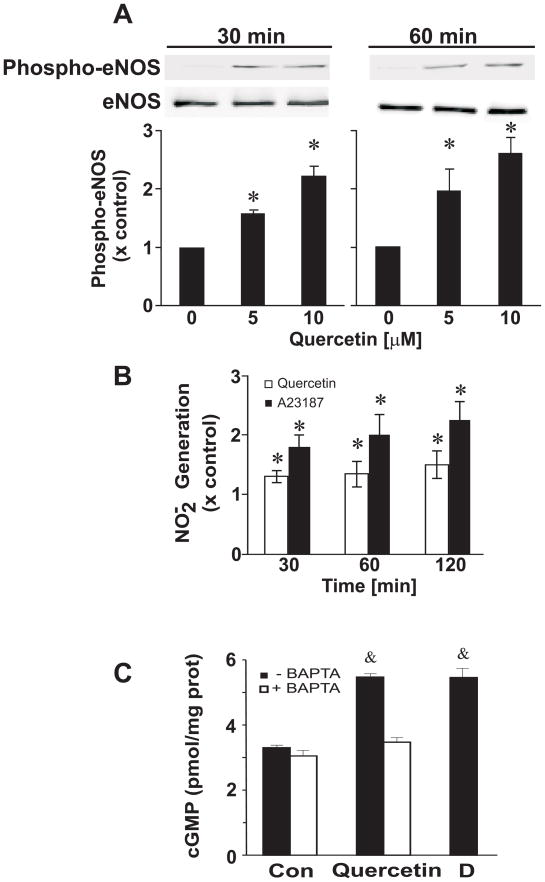
Several eNOS phosphorylation sites have been identified such as Ser1179, Thr497, and Ser116 that regulate eNOS activity. Short-term treatment (30 or 60 min) of quercetin (5 and 10 μM) stimulates phosphorylation of eNOS at Ser1179 in a dose-dependent manner in BAECs while the total eNOS protein levels remain unchanged (Figure 2A). Lower concentrations of quercetin (1 and 2.5 μM) stimulated the phosphorylation of eNOS at Ser1179, albeit not significant (not shown). Quercetin treatment did not alter Thr497 and Ser116 levels (not shown).
Figure 2. Quercetin stimulates eNOS phosphorylation at Ser1179, NO production, and cGMP.
Confluent BAECs were treated with 5 or 10 μM quercetin for 30 and 60 min. (A) Top panel is a representative Western blot; bottom panel is the densitometric intensity determined digitally. (B) Quercetin treatment stimulated NO release from BAECs. NO generation was determined in quercetin-treated confluent BAECs for the indicated times using Sievers nitric oxide analyzer (NOA). The levels of nitrite have been normalized to protein concentration (basal NO2− = 1.04 ± 0.09 nmoles/mg protein) and are reported as a percent of the vehicle control (DMSO) at each individual time point (30, 60, and 120 min). A23187 was used as a positive control. (C) Rat aortic ring segments were incubated with or without 25 μM BAPTA/AM for 30 min followed by the treatment of 5 μM quercetin for 60 min. The aortic ring segments were immediately snap frozen in liquid nitrogen and cGMP values were determined using the cGMP enzyme immunoassay assay from Cayman. Diethylenetriamine/NO (DETA/NO represented as D), a nitric oxide donor, was used as a positive control. Results were derived from at least four independent experiments and data were expressed as mean ± SEM. *, p<0.05 vs control; &, p<0.05 vs all groups.
Quercetin increases NO2− accumulation in BAECs
The ability of quercetin to stimulate eNOS phosphorylation at Ser1179 led to an increase in NO production over time in BAECs. The media was collected and analyzed for NO2− levels (indicative of eNOS-derived NO production) after treating cells with 5 μM quercetin for 30, 60, and 120 min. This NO2− accumulation in the media was normalized for cell protein content and reported as a percent over the DMSO control (basal NO2− = 1.04 ± 0.09 nmoles/mg protein). No changes in NO2− accumulation were observed for untreated or DMSO-treated cells at the same sampling times (not shown). Exposure of cells to quercetin increased NO2− accumulation 1.3-, 1.5-, and 1.8-fold over DMSO control at 30, 60, and 120 min, respectively (Fig. 2B). Calcium ionphore was used as a positive control.
Quercetin increases cGMP in aortic ring segments
The downstream effects of NO were tested by incubating rat aortic ring segments with 5 μM quercetin for 60 min. Quercetin increased cGMP levels and this effect was inhibited by treating the aortic ring segments with 25 μM BAPTA/AM for 30 min prior to the quercetin treatment. Diethylenetriamine/NO (D, DETA/NO), a nitric oxide donor, was used as a positive control (Fig 2C).
Quercetin increases intracellular Ca2+ levels in BAECs
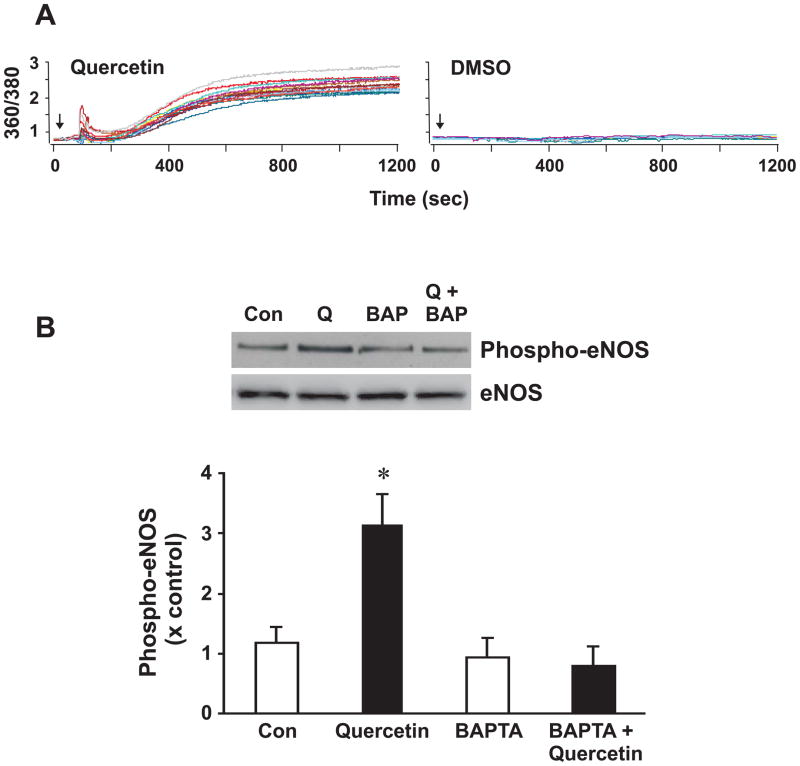
Previous studies demonstrated that polyphenols found in red wine elevate intracellular Ca2+ levels [31], and such increases enhance endothelial NO production [32]. Indeed, application of quercetin to BAECs increased the intracellular concentration of free calcium in BAECs, as determined by fura2/AM imaging (left panel, Fig 3A). These fura-2 signals peaked within the first 2 min of exposure to quercetin, and remained elevated until the removal of Ca2+ from the extracellular solution (not shown). On the other hand, vehicle controls using DMSO did not affect fura-2 signals (right panel, Fig 3A). The activation of eNOS by Ach or shear stress is accompanied by an increase in intracellular Ca2+ and a subsequent release of NO [33]. To test this, BAECs were pretreated with the Ca2+ chelator BAPTA/AM (25 μM for 30 min) and then stimulated with quercetin for 30 min. Fig 3B demonstrates that chelation of Ca2+ by BAPTA attenuates quercetin-stimulated eNOS phosphorylation at Ser1179. Moreover, excised rat aortic ring segments incubated with quercetin increased cGMP and this effect was blocked by the pretreatment of BAPTA/AM (Fig 3B). Rat aortic ring segments were incubated with BAPTA/AM or DMSO for 30 min followed by quercetin treatment (5μM) for 1 h.
Figure 3. Quercetin increases intracellular Ca2+ pools in BAECs while BAPTA/AM blocks quercetin-induced eNOS phosphorylation at Ser1179.
(A) BAECs were loaded with the fluorescent probe fura2/AM (5 μM) for 15 minutes, rinsed with HBSS, placed in a recording chamber mounted on a fixed-stage upright microscope, equilibrated and then treated with 2.5 μM quercetin (left panel) or DMSO (right panel). A representative recording from a single coverslip showing Ca2+ traces from individual cells following the treatment of quercetin. Each run had 10–20 cells per experiment. The arrows indicate when quercetin and DMSO were added. Results were derived from at least seven independent experiments. (B) BAECs were pretreated with 25 μM BAPTA/AM for 30 min followed by a 5 μM quercetin treatment for 30 min. Top panel is a representative Western blot of eNOS phosphorylation at Ser1179 and eNOS was used as a loading control; bottom panel is the densitometric intensity determined digitally. Data is represented as the mean ± SEM, n=4–6. *, p<0.05 vs control
Overexpression of catalase by adenovirus blocks quercetin-induced eNOS phosphorylation
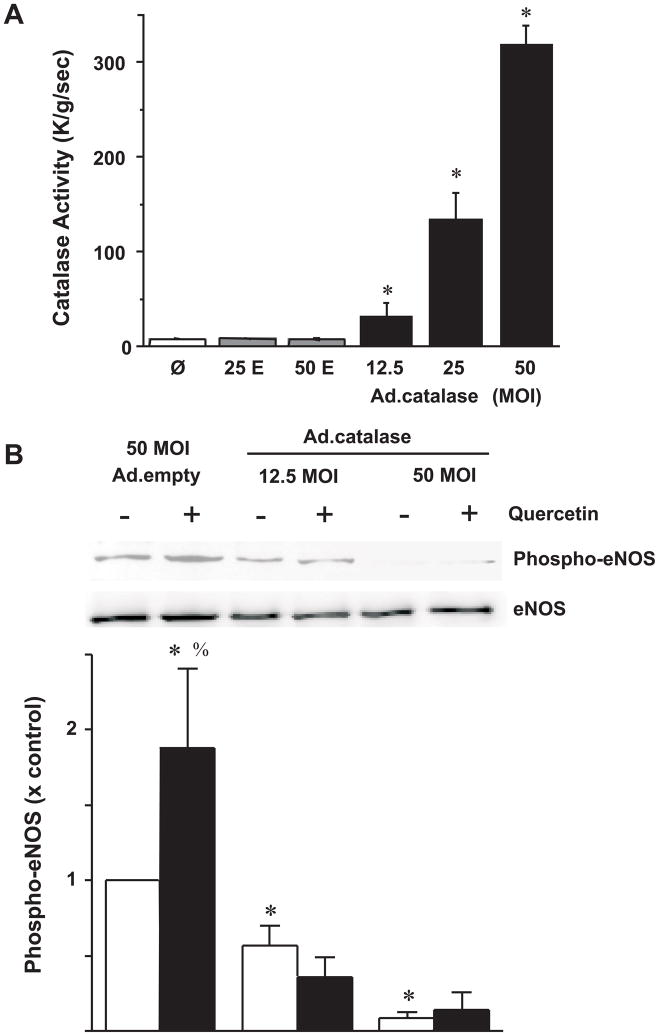
Quercetin has been shown to generate H2O2 via semi-quinone radical [34]. Rat aortic ring segments treated with 1, 10, or 50 μM H2O2 for 30 min markedly reduced the responses to PE (not shown) and consistent with previous findings [35, 36]. Additionally, H2O2 has been shown to phosphorylation eNOS at ser1179 in BAECs via PI3K-dependent signaling pathway [36]. Therefore, BAECs were transduced with adenovirus containing catalase (Ad.catalase) and incubated for 24 h to allow catalase protein and activity to increase significantly (Fig 4A). Overexpression of catalase dramatically diminished the basal phosphorylation levels of eNOS. More importantly, quercetin treatment (5 μM) for 30 min failed to stimulate eNOS phosphorylation at Ser1179 (Fig 4B) with densitometeric analysis (Fig 4C).
Figure 4. Overexpression of catalase inhibits quercetin-stimulated eNOS phosphorylation at Ser1179.
(A) Catalase transduction using 12.5, 25, 50 MOI Ad.Cat significantly increased catalase activity in BAECs compared to Ad.Empty transduced controls. (B) Catalase transduced BAECs were treated with DMSO or quercetin for 30 min. Top panel is a representative Western blot of eNOS phosphorylation at Ser1179 and eNOS was used as a loading control; bottom panel is the densitometric intensity determined digitally. Results are expressed as mean ± SEM, n=6. Ø, no treatment; 25E, 25 MOI Ad.Empty; 50E, 50 MOI Ad.Empty. *P<0.05, significant difference compared to all groups (non-transduced controls, 25 and 50 Ad.Empty transduced BAECs); *, p<0.05 vs Ad.Empty transduced BAECs treated only with DMSO; %, p<0.05- quercetin vs DMSO for respective adenovirus control (i.e.- BAECs transduced with 50 MOI Ad.Empty treated with quercetin compared to DMSO).
Endothelium derived hyperpolarization factor (EDHF) may also be responsible for quercetin-mediated vasorelaxation
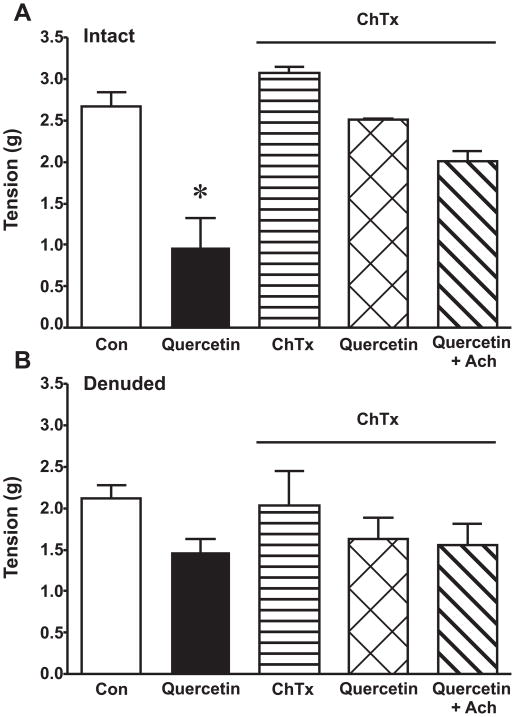
Quercetin treatment significantly induced endothelium-dependent relaxation compared to vehicle control in intact vessels (Fig 5A) but not in denuded vessels (Fig 5B). Pretreatment with charybdotoxin (ChTx), which blocks large- conductance (BKCa)- and intermediate-conductance (IKCa)-Ca2+-activated K+-channels, significantly attenuated the quercetin-mediated response in intact vessels (Fig 5A). ChTx did not have any effect on basal vessel tone. At the end of the experiments, acetylcholine (Ach) dose responses were performed to elicit maximal endothelium-dependent relaxation. There was no significant difference between quercetin and quercetin plus Ach with the pretreatment of ChTx or without (not shown). There were no significant differences in the denuded vessel for all of the treatments. As a control, an Ach dose response was performed to verify the endothelium was completely denuded (Fig 5B).
Figure 5. Quercetin treatment induces EDHF.
Rat aortic ring segments were equilibrated (basal) and then contracted with 70 mM KCl. The rings were rinsed and allowed to re-equilibrate back to baseline and then treated with or without 50 nM charybdotoxin (ChTx) for 30 min. Immediately after, the rings were contracted with another dose of 70 mM KCl (maximal contraction) followed by 5 μM quercetin treatment for 30 min in intact (A) and denuded vessels (B). At the end of the experiments, acetylcholine (Ach) dose responses were performed to ensure maximal relaxation. Results are presented as mean ± SEM, n=8–14. *, p<0.05, significant difference compared to all groups.
Discussion
This study reveals that quercetin induced vasorelaxation and increased cGMP levels in rat aortic vessels. Moreover, in vitro experiments indicated that quercetin treatment rapidly increased Ca2+, stimulated eNOS phosphorylation at Ser1179 resulting in NO generation in BAECs. Quercetin-stimulated eNOS phosphorylation was completely blocked by increasing cellular catalase enzymatic activity with the transduction of Ad.Cat.
Investigation into the underlying mechanism(s) revealed that quercetin-induced vasorelaxation could be regulated, in part, by the formation of an oxidant. More studies are needed to verify these findings; however cell culture experiments provided some insight. The transduction of catalase activity via Ad.Cat ameliorated quercetin stimulated eNOS phosphorylation in BAECs.
New evidence supports that flavonoids, such as quercetin, not only have antioxidant capabilities but more importantly may act as signaling molecules [37]. In fact, it has been shown that quercetin can act as an antioxidant leading to the formation of quinones and prooxidants [38]. Furthermore, quercetin can be metabolized to a quinone/quinone methide metabolite such as o-semiquinone and o-quinone [39–41]. O-semiquinone has the capability to generate O2•− in cell culture [42] whereas o-quinone depletes glutathione (GSH) in the presence of excess ascorbate [41, 42]. The depletion of GSH and the generation O2•− through these quercetin metabolites suggest a prooxidant effect. Moreover, these quinone/quinone methide metabolites may trigger signaling pathways [37, 43]. In this context, quercetin and its cellular metabolites act more like signaling molecules than prooxidants that cause cell death per se. The formation of quinone/quinone methide metabolites may lead to a low level production of oxidants, which may in turn activate certain signaling cascades.
By acting as a sensor as well as a signaling molecule, H2O2 is an important regulator of signal transduction. An additional mechanism of action of quercetin then is through regulating H2O2 levels. H2O2 regulates endothelial cell function such as proliferation, inflammatory responses, apoptosis, and endothelium-dependent vasorelaxation [44]. The generation of H2O2 to act as a signaling molecule is tightly regulated and depends mainly on location of production and the surrounding antioxidant enzymatic activity. The dismutation of O2•− can occur spontaneously or catalytically by SOD generating H2O2. Interestingly, endogenously produced H2O2 is more effective in eliciting a signaling response compared to exogenously added H2O2 [45]. Moreover, minute intracellular fluxes in H2O2 concentrations may rapidly alter cell signaling responses in the endothelium resulting in endothelium-derived vasorelaxation.
Vascular endothelial cells have the capacity to release H2O2 [46]. Endothelium-derived H2O2 stimulates EDHF. The endothelium produces and releases several vasoactive mediators involved in vessel tone, such as NO, prostacyclins, and EDHF. RWPs have been shown to induce EDHF-dependent vasorelaxation in porcine coronary arteries [22]. Herein, quercetin stimulates vasodilation in intact vessels which is significantly attenuated by the pretreatment of ChTx. Quercetin does not stimulate relaxation significantly in denuded vessels (Fig 5). These results demonstrate that quercetin stimulates endothelium-dependent vasorelaxation. The inhibition of BKCa, IKCa, and some voltage-dependent K+-channels with ChTx blunts the response suggesting EDHF playing a role in quercetin-induced vasorelaxation.
Studies demonstrated that grape seed extracts (which are high in flavonoids and used to make red wine) and RWPs, stimulate endothelium-dependent relaxation through NO/NOS-dependent pathways by increasing cGMP levels in rat aortic vessels [19–21, 47]. The precise mechanism still remains to be elucidated. Additional studies demonstrated that RWP-induced vasorelaxation was actually impaired by the preincubation of porcine vessels with PEG-SOD or SOD mimetic (MnTMPyP). Surprisingly, PEG-catalase (but not native catalase) also impaired RWP-induced vasorelaxation suggesting that RWP potentially generate H2O2 or peroxide-like molecules [22, 48]. Additionally, RWPs stimulate vasorelaxation in small mesenteric arteries of rats is endothelium- and NOS-dependent. These effects were inhibited by SOD plus catalase or using the NADPH oxidase inhibitor DPI suggesting that RWP generate oxidants. In the presence of Ca2+, RWPs induced superoxide production [49]. The subsequent formation of H2O2 or the peroxide-like molecule may indeed mediate a signaling response resulting in RWP-induced vasorelaxation.
Exogenous H2O2 mediates vasorelaxation in aortas and is blunted by catalase but not SOD [50]. H2O2 treatment increases eNOS activity and NO bioavailability in primary cultured endothelial cells through the phosphorylation of eNOS at Ser1179 and the concomitant dephosphorylation at Thr495. The phosphorylation of eNOS at Ser1179, and not Thr495, is mediated by Ca2+ and the PI3K/Akt pathway [36]. Additionally, H2O2 dose-dependently increases Ca2+ in endothelial cells [51]. The removal of Ca2+ from buffers and/or aorta rings pretreated with BAPTA/AM significantly reduced H2O2-mediated vasorelaxation [35].
Other studies have shown that cellular responses to RWPs, such as resveratrol, are mediated through redox-sensitive pathways such as p38 MAPK, ERK1/2, PI3K [48, 52, 53]. Resveratrol can increase eNOS mRNA, protein, and promoter activity resulting in an increase eNOS activity and NO [54, 55]. Regardless of these mechanism(s), the physiological relevance cannot be overlooked. Herein, quercetin stimulated vasorelaxation in rat aortic vessels through the phosphorylation of eNOS at ser1179. Quercetin treatment did not alter Thr497 and Ser 116 levels (not shown) suggesting that the quercetin-mediated NO production may be due to the stimulation of eNOS phosphorylation at Ser1179. Several studies have shown that PI3K inhibitors block agonist-stimulated eNOS phosphorylation. At higher concentrations, quercetin has been shown to partially block the PI3K pathway [56]. Moreover, pretreatment of the PI3K pathway inhibitor wortmannin (100 nM) or LY294002 (10 μM) for 30 min failed to attenuate quercetin-stimulated eNOS phosphorylation at Ser1179 suggesting that this process is not mediated by the PI3K pathway. Moreover, quercetin did not significantly increase eNOS mRNA or protein (not shown).
Flavonoid and/or RWP studies report vasorelaxation through a NO synthase (NOS)- and PI3K-dependent pathway [19, 21, 22, 47], although the precise mechanism(s) still remains unclear. Black tea polyphenols (BTP) induced eNOS phosphorylation at Ser1177 and dephosphorylation of Thr495, increased cGMP levels, and enhanced conversion of L-[3H] arginine to L-[3H] citrulline in porcine aortic endothelial cells (PAECs) within 5 min [57]. The BTP-induced cGMP and conversion of arginine to citrulline was blocked by L-nitro-arginine methyl ester (L-NAME), the PI3K inhibitor LY294002, and a dominant negative p38 MAP kinase (MAPK) α suggesting that this effect is NOS, PI3K, and p38 MAPK dependent. Additionally, RWP-induced vasorelaxation was ablated by PI3K inhibitors (wortmannin and LY294002) and not affected by ERK1/2 (PD98059) nor p38 MAPK (SB203580) inhibitors [48]. These studies are highly suggestive that polyphenols mainly activate the redox-sensitive PI3K pathway inducing endothelium-dependent NO-mediate relaxation through polyphenol-stimulated eNOS phosphorylation.
Quercetin stimulates eNOS phosphorylation at ser1179 and perhaps sensitizes eNOS to lower concentrations of Ca2+. Moreover, quercetin increases the intracellular concentration of Ca2+, whereas Ca2+ chelation with BAPTA/AM inhibits quercetin-stimulated phosphorylation of Ser1179 in BAEC (Fig 3). In cultured endothelial cells, quercetin-mediated increases in Ca2+ may be responsible for the stimulation of eNOS phosphorylation at ser1179 eventually resulting in NO production. Not to diminish the fact that polyphenols are also widely accepted antioxidants and can scavenge ROS and reactive nitrogen species (RNS) such as O2•− and peroxynitrite (ONOO−) [58]. BTPs and RWPs can inhibit lipid peroxidation and increase the antioxidant capacity in human plasma in vitro [59, 60]. Additionally, lipid radicals serve as a sink for NO [61, 62] suggesting that polyphenolic antioxidant properties lead to an increase in NO bioavailability by reducing ROS and RNS, which are known sinks for NO consumption. This may help explain the inverse relationship between flavonoid intake and cardiovascular risk.
In summary, endothelial dysfunction is often associated with the lower NO bioavailability. We have shown that quercetin improves endothelial function by stimulating endothelium-dependent vasorelaxation ex vivo, eNOS phosphorylation in vitro thereby resulting in an increase in NO bioavailability. This suggests that NO bioavailability may play an important role in the quercetin-dependent attenuation of vascular dysfunction through endothelial dependent NO producing enzymes and EDHF. These findings serve to further elucidate potential mechanisms regarding the epidemiological evidence from several cohort studies demonstrating an inverse correlation with dietary intake of flavonoids and CVD.
Acknowledgments
This study has been supported in part by the National Institutes of Health AA12649 and AA/DK11589 and HL70071 (to D.A.P), American Heart Association #0525330B (to N.K.H.K) and The Alcohol Beverage Foundation and The Wine Institute.
Abbreviations
- Ach
acetylcholine
- AdCat
AdCMVCatalase
- AdLacZ
AdCMVLacZ
- Adempty
empty cassette
- ARE
antioxidant responsive element
- BAECs
bovine aortic endothelial cells
- BTP
black tea polyphenol
- ChTx
charybdotoxin
- CVD
cardiovascular diseases
- DCF
dichlorofluorescein
- DETA
diethylenetriamine
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- GSH
glutathione
- H2O2
hydrogen peroxide
- HBSS
Hank’s buffered salt solution
- KCl
potassium chloride
- L-NAME
L-nitro-arginine methyl ester
- LDL
low-density lipoprotein
- MOI
multiplicity of infection
- NO
nitric oxide
- NO2−
nitrite
- NOA
nitric oxide analyzer
- O2•−
superoxide
- p38
p38 mitogen-activated protein kinase
- PAGE
polyacrylamide gel electrophoresis
- PAECs
porcine aortic endothelial cells
- PBS
phosphate buffer saline
- PE
phenylephrine
- PI3K
phosphoinositide 3-kinases
- RNS
reactive nitrogen species
- ROIs
regions-of-interest
- ROS
reactive oxygen species
- RWP
red wine polyphenol
- SDS
sodium dodecyl sulfate
- TTBS
tween 20 tris-buffered saline
References
- 1.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 2.Vita JA. Tea consumption and cardiovascular disease: effects on endothelial function. J Nutr. 2003;133:3293S–3297S. doi: 10.1093/jn/133.10.3293S. [DOI] [PubMed] [Google Scholar]
- 3.Duffy SJ, Keaney JF, Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, Vita JA. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 4.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 5.Geleijnse JM, Launer LJ, van der Kuip DAM, Hofman A, Witteman JCM. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- 6.de Whalley CV, Rankin SM, Hoult JR, Jessup W, Leake DS. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem Pharmacol. 1990;39:1743–1750. doi: 10.1016/0006-2952(90)90120-a. [DOI] [PubMed] [Google Scholar]
- 7.Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, Violi F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- 8.Nijveldt RJ, van Nood E, van Hoorn DEC, Boelens PG, van Norren K, van Leeuwen PAM. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen SE, Frederiksen H, Struntze KK, Poulsen L. Dietary proanthocyanidins. Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol Nutr Food Res. 2005 doi: 10.1002/mnfr.200400082. [DOI] [PubMed] [Google Scholar]
- 10.Oak MH, El Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Mann GE, Rowlands DJ, Li FYL, de Winter P, Siow RCM. Activation of endothelial nitric oxide synthase by dietary isoflavones: Role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc Res. 2007;75:261–274. doi: 10.1016/j.cardiores.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Agag LH, Khoo NK, Binsack R, White CR, rley-Usmar V, Grenett HE, Booyse FM, Digerness SB, Zhou F, Parks DA. Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free Radic Biol Med. 2005;39:540–548. doi: 10.1016/j.freeradbiomed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Ralay RH, Diebolt M, Schott C, Andriantsitohaina R. Polyphenolic compounds from Cognac induce vasorelaxation in vitro and decrease post-ischaemic cardiac infarction after an oral administration. Fundam Clin Pharmacol. 2004;18:331–338. doi: 10.1111/j.1472-8206.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 14.Vinson JA, Teufel K, Wu N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis. 2001;156:67–72. doi: 10.1016/s0021-9150(00)00625-0. [DOI] [PubMed] [Google Scholar]
- 15.Cordova AC, Jackson LS, Berke-Schlessel DW, Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–439. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Dell’Agli M, Busciala A, Bosisio E. Vascular effects of wine polyphenols. Cardiovasc Res. 2004;63:593–602. doi: 10.1016/j.cardiores.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Stocker R, O’Halloran RA. Dealcoholized red wine decreases atherosclerosis in apolipoprotein E gene-deficient mice independently of inhibition of lipid peroxidation in the artery wall. Am J Clin Nutr. 2004;79:123–130. doi: 10.1093/ajcn/79.1.123. [DOI] [PubMed] [Google Scholar]
- 18.Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, Elis A, Aviram M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick DF, Hirschfield SL, Ricci T, Jantzen P, Coffey RG. Endothelium-dependent vasorelaxation caused by various plant extracts. J Cardiovasc Pharmacol. 1995;26:90–95. doi: 10.1097/00005344-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Benito S, Lopez D, Saiz MP, Buxaderas S, Sanchez J, Puig-Parellada P, Mitjavila MT. A flavonoid-rich diet increases nitric oxide production in rat aorta. Br J Pharmacol. 2002;135:910–916. doi: 10.1038/sj.bjp.0704534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriambeloson E, Kleschyov AL, Muller B, Beretz A, Stoclet JC, Andriantsitohaina R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br J Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndiaye M, Chataigneau T, Chataigneau M, Schini-Kerth VB. Red wine polyphenols induce EDHF-mediated relaxations in porcine coronary arteries through the redox-sensitive activation of the PI3-kinase/Akt pathway. Br J Pharmacol. 2004;142:1131–1136. doi: 10.1038/sj.bjp.0705774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, Rimbach G, Mueller MJ. Daily Quercetin Supplementation Dose-Dependently Increases Plasma Quercetin Concentrations in Healthy Humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 24.Gliszczynska-Swiglo A, van der WH, de Haan L, Tyrakowska B, Aarts JM, Rietjens IM. The role of quinone reductase (NQO1) and quinone chemistry in quercetin cytotoxicity. Toxicol In Vitro. 2003;17:423–431. doi: 10.1016/s0887-2333(03)00047-x. [DOI] [PubMed] [Google Scholar]
- 25.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Patel R, Eiserich JP, Zhou F, Kelpke S, Ma W, Parks DA, Darley-Usmar V, White CR. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol Heart Circ Physiol. 2001;281:H1469–H1475. doi: 10.1152/ajpheart.2001.281.4.H1469. [DOI] [PubMed] [Google Scholar]
- 27.Kershaw EE, Schupp M, Guan H-P, Gardner NP, Lazar MA, Flier JS. PPAR{gamma} regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293:E1736–1745. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam EWN, Zwacka R, Seftor EA, Nieva DRC, Davidson BL, Engelhardt JF, Hendrix MJC, Oberley LW. Effects of antioxidant enzyme overexpression on the invasive phenotype of hamster cheek pouch carcinoma cells. Free Radic Biol Med. 1999;27:572–579. doi: 10.1016/s0891-5849(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 29.Preuss M, Girnun GD, Darby CJ, Khoo N, Spector AA, Robbins ME. Role of antioxidant enzyme expression in the selective cytotoxic response of glioma cells to gamma-linolenic acid supplementation. Free Radic Biol Med. 2000;28:1143–1156. doi: 10.1016/s0891-5849(00)00210-0. [DOI] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 31.Martin S, Andriambeloson E, Takeda K, Andriantsitohaina R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br J Pharmacol. 2002;135:1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busse R, Mulsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990;265:133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- 33.Muller JM, Davis MJ, Kuo L, Chilian WM. Changes in coronary endothelial cell Ca2+ concentration during shear stress- and agonist-induced vasodilation. Am J Physiol Heart Circ Physiol. 1999;276:H1706–1714. doi: 10.1152/ajpheart.1999.276.5.H1706. [DOI] [PubMed] [Google Scholar]
- 34.Long LH, Clement MV, Halliwell B. Artifacts in Cell Culture: Rapid Generation of Hydrogen Peroxide on Addition of (−)-Epigallocatechin, (−)-Epigallocatechin Gallate, (+)-Catechin, and Quercetin to Commonly Used Cell Culture Media. Biochem Biophys Res Commun. 2000;273:50–53. doi: 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Zhang A, Altura BT, Altura BM. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta involvement of Ca2+ and other cellular metabolites. Gen Pharmacol. 1999;33:325–336. doi: 10.1016/s0306-3623(99)00019-1. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 37.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Choi EJ, Chee KM, Lee BH. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol. 2003;482:281–285. doi: 10.1016/j.ejphar.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 39.Awad HM, Boersma MG, Vervoort J, Rietjens IM. Peroxidase-catalyzed formation of quercetin quinone methide-glutathione adducts. Arch Biochem Biophys. 2000;378:224–233. doi: 10.1006/abbi.2000.1832. [DOI] [PubMed] [Google Scholar]
- 40.Awad HM, Boersma MG, Boeren S, van Bladeren PJ, Vervoort J, Rietjens IM. Structure-activity study on the quinone/quinone methide chemistry of flavonoids. Chem Res Toxicol. 2001;14:398–408. doi: 10.1021/tx000216e. [DOI] [PubMed] [Google Scholar]
- 41.Boots AW, Kubben N, Haenen GR, Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem Biophys Res Commun. 2003;308:560–565. doi: 10.1016/s0006-291x(03)01438-4. [DOI] [PubMed] [Google Scholar]
- 42.Metodiewa D, Jaiswal AK, Cenas N, Dickancaite E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med. 1999;26:107–116. doi: 10.1016/s0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 43.Canada AT, Giannella E, Nguyen TD, Mason RP. The production of reactive oxygen species by dietary flavonols. Free Radic Biol Med. 1990;9:441–449. doi: 10.1016/0891-5849(90)90022-b. [DOI] [PubMed] [Google Scholar]
- 44.Cai H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Choi MH, Lee IK, Kim GW, Kim BU, Han Y-H, Yu D-Y, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 46.Panus PC, Radi R, Chumley PH, Lillard RH, Freeman BA. Detection of H2O2 release from vascular endothelial cells. Free Radic Biol Med. 1993;14:217–223. doi: 10.1016/0891-5849(93)90013-k. [DOI] [PubMed] [Google Scholar]
- 47.Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 48.Ndiaye M, Chataigneau T, Lobysheva I, Chataigneau M, Schini-Kerth VB. Red wine polyphenols-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2004 doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- 49.Duarte J, Andriambeloson E, Diebolt M, Andriantsitohaina R. Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol Res. 2004;53:595–602. [PubMed] [Google Scholar]
- 50.Fraile ML, Conde MV, Sanz L, Moreno MJ, Marco EJ, Lopez de Pablo AL. Different influence of superoxide anions and hydrogen peroxide on endothelial function of isolated cat cerebral and pulmonary arteries. Gen Pharmacol. 1994;25:1197–1205. doi: 10.1016/0306-3623(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 51.Doan TN, Gentry DL, Taylor AA, Elliott SJ. Hydrogen peroxide activates agonist-sensitive Ca(2+)-flux pathways in canine venous endothelial cells. Biochem J. 1994;297(Pt 1):209–215. doi: 10.1042/bj2970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280:7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Förstermann U. Resveratrol: A Multifunctional Compound Improving Endothelial Function. Cardiovascular Drugs and Therapy. 2009 doi: 10.1007/s10557-009-6209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 55.Wallerath T, Poleo D, Li H, Forstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: a mechanism that may contribute to its beneficial cardiovascular effects. J Am Coll Cardiol. 2003;41:471–478. doi: 10.1016/s0735-1097(02)02826-7. [DOI] [PubMed] [Google Scholar]
- 56.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 57.Anter E, Thomas SR, Schulz E, Shapira OM, Vita JA, Keaney JF., Jr Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. J Biol Chem. 2004;279:46637–46643. doi: 10.1074/jbc.M405547200. [DOI] [PubMed] [Google Scholar]
- 58.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 59.Cherubini A, Beal MF, Frei B. Black tea increases the resistance of human plasma to lipid peroxidation in vitro, but not ex vivo. Free Radic Biol Med. 1999;27:381–387. doi: 10.1016/s0891-5849(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 60.Carbonneau MA, Leger CL, Monnier L, Bonnet C, Michel F, Fouret G, Dedieu F, Descomps B. Supplementation with wine phenolic compounds increases the antioxidant capacity of plasma and vitamin E of low-density lipoprotein without changing the lipoprotein Cu(2+)-oxidizability: possible explanation by phenolic location. Eur J Clin Nutr. 1997;51:682–690. doi: 10.1038/sj.ejcn.1600464. [DOI] [PubMed] [Google Scholar]
- 61.O’Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- 62.Kelley EE, Wagner BA, Buettner GR, Burns CP. Nitric oxide inhibits iron-induced lipid peroxidation in HL-60 cells. Arch Biochem Biophys. 1999;370:97–104. doi: 10.1006/abbi.1999.1386. [DOI] [PubMed] [Google Scholar]