This study shows substantial variation in adherence to guideline recommendations, with both overuse and underuse of surveillance visits and tests.
Abstract
Purpose:
To describe the patterns of follow-up care provided to a population-based cohort of breast cancer survivors, and to assess factors associated with adherence to guidelines on follow-up care.
Patients and Methods:
We conducted a retrospective longitudinal study of all women with surgically treated breast cancer who were without evidence of recurrence, advanced breast cancer, or new primary cancer and were diagnosed in Ontario, Canada, within a 2-year period (n = 11,219). They were followed for 5 years. The cohort was identified through the Ontario Cancer Registry, and individuals were linked across population-based administrative health databases. Frequency of and adherence to guideline recommendations for oncologist and primary care physician (PCP) visits; surveillance imaging for metastatic disease; and surveillance mammograms by year from diagnosis, age group, and income quintile were analyzed. Factors associated with adherence to guideline recommendations were analyzed.
Results:
Most women saw both oncologists and PCPs in each follow-up year. Approximately two thirds had surveillance mammograms in each follow-up year. Overall, two thirds had either fewer or greater than recommended oncology visits, one quarter had fewer than recommended surveillance mammograms, and half had greater than recommended surveillance imaging for metastatic disease.
Conclusion:
This population-based study shows substantial variation in adherence to guideline recommendations, with both overuse and underuse of surveillance visits and tests. Most importantly, a substantial proportion are receiving more than recommended imaging for metastatic disease but fewer than recommended mammograms for detection of local recurrence or new primary cancer, for which effective intervention is possible.
Introduction
Over the past 15 years, there has been growing interest in the practice of routine follow-up of breast cancer survivors. Studies have evaluated aspects of follow-up such as intensive surveillance strategies versus minimalist strategies,1,2 primary care versus specialist-based strategies,3,4 and mammography surveillance.5,6 These studies have led to some degree of consistency in guideline recommendations on follow-up.7,8 However, little is known about how follow-up care is provided to breast cancer survivors at a population level, or the extent to which this is consistent with guideline recommendations. The use of cancer registries linked with administrative health databases has been proposed as a valuable tool to study this question.9–11 In the United States, the Surveillance Epidemiology and End Results and Medicare databases have been used to this end but are limited by being applicable only to those over 65 years old.12 In Canada, the administrative health databases of the single-payer universal health care system enable the study of this question at the population level.
The objective of this study is to describe the patterns of follow-up care provided to breast cancer survivors and to assess factors associated with adherence to guidelines on follow-up care.
Patients and Methods
Study Cohort
We conducted a retrospective longitudinal study of a population-based sample of all women who were diagnosed with breast cancer in Ontario, Canada, within a 2-year period and followed for 5 years.
The study cohort was identified through the Ontario Cancer Registry and consists of all women who were diagnosed with breast cancer in calendar years 1998 and 1999 and were in the Registered Persons Database (which contains demographic information) and eligible for the Ontario Health Insurance Plan (OHIP), the health insurance plan available to all residents of Ontario. Women were excluded if they had a previous primary cancer, did not undergo primary breast cancer surgery, had evidence of advanced cancer or of a possible recurrence within the 1st year after diagnosis. This process identified an initial study cohort of 11,219 women who were 1 year past the date of diagnosis. Women were censored in each of 4 subsequent follow-up years if they were no longer eligible for OHIP (n = 73), died (n = 762), developed a new primary cancer (n = 193), or experienced breast cancer recurrence (based on OHIP fee codes for chemotherapy, radiation therapy, or breast surgery; n = 2,092). Patients were censored 90 days earlier than the date of new primary cancer or breast cancer recurrence to exclude the imaging or visits associated with these events. For patients censored as a result of death or OHIP ineligibility, the actual date was used. Exclusion and censoring criteria were set conservatively to ensure a cohort that was on well “routine” follow-up. Each individual in the cohort was followed for a total of 5 years from the date of diagnosis. The follow-up period was defined as 1 year from the date of diagnosis to 5 years from the date of diagnosis, or sooner if censored.
Data Sources and Measures
For the entire study cohort, scrambled anonymized health care numbers were used to link individual patients across the databases used in this study: the OHIP database for physician visits and procedures; the Canadian Institute for Health Information Hospital Discharge Abstract Database for hospitalizations; Ontario Cancer Registry for new primary cancers, and vital statistics for deaths.
Physician visits were defined as visits to a primary care physician (PCP; either general practitioners or family physicians) or oncologist (surgical, medical, or radiation) according to OHIP records for out-patient visits (including office, home, or nursing home visits). Physician type was determined by linking the OHIP physician number to the Institute of Clinical Evaluative Sciences (ICES) physician database and the ICES surgical oncologist database. For all physician visits, fee codes for inpatient visits, laboratory tests, radiological examinations, and surgical procedures were excluded. Multiple fee codes billed by the same physician on the same day were counted as one visit.
Imaging investigations were measured by OHIP records for imaging procedures commencing 1 year from the date of diagnosis and ending at study end or date of censoring. In addition to mammograms, other imaging investigations included in the analysis were limited to those traditionally used for surveillance for metastatic breast cancer: bone scans; chest imaging with chest x-rays or chest computerized tomographic scans (CT); abdomen/pelvic imaging with ultrasounds, CT scan, or magnetic resonance imaging (MRI).
Socioeconomic status was based on neighborhood income quintile (where Q1 is the poorest and Q5 is the wealthiest) as calculated by the postal code conversion file from Statistics Canada.13 Comorbidity was classified according to the Charlson comorbidity index.14,15 Continuity of care was measured by the Usual Provider Continuity (UPC) index. The index was calculated separately for PCPs and for oncologists. For PCPs, the index was calculated by dividing the number of visits to the usual PCP in the previous 2 years by the total number of visits to a PCP. For oncologists, the index was calculated for visits to oncologists over the follow-up period. Emergency department and inpatient visits were excluded from the calculation. The UPC is calculated only for individuals with at least three visits. The usual provider is defined as the PCP or oncologist who provided the greatest proportion of care. A score of 1 represents perfect continuity; low continuity is defined as a score of ≤ 0.75.16 Ethics approval was obtained from the ICES Research Ethics Board in Toronto, Ontario, Canada.
Analysis
Descriptive statistics were calculated for the frequency of oncology, PCP, and other physician visits by follow-up year, age group, and income quintile. Descriptive statistics were calculated for the frequency of imaging investigations by follow-up year, age group, and income quintile. For each type of imaging investigation (including mammograms), the investigation was classified as surveillance (> 330 days from the last test) or diagnostic (≤ 330 days from the last test). For surveillance mammograms, patients with bilateral mastectomies were excluded.
Following the approach of Cooper et al 2007,10 Lafata et al,17 and Keating et al,12 adherence to guideline recommendations for oncologist visits, surveillance mammograms, and surveillance imaging investigations were classified as consistent with recommendations, less than recommended, or greater than recommended. Adherence per year was defined as three or four visits per year in years 2 and 3, two visits per year in years 4 and 5, one mammogram per year, and no imaging for metastatic disease. Adherence by age group and income quintile over the 4 years of follow-up was defined as six to eight visits over years 2 and 3, three to four visits over years 4 and 5, three to five mammograms over 4 years, and no imaging for metastatic disease.
We used generalized estimation equation (GEE) methods to analyze follow-up data. GEE methods are commonly used for the analysis of different types of correlated data. The main advantage of GEE resides in the unbiased estimation of population-averaged regression coefficients despite possible misspecification of the correlation structure.18 Binary outcomes were analyzed using logistic regression, and rates (mean number of visits) were analyzed using Poisson regression. χ2 tests were used to compare several frequencies across age groups.
All test statistics were two-sided, and P values less than 0.05 were considered statistically significant. Analyses were performed with SAS 9.1 (SAS Institute, Cary, NC).
Results
Characteristics of the breast cancer survivor cohort are presented in Table 1. The average age at diagnosis was 60.1 (standard deviation [SD] = 13.7) years. Patients were followed for a mean of 52.9 months (SD = 14.8). In this cohort, 19.0% developed a breast cancer recurrence over the 5 years and the overall 5-year survival was 86.3%.
Table 1.
Characteristics of Patients
| Characteristic | No. | % |
|---|---|---|
| Total No. of patients | 11,219 | 100 |
| Age, years | ||
| Mean | 60.1 | |
| SD | 13.7 | |
| Age group, years | ||
| ≤ 49 | 2,779 | 24.8 |
| 50-64 | 4,017 | 35.8 |
| 65-79 | 3,524 | 31.4 |
| ≥ 80 | 899 | 8.0 |
| Income quintile | ||
| Unknown | 143 | 1.3 |
| 1 (lowest) | 1,883 | 16.8 |
| 2 | 2,307 | 20.6 |
| 3 | 2,225 | 19.5 |
| 4 | 2,151 | 19.2 |
| 5 (highest) | 2,510 | 22.4 |
| Geographic location | ||
| Unknown | 9 | 0.1 |
| Urban | 9,602 | 85.6 |
| Rural | 1,608 | 14.3 |
| Type of initial surgery | ||
| Lumpectomy | 8,781 | 78.3 |
| Unilateral mastectomy | 2,255 | 20.1 |
| Bilateral mastectomy | 63 | 0.6 |
| Other | 120 | 1.1 |
| Charlson comorbidity index | ||
| 0 | 10,705 | 95.4 |
| 1 | 362 | 3.2 |
| 2 | 103 | 0.9 |
| 3+ | 49 | 0.4 |
| PCP continuity of care | ||
| Not calculated* | 1,221 | 10.9 |
| High UPC | 6,300 | 56.1 |
| Low UPC | 3,698 | 33.0 |
| Index | ||
| Mean | 0.80 | |
| SD | 0.20 | |
| Oncologist continuity of care | ||
| Not calculated* | 1,864 | 16.6 |
| High UPC | 2,471 | 22.0 |
| Low UPC | 6,884 | 61.4 |
| Index | ||
| Mean | 0.62 | |
| SD | 0.22 | |
| New primary cancer within 5 years | 303 | 2.7 |
| Recurrence of breast cancer within 5 years | 2132 | 19.0 |
| Alive at 5 years | 9,680 | 86.3 |
Abbreviations: SD, standard deviation; PCP, primary care physician; UPC, Usual Provider Continuity index.
Continuity of care not calculated if fewer than three visits.
Physician Visits
The average number of oncology visits diminished over time (average 4.1 in year 2 to 1.9 in year 5; P < .05; Table 2) but was similar across income quintiles (data not shown) and age groups, except age ≥ 80, where it was fewer (average 2.0; Table A1, online only). However, the average number of PCP visits remained relatively constant across follow-up years. Oldest and lowest income patients had more PCP visits (average of 12.1 and 7.8, respectively; P < .05).
Table 2.
Mean (standard error) No. of Physician Visits by Specialty and Follow-Up Year
| Physician Specialty | Year 2 (n = 11,219) |
Year 3 (n = 10,026) |
Year 4 (n = 9,297) |
Year 5 (n = 8,624) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Primary care* | 6.9 | 0.02 | 6.7 | 0.02 | 6.6 | 0.02 | 6.6 | 0.02 |
| Oncology* | 4.1 | 0.01 | 2.9 | 0.01 | 2.3 | 0.01 | 1.9 | 0.01 |
| Medical* | 1.4 | 0.01 | 1.1 | 0.01 | 0.8 | 0.01 | 0.7 | 0.01 |
| Radiation* | 0.8 | 0.01 | 0.5 | 0.01 | 0.4 | 0.01 | 0.3 | 0.01 |
| Surgical* | 1.9 | 0.01 | 1.3 | 0.01 | 1.1 | 0.01 | 1.0 | 0.01 |
| Other* | 4.8 | 0.02 | 4.5 | 0.02 | 4.2 | 0.02 | 4.3 | 0.02 |
NOTE. No. of visits is calculated as mean per patient per patient year.
Abbreviation: SE, standard error.
P < .05.
Most women saw both oncologists and PCPs in each follow-up year (Table 3), regardless of age (Table A2, online only), or income (data not shown). However, the proportion seeing both oncologists and PCPs diminished significantly over time (from 81.1% in year 2 to 66.6% in year 5; P < .05) (Table 3), older age (from 90.5% age ≤ 49 to 73.6% age ≥ 80; P < .05 (Table A2, online only), and lower income quintile (from 89.9% for highest to 88.3% for lowest; P < .05) (data not shown). Over half in each age group, except age ≥ 80 (31.6%), and each income quintile were seeing multiple oncologists. For the overall sample, the number diminished from 49.2% in year 2 to 23.8% in year 5. Under 12% in each year, age group, and income quintile saw only a PCP except in the fourth (17.3%) and fifth (23.0%) years and oldest age group (21.8%).
Table 3.
Distribution of Physician Visits by Physician Specialty and Follow-Up Year
| Physician Specialty | Year 2 (n = 11,219) |
Year 3 (n = 10,026) |
Year 4 (n = 9,297) |
Year 5 (n = 8,624) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean No. of Visits | % With at Least One Visit | Mean No. of Visits | % With at Least One Visit | Mean No. of Visits | % With at Least One Visit | Mean No. of Visits | % With at Least One Visit | |
| PCP only*† | 10.5 | 8.0 | 10.0 | 12.3 | 9.2 | 17.3 | 9.0 | 23.0 |
| Oncology only† | ||||||||
| Medical | 2.9 | 1.7 | 2.5 | 1.9 | 2.0 | 2.0 | 1.8 | 1.8 |
| Radiation | 2.1 | 0.7 | 2.0 | 0.8 | 1.5 | 0.8 | 1.4 | 0.8 |
| Surgical | 3.4 | 1.5 | 3.4 | 1.4 | 3.7 | 1.7 | 3.3 | 1.6 |
| Multiple | 6.3 | 4.9 | 4.7 | 3.6 | 4.9 | 3.0 | 4.6 | 2.2 |
| PCP and oncology† | ||||||||
| PCP and medical | 9.6 | 11.3 | 8.6 | 16.5 | 8.1 | 18.4 | 8.1 | 17.6 |
| PCP and radiation | 8.8 | 7.5 | 8.5 | 8.2 | 8.4 | 9.2 | 7.9 | 9.3 |
| PCP and surgical | 11.5 | 13.1 | 10.2 | 13.9 | 10.0 | 14.7 | 10.2 | 15.9 |
| PCP and multiple | 12.9 | 49.2 | 11.5 | 38.4 | 10.8 | 29.5 | 10.4 | 23.8 |
| Other physician only | 5.9 | 0.7 | 7.4 | 0.8 | 5.2 | 1.1 | 6.9 | 1.4 |
| No physician | 1.4 | 2.1 | 2.3 | 2.6 | ||||
| Days between oncology visits | ||||||||
| Median† | ||||||||
| Interquartile range | 46 | 83 | 89 | 136 | 113 | 148 | 128 | 157 |
Abbreviation: PCP, primary care physician.
PCP only = Only PCP visits included in the counts, but patients may have also seen other physicians.
P < .05.
Imaging Investigations
Approximately two thirds of women had a surveillance mammogram in each follow-up year (Table 4) and across age groups (Table A3; online only), and income quintiles (data not shown), except in the oldest age group (25.5%) and lowest income quintile (59.4%) (P < .05). A quarter of women had surveillance chest imaging in each follow-up year (Table 4).
Table 4.
No. of Surveillance and Diagnostic Imaging Tests by Follow-Up Year
| Test | Year 2 (n = 11,219) |
Year 3 (n = 10,026) |
Year 4 (n = 9,297) |
Year 5 (n = 8,624) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | % With at Least One Test | Mean | SE | % With at Least One Test | Mean | SE | % With at Least One Test | Mean | SE | % With at Least One Test | |
| Mammography | ||||||||||||
| Surveillance* | 0.69 | 0.01 | 63.4 | 0.72 | 0.01 | 68.0 | 0.73 | 0.01 | 69.0 | 0.73 | 0.01 | 67.4 |
| Diagnostic† | 0.25 | 0.01 | 18.6 | 0.17 | 0.02 | 13.3 | 0.13 | 0.02 | 10.5 | 0.11 | 0.02 | 8.8 |
| Chest imaging | ||||||||||||
| Surveillance* | 0.29 | 0.01 | 27.6 | 0.28 | 0.01 | 26.7 | 0.26 | 0.01 | 24.7 | 0.25 | 0.01 | 24.0 |
| Diagnostic* | 0.31 | 0.02 | 16.5 | 0.26 | 0.02 | 15.3 | 0.23 | 0.02 | 13.1 | 0.23 | 0.02 | 12.9 |
| Bone scan | ||||||||||||
| Surveillance* | 0.16 | 0.01 | 14.6 | 0.12 | 0.02 | 11.6 | 0.10 | 0.02 | 10.0 | 0.09 | 0.02 | 8.7 |
| Diagnostic | 0.04 | 0.04 | 3.3 | 0.03 | 0.04 | 2.5 | 0.02 | 0.04 | 1.7 | 0.02 | 0.05 | 1.5 |
| Abdomen/pelvis imaging | ||||||||||||
| Surveillance* | 0.23 | 0.01 | 22.0 | 0.21 | 0.01 | 20.3 | 0.20 | 0.01 | 19.1 | 0.20 | 0.01 | 19.0 |
| Diagnostic* | 0.18 | 0.02 | 11.1 | 0.18 | 0.02 | 10.8 | 0.16 | 0.02 | 10.3 | 0.16 | 0.02 | 9.4 |
| Ultrasound, intracavitary/transvaginal | ||||||||||||
| Surveillance* | 0.09 | 0.02 | 8.6 | 0.08 | 0.02 | 8.0 | 0.08 | 0.02 | 7.8 | 0.08 | 0.02 | 7.5 |
| Diagnostic† | 0.05 | 0.03 | 3.2 | 0.05 | 0.03 | 3.3 | 0.05 | 0.03 | 3.3 | 0.04 | 0.04 | 3.1 |
P < .0001.
P < .001.
Adherence to Guidelines
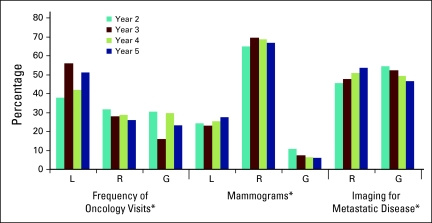
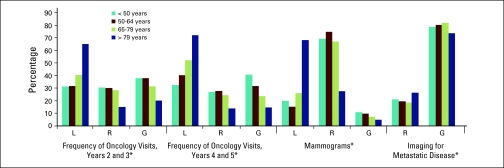
Follow-up care was consistent with guideline recommendations for between one quarter and one third of women for frequency of oncology visits, and two thirds of women for frequency of surveillance mammograms. Overall, approximately half of women had more than recommended surveillance imaging for metastatic disease (Figure 1). The proportion varied significantly by year of follow-up (P < .05) and age (Figure A1, online only). In the oldest age group, 71.9% had fewer than recommended oncology visits and 65.0% had fewer than recommended mammograms (P < .05). The differences were small (< 7%) but significant (P < .05) for each of these factors across income quintiles (data not shown).
Figure 1.
Adherence to guidelines by follow-up year, defined as three or four visits per year in years 2 and 3; two visits per year in years 4 and 5; one mammogram per year. L, less than recommended; R, recommended; G, greater than recommended. (*) P < .05.
The odds ratios for factors associated with adherence to guideline recommendations are shown in Table 5. Age ≤ 49, having a mastectomy, seeing multiple oncologists, and low oncology continuity index all increased the odds of having greater than recommended oncology visits. Older age, rural residence, and higher comorbidity increased the odds of having fewer than recommended mammograms, whereas seeing an oncologist was associated with reduced odds of fewer than recommended mammograms. Higher comorbidity, having a mastectomy, and seeing a PCP plus oncologists all increased the odds of having greater than recommended imaging for metastatic disease. Regression analysis with and without age, or with and without comorbidity, yielded similar estimates for both age and comorbidity effects, indicating that the effects of age and comorbidity on adherence to guideline recommendations are nearly independent of each other.
Table 5.
Adjusted Odds Ratios for Adherence to Guidelines
| Variable | Greater Than Recommended Oncology Visits |
Less Than Recommended Mammograms |
Greater Than Recommended Imaging for Metastatic Disease |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age group, years | ||||||
| ≤ 49 | Reference | Reference | Reference | |||
| 50-64 | 0.81 | 0.72 to 0.88 | 0.86 | 0.79 to 0.94 | 0.96 | 0.90 to 1.03 |
| 65-79 | 0.73 | 0.67 to 0.80 | 1.18 | 1.07 to 1.29 | 0.97 | 0.90 to 1.04 |
| ≥ 80 | 0.88 | 0.73 to 1.05 | 3.49 | 2.96 to 4.10 | 0.82 | 0.73 to 0.93 |
| Income quintile | ||||||
| 1 | Reference | Reference | Reference | |||
| 2 | 1.00 | 0.89 to 1.11 | 0.98 | 0.88 to 1.10 | 0.96 | 0.88 to 1.05 |
| 3 | 0.95 | 0.85 to 1.07 | 0.92 | 0.82 to 1.04 | 1.00 | 0.92 to 1.10 |
| 4 | 0.98 | 0.88 to 1.09 | 0.89 | 0.80 to 1.00 | 1.04 | 0.95 to 1.13 |
| 5 | 0.91 | 0.82 to 1.02 | 0.95 | 0.85 to 1.06 | 0.95 | 0.87 to 1.03 |
| Rural v urban | 0.91 | 0.82 to 1.01 | 1.11 | 1.01 to 1.23 | 0.92 | 0.86 to 1.00 |
| Comorbidity | ||||||
| 0 | Reference | Reference | Reference | |||
| 1 | 1.00 | 0.81 to 1.23 | 1.41 | 1.17 to 1.71 | 1.19 | 1.04 to 1.36 |
| 2 | 1.15 | 0.71 to 1.88 | 1.56 | 1.07 to 2.26 | 1.42 | 1.08 to 1.87 |
| 3+ | 1.02 | 0.60 to 1.72 | 3.05 | 1.68 to 5.55 | 1.52 | 1.10 to 2.11 |
| Surgery type | ||||||
| Lumpectomy | Reference | Reference | Reference | |||
| Mastectomy | 1.29 | 1.18 to 1.41 | 1.39 | 1.27 to 1.52 | 1.20 | 1.12 to 1.28 |
| Physician type | ||||||
| Oncology only | Reference | Reference | Reference | |||
| PCP only | – | 2.02 | 1.73 to 2.35 | 1.23 | 1.07 to 1.42 | |
| PCP + single oncologist | 0.24 | 0.21 to 0.28 | 0.76 | 0.66 to 0.87 | 1.63 | 1.46 to 1.83 |
| PCP + multiple oncologists | 1.89 | 1.67 to 2.13 | 0.52 | 0.45 to 0.60 | 2.31 | 2.06 to 2.59 |
| Low GP/FP UPC v high | 1.06 | 0.99 to 1.14 | 1.07 | 1.00 to 1.16 | 1.04 | 0.99 to 1.10 |
| Low Oncologist UPC v high | 1.28 | 1.16 to 1.41 | 1.06 | 0.97 to 1.15 | 1.03 | 0.97 to 1.10 |
NOTE. Boldface indicates statistically significant values.
Abbreviations: OR, odds ratio; PCP, primary care physician; GP/FP, general practitioner/family practice; UPC, Usual Provider Continuity index.
Discussion
Most women in this Canadian cohort had regular follow-up visits with an oncologist throughout the 4 years of follow-up. This is in contrast with the finding by Keating et al that most women did not see a cancer specialist annually.12 They studied US women over age 65 who were recipients of Medicare and thus comparable to our cohort in terms of access to health care coverage. Nonetheless, in our study approximately 80% of women saw at least one oncologist in each of the 2nd through 4th years of follow-up, decreasing to 73% in the 5th year; this figure was > 90% in each age group except for ≥ 80 (76%). Follow-up exclusively by PCPs was rare except in the fifth year or for patients age ≥ 80, when approximately a quarter of women saw PCPs exclusively. Otherwise, > 80% of women in each year of follow-up and in each age group saw a combination of PCP and at least one type of oncology specialist, with almost half seeing multiple oncology specialists. Differences by income quintile were small (< 2%). During the period of this study one trial evaluating exclusive PCP follow-up had been published.4 The second larger trial3 and guideline statements supporting PCP follow-up7,8 were not published until after the period of this study (1998 to 2004).
Adherence to Guidelines
The study shows substantial variation in adherence to guideline recommendations. Two thirds of women had either fewer or greater than recommended oncology visits. Half of women had greater than recommended surveillance imaging for metastatic disease, whereas a quarter had fewer than recommended surveillance mammograms for detection of local recurrence or new primary cancer, for which effective intervention is possible. Most older women had fewer than recommended visits (71.9%) and fewer than recommended mammograms (65%). Although there were significant differences by income quintile, the proportional differences were small (< 7% between highest and lowest income quintile), and the statistical significance reflects the large sample size.
The American Society of Clinical Oncology (ASCO) published guidelines on breast cancer follow-up in 199719 and updated them in 199820 and 2006.7 The Canadian guidelines were published in 199821 and updated in 2005.8 Although guidelines have been published elsewhere,22 it is the ASCO and Canadian guidelines that would have been most influential in Ontario during the period of this study. These two guidelines give similar recommendations for mammography surveillance (recommended annually) and surveillance for metastatic disease (not recommended). However, the Canadian guidelines do not specify the frequency for follow-up visits while the ASCO guidelines are more prescriptive. Taking a conservative approach, we used the frequency of visits recommended by the ASCO guidelines to define our adherence classification for frequency of follow-up visits. Similarly we have included only oncology visits in this classification, recognizing that, in addition, a proportion of visits to PCPs would have been for breast cancer follow-up.23 Consequently, our classification of greater than recommended oncologist visits is conservative, and the proportion of patients receiving greater than recommended follow-up visits is likely to be much larger, reflecting substantial duplication of care. Certainly, the number of patients seeing multiple different oncology specialists and the associated low continuity of care index highlights the concern about duplication of care.
Mammograms
Mammography surveillance is the only routine investigation recommended.7,8 Although there has never been a formal evaluation,24 several observational studies point to the value of these mammograms to detect locoregional recurrence6, which may result in reduced breast cancer mortality.5,9,25 Our findings are consistent with others showing underuse of surveillance mammograms in each year, age group, and income quintile.26,27 Specifically, studies have consistently found that older age is associated with fewer than recommended mammograms.12,28 In addition, our finding that women with mastectomies were less likely to have mammograms has been reported by others.26 The protective effect of surveillance mammograms on breast cancer mortality was found by Lash et al9 to be strongest in women with mastectomies or > 80 years of age, the very women least likely to have guideline-recommended mammograms in this study.
As with this study, other studies have consistently found that women followed by an oncology specialists are more likely to have surveillance mammograms.29–31 Earle et al noted such a phenomenon in breast and colorectal cancer patients: surveillance procedures for the index cancer occurred less frequently, whereas other health maintenance procedures occurred more frequently, for patients under exclusive PCP care.30,32 Snyder et al attribute this to a lack of clear allocation of responsibility.32 In a randomized clinical trial (RCT) in which allocation was made to either exclusive PCP care or oncology care, mammograms occurred with equal frequency in both groups, supporting this contention.33
Coordination of Care
Both ASCO and Canadian guidelines recommend against routine imaging investigations for metastatic disease based on the results of two RCTs.1,2 The relationship between more than recommended imaging for metastatic disease and both multiple oncology specialists and low continuity of oncology care supports the proposition that better coordination of care, as called for by the guidelines,7,8 may reduce unnecessary testing.12
Strengths and Limitations
We examined follow-up care provided to a population-based cohort of breast cancer survivors in a health care system with universal health insurance, so it is a true reflection of real-world care. The study period extended to 5 years after diagnosis, and thus captures the full period of most intensive follow-up. In addition, there is almost universal capture of imaging investigations and physician visits.
Nevertheless this study has some important limitations. Information about tumor size, nodal status and other prognostic factors such as receptor status and tumor grade are not available in our databases. We were, therefore, not able to use these factors as potential explanatory variables in our models. Similarly, we do not have any measure of laboratory investigations such a blood counts, liver function tests, and tumor markers. These are not recommended according to either ASCO or Canadian guidelines. Consequently our classification of surveillance tests includes only imaging and does not take into consideration these laboratory investigations. We endeavored to distinguish between diagnostic versus surveillance imaging by including in the latter category only those tests performed annually so that only one imaging test per year was counted as surveillance.5 However, this approach has the potential that a proportion of tests are misclassified, likely leading to an underestimation of surveillance testing.12 Other investigators who have compared claims data with medical record review found good agreement between classification of surveillance care from claims data as compared with medical record review,10 and found that the majority of testing is indeed for surveillance rather than diagnostic testing (82% versus 11%).34
Conclusion
This population based study shows substantial variation in practice, with both overuse and underuse of surveillance visits and tests. Most important, a substantial proportion of patients are receiving fewer than recommended surveillance mammograms and more than recommended imaging tests for metastatic disease. Almost half of patients were seeing multiple oncology specialists in addition to their PCP. Despite this frequency of visits and multiplicity of providers, women were not receiving recommended mammograms, which are arguably the most important surveillance investigation. Similar findings have been noted in other studies and considered to be a consequence of unclear allocation of responsibilities for follow-up care among providers. Survivorship care plans, which have been proposed as tools to specify procedures and responsibilities and improve coordination of care,35 can potentially address this problem. This is currently being evaluated through a multicenter RCT.
Acknowledgment
We thank Nadia Gunraj and Refik Saskin for programming. This study was supported by the Institute of Clinical Evaluative Sciences (ICES), with funds from the Ontario Ministry of Health and Long-Term Care (MOHLTC), and by Cancer Care Ontario. The research is independent, and no endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Appendix
Table A1.
Mean No. of Physician Visits by Physician Specialty and Patient Age
| Physician Specialty | Age Group (years) |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 49 (n = 2,779) |
50-64 (n = 4,017) |
65-79 (n = 3,524) |
≥ 80 (n = 899) |
|||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| PCP* | 5.2 | 0.05 | 5.9 | 0.04 | 7.7 | 0.05 | 12.1 | 0.14 |
| Oncology* | 3.3 | 0.04 | 3.0 | 0.03 | 2.6 | 0.03 | 2.0 | 0.07 |
| Medical* | 1.3 | 0.03 | 1.1 | 0.03 | 0.8 | 0.03 | 0.5 | 0.08 |
| Radiation* | 0.5 | 0.03 | 0.6 | 0.03 | 0.5 | 0.03 | 0.3 | 0.09 |
| Surgical* | 1.5 | 0.03 | 1.3 | 0.02 | 1.3 | 0.03 | 1.2 | 0.07 |
| Other* | 3.2 | 0.04 | 3.8 | 0.04 | 5.7 | 0.05 | 6.4 | 0.1 |
NOTE. No. of visits is calculated as mean per patient per patient year.
Abbreviation: PCP, primary care physician.
P < .05.
Table A2.
Distribution of Physician Visits by Physician Specialty and Patient Age
| Physician Specialty | Age Group (years) |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 49 (n = 2,779) |
50-64 (n = 4,017) |
65-79 (n = 3,524) |
≥ 80 (n = 899) |
|||||
| Mean No. of Visits | % With at Least One Visit | Mean No. of Visits | % With at Least One Visit | Mean No. of Visits | % With at Least One Visit | Mean No. of Visits | % With at Least One Visit | |
| PCP only*† | 4.8 | 3.5 | 6.3 | 3.1 | 9.2 | 5.9 | 16.4 | 21.8 |
| Oncology only† | ||||||||
| Medical | 2.2 | 0.9 | 2.3 | 0.8 | 1.7 | 0.6 | 6.8 | 0.2 |
| Radiation | 1.2 | 0.4 | 1.7 | 0.3 | 0.8 | 0.3 | 0.0 | 0.0 |
| Surgical | 1.8 | 0.8 | 3.2 | 0.7 | 1.5 | 1.0 | 5.6 | 1.0 |
| Multiple | 4.8 | 2.7 | 4.0 | 2.6 | 4.2 | 1.8 | 5.6 | 1.1 |
| PCP and oncology† | ||||||||
| Medical | 6.1 | 8.6 | 6.9 | 8.1 | 9.3 | 9.3 | 11.0 | 8.7 |
| Radiation | 5.8 | 2.7 | 6.7 | 5.2 | 8.2 | 6.2 | 11.6 | 4.2 |
| Surgical | 7.8 | 9.3 | 7.7 | 10.1 | 10.2 | 16.7 | 14.4 | 29.1 |
| Multiple | 9.1 | 69.5 | 9.7 | 67.7 | 11.1 | 56.9 | 13.5 | 31.6 |
| Other specialty only | 1.1 | 0.1 | 1.6 | 0.4 | 4.3 | 0.3 | 3.8 | 1.1 |
| No physician | 1.5 | 1.0 | 1.1 | |||||
| Days between oncology visits | ||||||||
| Median† | 72 | 84 | 85 | 53 | ||||
| Interquartile range | 116 | 137 | 152 | 160 | ||||
NOTE. No. of visits is calculated as mean per patient per patient year.
Abbreviation: PCP, primary care physician.
Only PCP visits included in the counts, but patients may have also seen other types of physicians.
P < .05.
Table A3.
Mean (standard error) No. of Surveillance Imaging Tests by Patient Age
| Test | Age Group (years) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 49 (n = 2,779) |
50-64 (n = 4,017) |
65-79 (n = 3,524) |
≥ 80 (n = 899) |
|||||||||
| Mean No. of Tests | SE | % With at Least Three Tests in 4 Years | Mean No. of Tests | SE | % With at Least Three Tests in 4 Years | Mean No. of Tests | SE | % With at Least Three Tests in 4 Years | Mean No. of Tests | SE | % With at Least Three Tests in 4 Years | |
| Mammography* | 0.75 | 0.02 | 65.5 | 0.77 | 0.02 | 71.0 | 0.70 | 0.02 | 61.1 | 0.38 | 0.04 | 25.5 |
| Chest imaging* | 0.24 | 0.02 | 9.4 | 0.26 | 0.01 | 9.6 | 0.30 | 0.01 | 10.4 | 0.30 | 0.03 | 11.2 |
| Bone scan* | 0.14 | 0.02 | 5.4 | 0.12 | 0.02 | 3.8 | 0.11 | 0.02 | 2.4 | 0.07 | 0.05 | 2.3 |
| Abdomen/pelvis imaging* | 0.24 | 0.02 | 8.6 | 0.22 | 0.01 | 7.0 | 0.19 | 0.02 | 5.8 | 0.13 | 0.04 | 4.2 |
| Ultrasound, intracavitary/transvaginal | 0.13 | 0.02 | 3.9 | 0.09 | 0.02 | 2.5 | 0.05 | 0.03 | 1.1 | 0.01 | 0.1 | 0.1 |
P < .0001.
Figure A1.
Adherence to guidelines by patient age, defined as six to eight visits over years 2 and 3, three to four visits over years 4 and 5, three to five mammograms over 4 years. L, less than recommended; R, recommended; G, greater than recommended. (*) P < .05.
Authors' Disclosure of Potential Conflicts of Interest.
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Eva Grunfeld, David C. Hodgson, M. Elisabeth Del Giudice
Data analysis and interpretation: Eva Grunfeld, Rahim Moineddin, David C. Hodgson, M. Elisabeth Del Giudice
Manuscript writing: Eva Grunfeld
Final approval of manuscript: Eva Grunfeld, David C. Hodgson, M. Elisabeth Del Giudice, Rahim Moineddin
References
- 1.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. national Research Council Project on Breast Cancer follow-up. JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 2.Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The Givio Investigators. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- 3.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: A comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–855. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld E, Mant D, Yudkin P, et al. Routine follow-up of breast cancer in primary care: Randomised trial. BMJ. 1996;313:665–669. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paszat L, Sutradhar R, Grunfeld E, et al. Outcomes of surveillance mammography after treatment of primary breast cancer: A population-based case series. Breast Cancer Res Treat. 2009;114:169–178. doi: 10.1007/s10549-008-9986-4. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery DA, Krupa K, Cooke TG. Follow-up in breast cancer: Does routine clinical examination improve outcome? A systematic review of the literature. Br J Cancer. 2007;97:1632–1641. doi: 10.1038/sj.bjc.6604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld E, Dhesy-Thind S, Levine M, et al. Clinical practice guidelines for the care and treatment of breast cancer: Follow-up after treatment for breast cancer (summary of the 2005 update) CMAJ. 2005;172:1319–1320. doi: 10.1503/cmaj.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lash TL, Fox MP, Buist DS, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 10.Cooper GS, Schultz L, Simpkins J, et al. The utility of administrative data for measuring adherence to cancer surveillance care guidelines. Med Care. 2007;45:66–72. doi: 10.1097/01.mlr.0000241107.15133.54. [DOI] [PubMed] [Google Scholar]
- 11.Earle CC, Nattinger AB, Potosky A, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40:75–81. doi: 10.1097/00005650-200208001-00011. [DOI] [PubMed] [Google Scholar]
- 12.Keating NL, Landrum MB, Guadagnoli E, et al. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25:1074–1081. doi: 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins R. Statistics Canada. Canada: Ottawa, Ontario; 2001. PCCF+ Version 3G User's Guide (Geocodes/PCCF). Automated Geographic Coding Based on the Statistics Canada Postal Code Conversion Files, Including Postal Codes to June 2001. [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Ionescu-Ittu R, McCusker J, Ciampi A, et al. Continuity of primary care and emergency department utilization among elderly people. CMAJ. 2007;177:1362–1368. doi: 10.1503/cmaj.061615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 18.Ghisletta P, Spini R. An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals. J Educ Behav Stat. 2004;29:421–437. [Google Scholar]
- 19.Recommended breast cancer surveillance guidelines. American Society of Clinical Oncology. J Clin Oncol. 1997;15:2149–2156. doi: 10.1200/JCO.1997.15.5.2149. [DOI] [PubMed] [Google Scholar]
- 20.Smith TJ, Davidson NE, Schapira DV, et al. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17:1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- 21.Steering Committee on Clinical Practice Guidelines for the Care Treatment of Breast Cancer. Clinical Practice Guidelines for the Care and Treatment of Breast Cancer 9. Follow-up for treatment for breast cancer. CMAJ. 1998;158:S65–S70. [PubMed] [Google Scholar]
- 22.National Health Service. Research evidence for the manual update. London, United Kingdom: Department of Health; 2002. Improving the outcomes in breast cancer. [Google Scholar]
- 23.Worster A, Wood ML, McWhinney IR, et al. Who provides follow-up care for patients with breast cancer? Can Fam Phys. 1995;41:1314–1319. [PMC free article] [PubMed] [Google Scholar]
- 24.Grunfeld E, Noorani H, McGahan L, et al. Surveillance mammography after treatment of primary breast cancer: A systematic review. Breast. 2002;11:228–235. doi: 10.1054/brst.2001.0404. [DOI] [PubMed] [Google Scholar]
- 25.Lash TL, Fox MP, Silliman RA. Reduced mortality rate associated with annual mammograms after breast cancer therapy. Breast J. 2006;12:2–6. doi: 10.1111/j.1075-122X.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 26.Mandelblatt JS, Lawrence WF, Cullen J, et al. Patterns of care in early-stage breast cancer survivors in the first year after cessation of active treatment. J Clin Oncol. 2006;24:77–84. doi: 10.1200/JCO.2005.02.2681. [DOI] [PubMed] [Google Scholar]
- 27.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38:281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Geller BM, Kerlikowske K, Carney PA, et al. Mammography surveillance following breast cancer. Breast Cancer Res Treat. 2003;81:107–115. doi: 10.1023/A:1025794629878. [DOI] [PubMed] [Google Scholar]
- 29.Field TS, Doubeni C, Fox MP, et al. Under utilization of surveillance mammography among older breast cancer survivors. J Gen Intern Med. 2008;23:158–163. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earle CC, Burstein HJ, Winer EP, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 31.Keating NL, Landrum MB, Guadagnoli E, et al. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 32.Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: A 5-year longitudinal study. J Clin Oncol. 2008;26:1073–1079. doi: 10.1200/JCO.2007.11.9859. [DOI] [PubMed] [Google Scholar]
- 33.Grunfeld E, Julian JA, Levine MN, et al. A randomized controlled trial (RCT) of long-term follow-up for early stage breast cancer comparing family physician to specialist care: A report of secondary outcomes. Proc Am Soc Clin Oncol. 2006;24:301s. doi: 10.1200/JCO.2005.03.2235. abstr 6003. [DOI] [PubMed] [Google Scholar]
- 34.Cooper GS, Johnson CC, Lamerato L, et al. Use of guideline recommended follow-up care in cancer survivors: Routine or diagnostic indications? Med Care. 2006 Jun;44:590–594. doi: 10.1097/01.mlr.0000215902.50543.77. [DOI] [PubMed] [Google Scholar]
- 35.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26:759–767. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]