Abstract
Galectin-1 (Gal-1) is important in immune function and muscle regeneration, but its expression and localization in adult tissues and primary leukocytes remain unclear. To address this, we generated a specific monoclonal antibody against Gal-1, termed αhGal-1, and defined a sequential peptide epitope that it recognizes, which is preserved in human and porcine Gal-1, but not in murine Gal-1. Using αhGal-1, we found that Gal-1 is expressed in a wide range of porcine tissues, including striated muscle, liver, lung, brain, kidney, spleen, and intestine. In most types of cells, Gal-1 exhibits diffuse cytosolic expression, but in cells within the splenic red pulp, Gal-1 showed both cytosolic and nuclear localization. Gal-1 was also expressed in arterial walls and exhibited prominent cytosolic and nuclear staining in cultured human endothelial cells. However, human peripheral leukocytes and promyelocytic HL60 cells lack detectable Gal-1 and also showed very low levels of Gal-1 mRNA. In striking contrast, Gal-1 exhibited an organized cytosolic staining pattern within striated muscle tissue of cardiac and skeletal muscle and colocalized with sarcomeric actin on I bands. These results provide insights into previously defined roles for Gal-1 in inflammation, immune regulation and muscle biology.
Keywords: galectin-1 expression, leukocytes, monoclonal antibody, muscle, tissue localization
Introduction
Immunological homeostasis relies upon proper regulation of many aspects of leukocyte behavior, including trafficking, cytokine secretion, and cellular differentiation. In addition, efficient inflammatory resolution relies upon significant contraction of leukocyte populations (Antia et al. 2005). Failure to appropriately eliminate leukocytes often exacerbates leukocyte-mediated damage of viable tissue and can eventually result in autoimmunity (Danial and Korsmeyer 2004).
Several factors, including members of the galectin and tumor necrosis factor (TNF) families, appear to play key roles in the regulation of leukocyte turnover (Liu 2000; Simon 2003a,b). TNF family members regulate leukocyte turnover through the induction of apoptotic cell death (Simon 2003a,b), whereas galectins appear to regulate leukocyte turnover through both apoptotic and non-apoptotic mechanisms, depending on the cell type and activation state (Stowell et al. 2007; Toscano et al. 2007; Stowell, Arthur, Mehta, et al. 2008; Stowell, Arthur, Slanina, et al. 2008; Stowell, Qian, et al. 2008). For galectin-1 (Gal-1), these immunoregulatory effects are related to induction of regulatory cytokines (IL-27 and IL-10), control of T cell recruitment and regulation of the functions of tolerogenic dendritic cells and CD4 CD25 Foxp3 Treg cells (van der Leij et al. 2004; Garin et al. 2007; Norling et al. 2008; Stowell, Arthur, Mehta, et al. 2008; Stowell, Arthur, Slanina, et al. 2008; Stowell, Qian, et al. 2008; Ilarregui et al. 2009).
In addition to the effects of galectins on leukocyte function (Rabinovich et al. 2002), many studies suggest a role for Gal-1, a prototypical galectin and the most well studied galectin to date, in the development and regeneration of muscle (Camby et al. 2006). Gal-1-null mice experience reduced myofiber formation and reduced myofiber regeneration following injury (Watt et al. 2004; Georgiadis et al. 2007), also strongly suggesting that, in addition to regulating immunity, Gal-1 also regulates fundamental processes of muscle development and function. Gal-1 induces myoblast differentiation in vitro and exhibits unique expression patterns during muscle development in vivo (Watt et al. 2004; Georgiadis et al. 2007). In addition, it was recently demonstrated that, while Gal-1 plays a minimal role during embryonic myogenesis (Shoji et al. 2009), it plays a more important role in adult myogenesis (Chan et al. 2006; Shao et al. 2009) as well as contributes to muscle development in the zebrafish Danio rerio (Ahmed et al. 2009).
Although Gal-1 appears to regulate fundamental aspects of immune function and muscle development and regeneration, the expression and localization of Gal-1 in adult tissue and primary leukocytes remains enigmatic. In this study, we examined the expression and localization of Gal-1 using an epitope-defined monoclonal antibody. These results provide insights into previously defined roles for Gal-1.
Results
Monoclonal antibody, αhGal-1, displays specificity for hGal-1 in multiple formats
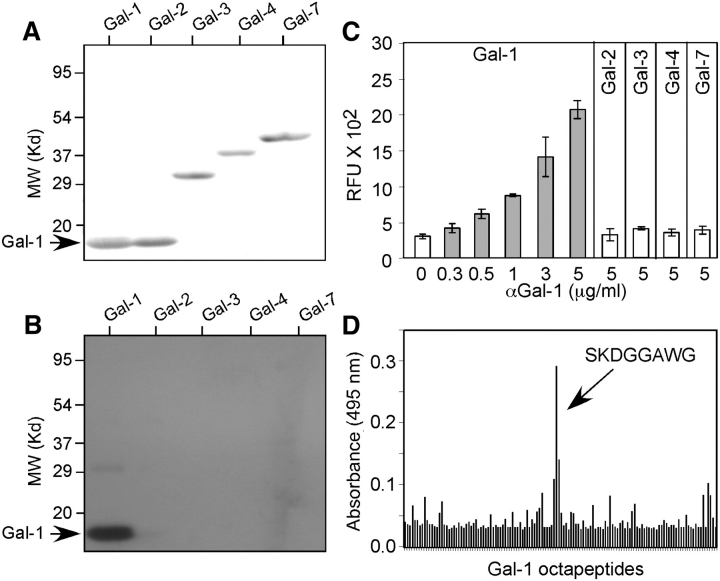
To date, 15 members of the galectin family have been identified in vertebrates, 11 of these are expressed in humans (Gal-1, Gal-2, Gal-3, Gal-4, Gal-7, Gal-8, Gal-9, Gal-10, Gal-12, Gal-13 and Gal-14), and all share amino acid sequences (Cooper 2002). Thus, to define the location and expression of Gal-1, we developed a highly specific anti-Gal-1 monoclonal antibody. To accomplish this, we generated hybridomas from mice immunized with recombinant human Gal-1 and screened these clones against a panel of recombinant human galectins. Although several hybridomas produced antibodies which showed significant binding toward Gal-1, one clone, hereafter called αhGal-1, exhibited robust binding to Gal-1 and did not cross-react with other recombinant human galectins, including Gal-2, Gal-3, Gal-4 and Gal-7, under either native or denaturing conditions (Figure 1A–C).
Fig. 1.
αhGal-1 specifically recognizes human Gal-1. (A) SDS-PAGE followed by transfer to nitrocellulose of recombinant Gal-1, Gal-2, Gal-3, Gal-4 and Gal-7 followed by Ponceau S stain. (B) SDS-PAGE followed by transfer to nitrocellulose of recombinant Gal-1, Gal-2, Gal-3, Gal-4 and Gal-7 and Western blot analysis using αhGal-1. (C) Recombinant Gal-1, Gal-2, Gal-3, Gal-4 and Gal-7 were captured on a polystyrene microtiter plate followed by detection with αhGal-1 using the concentrations indicated. (D) Octapeptides spanning human Gal-1 were generated as outlined in the Materials and methods. αhGal-1 was incubated with each pin octapeptide, and positive binding was detected with an alkaline phosphatase-labeled secondary antibody.
The ability of αhGal-1 to detect Gal-1 under both native and denaturing conditions suggested recognition of a surface-exposed sequential epitope within the Gal-1 sequence. To determine the epitope recognized by αhGal-1, we utilized a solid-phase epitope mapping system (James and Harley 1996) of overlapping octapeptide sequences covering the entire length of Gal-1. αhGal-1 specifically recognized the amino acid sequence SKDGGAWG (Figure 1D). Importantly, SKDGGAWG lies on the surface of Gal-1 (Lopez-Lucendo et al. 2004), which corroborated the ability of αhGal-1 to recognize both denatured and native Gal-1. Sequence comparison analysis between Gal-1 and other galectin family members demonstrated that Gal-2 and Gal-7 display the highest percent identity over this epitope region (Table 1). However, αhGal-1 did not recognize either Gal-2 or Gal-7 in either a solid-phase assay system or Western blot analysis (Figure 1B and C). This sequential epitope is not conserved in murine Gal-1, which has the sequence TKEDGTWG (Wells and Mallucci 1991), and αhGal-1 did not recognize the mouse Gal-1, as expected (data not shown). This epitope is identical between the human and porcine Gal-1 sequence (Merkle et al. 1989; Qiu et al. 2008), but it is partially conserved in bovine Gal-1, which has the sequence SKDAGAWG (Ahmed et al. 1996). Thus, we conclude that αhGal-1 shows specific binding only to this sequential epitope SKDGGAWG in human and porcine Gal-1.
Table I.
Comparison of the human αhGal-1 epitope with nearest related sequences in other members of the human galectin familya
| Galectin family member | Sequence | Epitope identity % | Galectin family member | Sequence | Epitope identity % |
|---|---|---|---|---|---|
| Gal-1 | SKDGGAWG | – | Gal-8-C | SFLQESWG | 38 |
| Gal-2 | SLDGSNWG | 63 | Gal-9-N | TRQNGSWG | 38 |
| Gal-3-Nb | QGWPGAWG | 38 | Gal-9-C | TQIDNSWG | 25 |
| Gal-3-C | TKLDNNWG | 38 | Gal-10 | TLAWISWG | 25 |
| Gal-4-N | TLQGGKWG | 50 | Gal-12-N | TLHGGRWQ | 25 |
| Gal-4-C | SLLNGSWG | 50 | Gal-12-C | LAWISRWG | 25 |
| Gal-7 | SKEQGSWG | 63 | Gal-13 | RREFGIWM | 38 |
| Gal-8-N | TLINEKWG | 25 |
Residues in bold are those that differ from the epitope sequence of human Gal-1.
Denotes the N- or C-terminal regions of the protein.
αhGal-1 recognizes Gal-1 bound to ligand
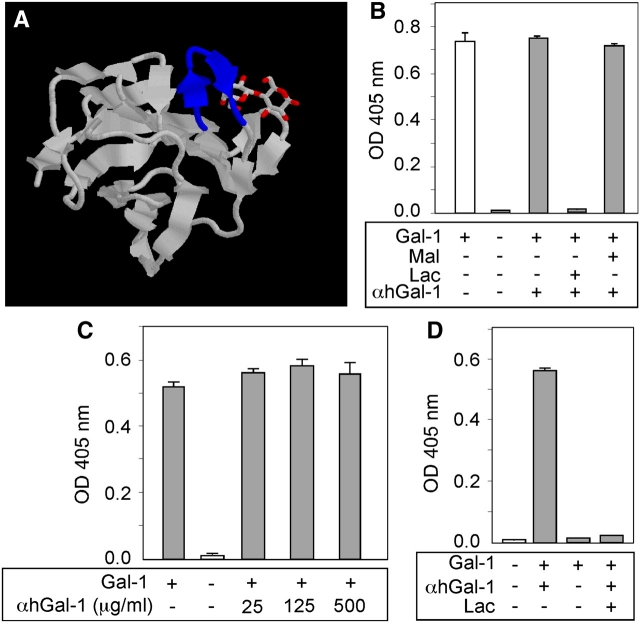
The αhGal-1 epitope SKDGGAWG lies in close proximity to the carbohydrate recognition domain of the protein (Figure 2A) (Lopez-Lucendo et al. 2004), which raised the possibility that αhGal-1 might be a function-blocking antibody or alternatively could not recognize Gal-1 bound to ligand. If this latter case were true, then αhGal-1 might not be useful in histochemical approaches where Gal-1 might be complexed with ligands. To test this, we pre-incubated biotinylated Gal-1 with αhGal-1, followed by incubation of this complex with laminin, a previously described ligand for Gal-1 (Cooper et al. 1991; Castronovo et al. 1992; Zhou and Cummings 1993). Pre-incubation of αhGal-1 with Gal-1 did not reduce Gal-1 binding to laminin (Figure 2B). Importantly, Gal-1 binding to laminin required recognition of specific glycans on laminin because lactose, but not maltose, inhibited binding (Figure 2B). Similar results occurred over a wide range of αhGal-1 concentrations (Figure 2C). The observation that αhGal-1 does not block Gal-1-ligand interactions suggested that αhGal-1 likely recognizes Gal-1 following ligand binding. To test this, we examined the ability of αhGal-1 to directly detect Gal-1 once bound to laminin. αhGal-1 detected Gal-1 following pre-incubation of Gal-1 alone with laminin (Figure 2D). Inclusion of lactose precluded detection (Figure 2D), which demonstrated that αhGal-1 specificity recognized Gal-1 in this assay. Taken together, these results demonstrate that αhGal-1 does not inhibit Gal-1 ligand interactions and that αhGal-1 recognizes Gal-1 bound to ligand.
Fig. 2.
αhGal-1 recognizes Gal-1 bound to ligand. (A) Co-crystal of Gal-1 complexed with lactose as ligand (Lopez-Lucendo et al. 2004). The epitope sequence bound by αhGal-1 is highlighted in blue. (B) αhGal-1 was incubated with biotinylated Gal-1 with or without lactose or maltose as indicated for 30 min followed by incubation with laminin for 30 min. Bound Gal-1 was detected using horseradish peroxidase (HRP)-labeled streptavidin. (C) αhGal-1 was incubated with Gal-1 as in (B) (at the indicated concentrations) followed by incubation with laminin and detection of bound Gal-1 with HRP-streptavidin. (D) Gal-1 was incubated with laminin for 30 min followed by detection using αhGal-1.
Gal-1 expression in tissue
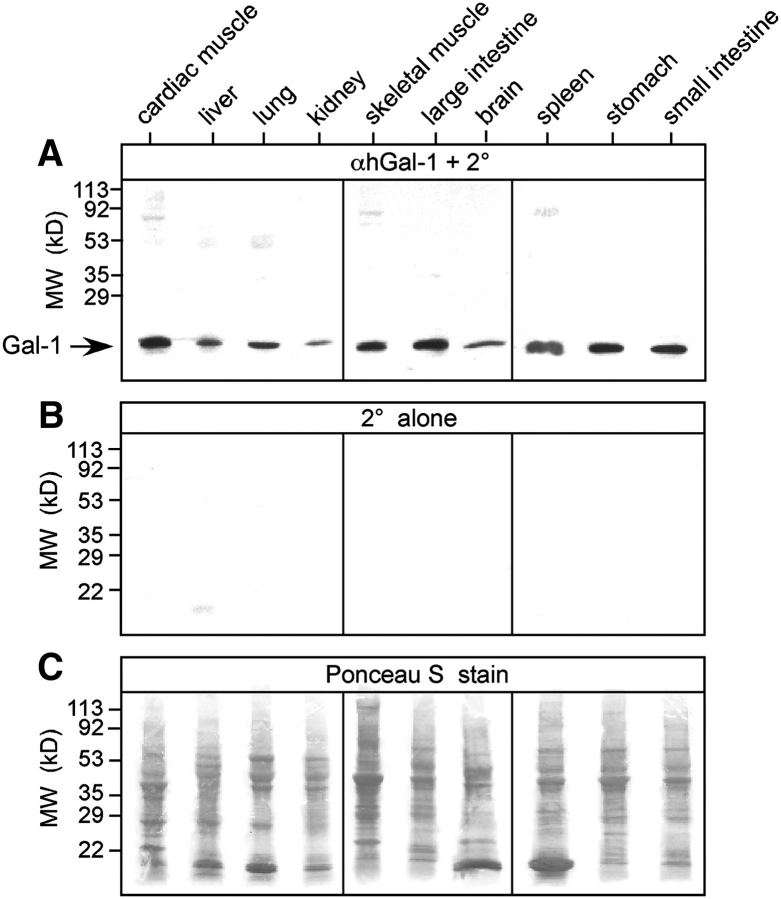
We next explored Gal-1 expression among different tissues using Western blot analysis. Because of the conservation of the αhGal-1 epitope between porcine and human Gal-1, we examined porcine tissue due to the ease of obtaining sufficient quantities of this tissue needed for analysis. Samples were directly solubilized in sodium dodecyl sulfate (SDS) sample buffer to minimize underestimation of Gal-1 content. We used this direct approach, since Gal-1 might be associated with macromolecular ligands upon tissue solubilization, and we were concerned that some protein might be lost if samples are homogenized and centrifuged to prepare extracts (Leppanen et al. 2005). Western blot analyses of tissue preparations revealed a single prominent immunoreactive band at 14 kDa that had identical electrophoretic mobility to recombinant Gal-1 (Figure 1B). Importantly, incubation of extracts with the secondary antibody alone failed to produce similar immunoreactivity (Figure 3A and B), demonstrating specificity of the αhGal-1 binding. Gal-1 was present in all tissues examined, with varying levels of expression between different tissues (Figure 3A), although similar amounts of total protein were extracted from each tissue (Figure 3C). Gal-1 displayed higher expression in tissues lining the alimentary tract and striated muscle tissue, with less, but detectable, expression in tissue isolated from the kidney and cerebrum (Figure 3A). These results demonstrate that αhGal-1 specifically recognized Gal-1 and that Gal-1 is present in all tissues examined.
Fig. 3.
Gal-1 exhibits expression in multiple tissues. Equal wet weights of each tissue were solubilized in SDS and subjected to SDS-PAGE. (A) Immunoblot of each respective tissue as indicated using αhGal-1 and secondary antibody (2°). (B) Incubation of membranes with secondary antibody alone (2° alone). (C) Ponceau S stains of each membrane prior to incubation with αhGal-1 in (A).
Galectin-1 localization in cells
Gal-1 displayed a diffuse localization, with significant cytosolic staining in cells from most tissues examined, as shown for kidney, liver, spleen, intestine, brain and stomach (Figure 4A–H). Staining by αhGal-1 was specific for Gal-1 since secondary antibody alone or an isotype control followed by secondary antibody did not show staining (data not shown). In addition to the primarily cytoplasmic localization observed in most tissues, human umbilical vein endothelial cells (HUVECs) as well as red pulp from spleen displayed some nuclear localization of Gal-1 (Figure 5).
Fig. 4.
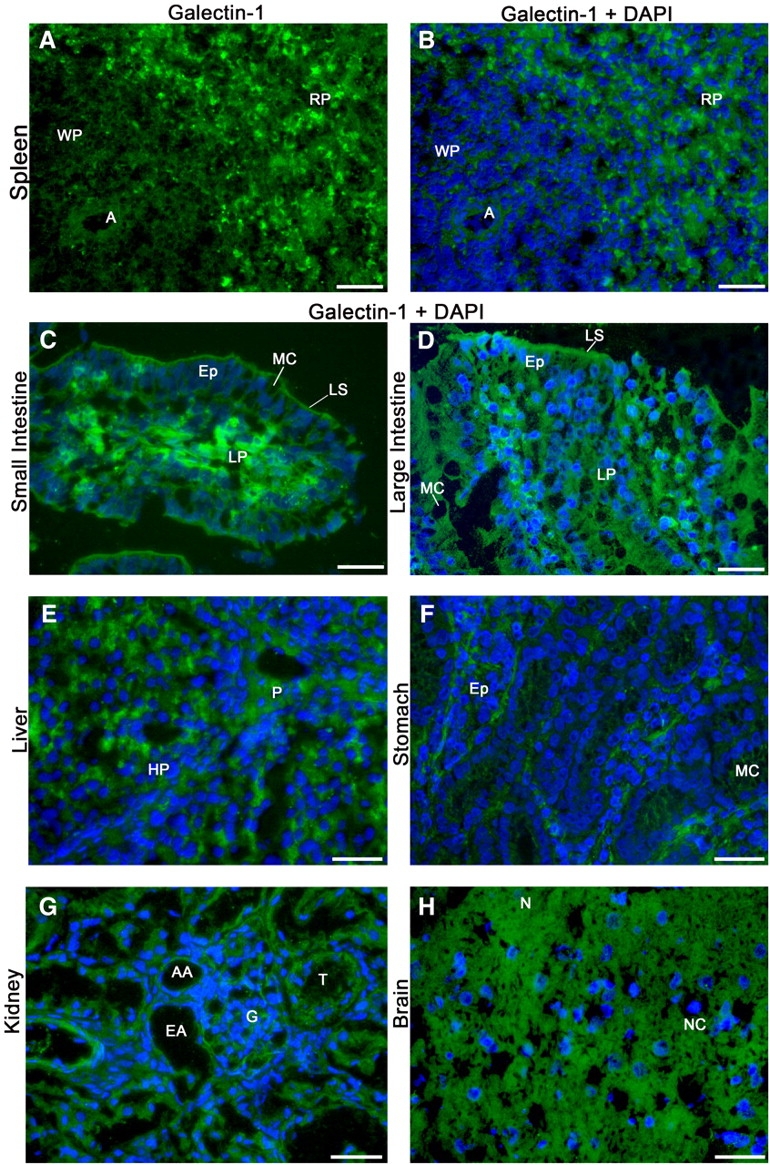
Gal-1 exhibits diffuse cytosolic localization in various adult tissues. Frozen sections of porcine tissue were subjected to immunofluorescence analysis using αhGal-1 and secondary antibody (2°) followed by analysis on a Leica DM 6000 M microscope. Deconvolution was performed using the Leica LAS AF software (version: 1.8.0 build 1346). (A) αhGal-1 stain of spleen (A, arteriole; WP, white pulp; RP, red pulp); (B–H) αhGal-1 and 4′,6′-diamidino-2-phenylindole (DAPI) stain of (B) spleen (A, arteriole; WP, white pulp; RP, red pulp); (C) small intestine (MC, mucous cell; Ep, epithelium; LS, lumenal surface; LP, lamina propria); (D) large intestine (MC, mucous cell; Ep, epithelium; LS, lumenal surface; LP, lamina propria); (E) liver (P, portal space; HP, hepatica plate); (F) stomach (MC, mucous cell; Ep, epithelium cells forming gastric glands); (G) kidney (T, tubule; AA, afferent arteriole; EA, efferent arteriole; G, glomerulus); (H) brain (NC, nerve cells; N, neuropil and glia). Bar = 25 µm
Fig. 5.
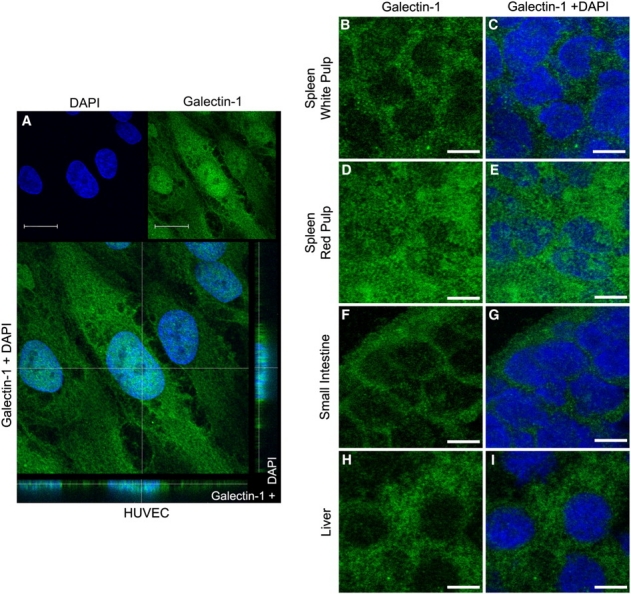
Gal-1 exhibits diffuse cytosolic as well as nuclear localization in cells from different tissues. (A) Confocal analysis of HUVEC cells immunostained with αhGal-1 and DAPI as indicated. Gal-1 is localized in the nucleus and cytoplasm as seen in the confocal transversal section (Z axis image) in the bottom and side of panel (A). Bar = 20 μm. (B–I) Confocal analysis through the middle sections, as the plane of focus cuts through the nucleus sections of the spleen white pulp, spleen red pulp, small intestine and liver. The sections were stained with αhGal-1 (B, D, F and H) or double-stained with DAPI (C, E, G and I) as indicated. Bar = 5 µm.
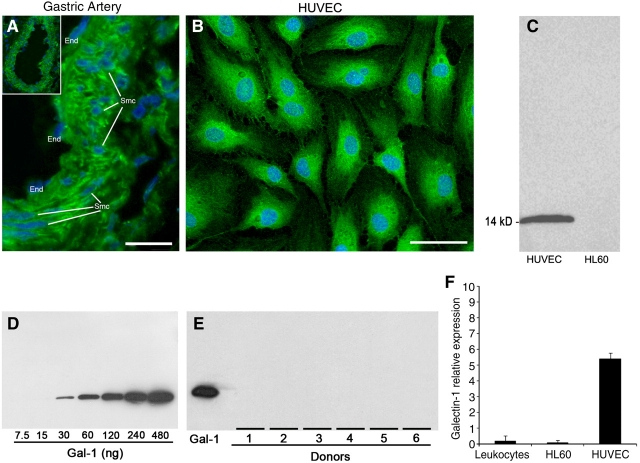
Importantly, αhGal-1 also appeared to display significant staining of smooth muscle cells and of endothelial cells lining the lumen of the artery (Figure 6A). To further explore Gal-1 expression by the endothelium in more detail, we examined Gal-1 expression in HUVECs. In accord with previous results (Baum et al. 1995), Gal-1 was highly expressed within HUVECs (Figure 6B and C). Because Gal-1 has significant effects on leukocytes, we next examined Gal-1 expression in several leukocyte populations. To perform these analyses quantitatively, we demonstrated that αhGal-1 could detect as little as 30 ng of protein in Western blots (Figure 6D). Interestingly, we did not detect Gal-1 expression in HL60 cells (Figure 6C), human neutrophils or MOLT-4 cells (data not shown), although αhGal-1 detected Gal-1 in HUVECs (Figure 6C). Consistent with this, αhGal-1 did not detect Gal-1 expression in peripheral leukocytes from six different donors (Figure 6E). Thus, we conclude that these cells lack detectable Gal-1. We also examined Gal-1 mRNA levels in these cell populations. Only low levels of Gal-1 mRNA were detected in either peripheral leukocytes or HL60 cells, while HUVECS showed higher levels of Gal-1 mRNA (Figure 6F). The low level expression of Gal-1 transcripts in HL60 cells is consistent with prior studies in which cDNA for human Gal-1 was cloned from these cells (Couraud et al. 1989). This suggests that, while Gal-1 is widely expressed throughout various tissues, peripheral leukocytes do not express significant amounts of Gal-1.
Fig. 6.
Gal-1 expression occurs in vascular tissue but not leukocytes. (A) Deconvolution image of frozen sections of a porcine gastric artery stained with αhGal-1 and DAPI using low (insert, ×20) and high magnification (×60) as outlined in Materials and methods. The endothelial cells (End) and smooth muscle cells (Smc) are indicated in the panel. Bar = 20 µm. (B) Human umbilical vein endothelial cells (HUVECs) were subjected to immunofluorescence analysis using αhGal-1 and DAPI and Leica DM 6000 M microscope, as in Figure 4. Bar = 25 µm. (C) Western blot analysis using αhGal-1 of extracts from HL60 cells or HUVECs as indicated. (D) Western blot analysis using ahGal-1 of various concentrations of recombinant Gal-1 as indicated. (E) Western blot analysis using ahGal-1 of recombinant Gal-1 or peripheral leukocytes from several donors. Western blot results are representative of three independent experiments. (F) Determination of the relative expression of cDNA for Gal-1 from total mRNA from pooled peripheral leukocytes, HL60, or HUVEC cells. Results were obtained using real-time PCR and represent accumulative data from three independent experiments performed in triplicate
Gal-1 expression in muscle
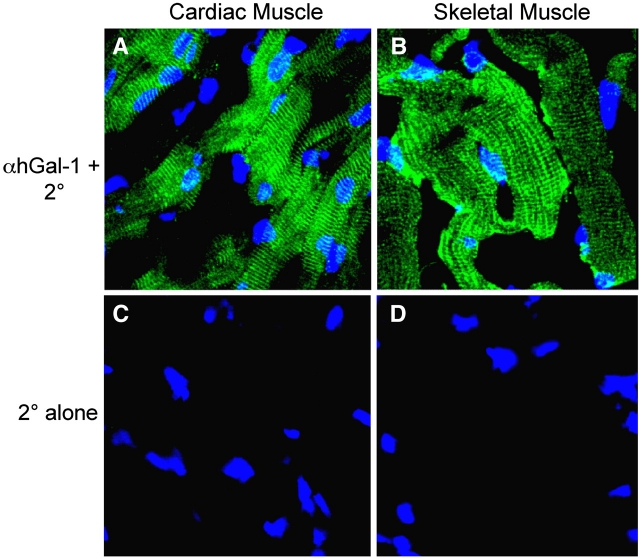
In stark contrast to its diffuse cytoplasmic localization in cells as described above, Gal-1 was expressed in an organized, striated staining pattern in cardiac and skeletal muscle tissue (Figure 7). Smooth muscle within the tunica media of gastric artery stained strongly with αhGal-1 (Figure 6A). However, unlike αhGal-1 staining in striated muscle, αhGal-1 displayed a diffuse staining pattern in smooth muscle (Figure 6A), similar to that observed in liver, kidney, brain, stomach, intestine and spleen (Figure 4A–H).
Fig. 7.
Gal-1 displays organized cytosolic localization in striated tissues. Frozen sections of cardiac or skeletal muscle tissue for porcine tissue were subjected to confocal analysis using αhGal-1 and secondary antibodies or secondary alone as indicated. (A–B) αhGal-1 stain of (A) cardiac muscle and (B) skeletal muscle. (C–D) Staining with the secondary antibody alone is shown for (C) cardiac muscle and (D) skeletal muscle
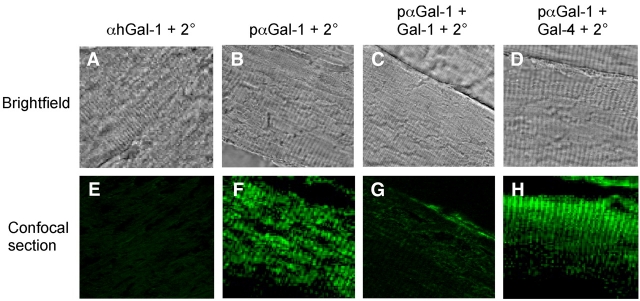
To ensure that the unique intracellular localization of Gal-1 in striated tissue did not reflect cross reactivity between αhGal-1 and an unknown muscle specific antigen, we next examined localization of Gal-1 using an alternative affinity-purified polyclonal antibody preparation against Gal-1 (pαGal-1) and compared this to the staining observed following detection with αhGal-1. To accomplish this, we first determined whether pαGal-1 might recognize epitopes on Gal-1 distinct from that recognized by αhGal-1. The αhGal-1 epitope, SKDGGAWG, contains a critical lysine residue, which suggested that potential lysine modification following tissue fixation with paraformaldehyde could preclude Gal-1 recognition by αhGal-1. Consistent with this, αhGal-1 failed to detect Gal-1 within fixed tissue (Figure 8A and E). However, staining of skeletal muscle with pαGal-1 produced the same staining pattern observed when frozen sections were stained with αhGal-1 (Figure 8B and F). To determine whether pαGal-1 specifically recognized Gal-1 within muscle tissue, we pre-incubated pαGal-1 with recombinant forms of either Gal-1 or Gal-4 as a control. Pre-incubation of pαGal-1 with Gal-1 blocked staining, while pre-incubation with Gal-4 had no effect (Figure 8C, D, G and H). Costaining of pαGal-1 with two different highly organized muscle markers demonstrates that Gal-1 colocalizes with sarcomeric actin (Figure 9D–F) but not myosin (Figure 9A–C) in striated muscles. This indicates selective localization of Gal-1 in I bands of striated muscle (Figure 9G). Taken together, these results demonstrate that Gal-1 exhibits high expression in multiple tissues, which remains primarily cytosolic with some detectable nuclear localization yet exhibits an unprecedented organization within striated muscle cells.
Fig. 8.
Gal-1 exhibits discrete localization within skeletal muscle tissue as detected using a polyclonal anti-Gal-1. (A–D) Brightfield images of paraformaldehyde (PFA)-fixed skeletal muscle subjected to PFA, fixed skeletal muscle subjected to immunohistochemical analysis of muscle tissue using (A) αhGal-1, (B) pαGal-1, (C) pαGal-1 pre-incubated with recombinant Gal-1 and (D) pαGal-1 pre-incubated with recombinant Gal-4. (E–H) Confocal analysis of PFA-fixed skeletal muscle subjected to immunofluorescence analysis of muscle tissue using (E) αhGal-1, (F) pαGal-1, (G) pαGal-1pre-incubated with recombinant Gal-1 and (H) pαGal-1 pre-incubated with recombinant Gal-4
Fig. 9.
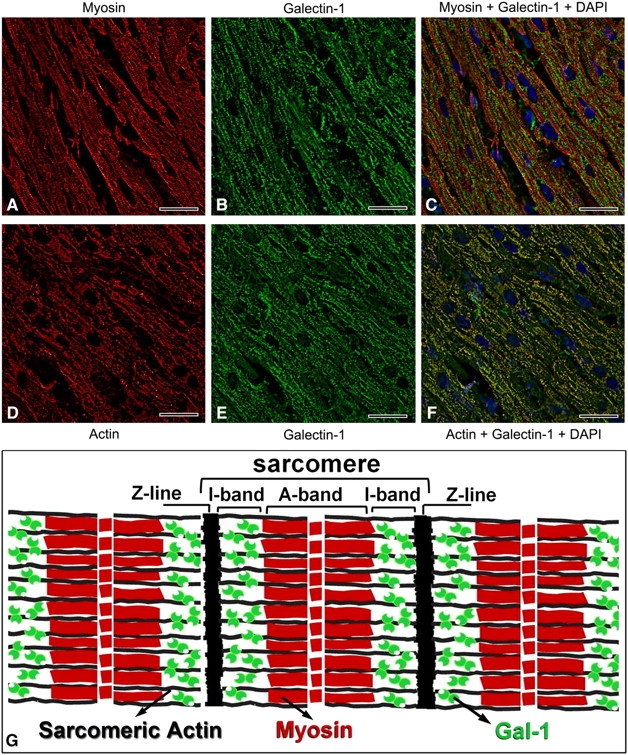
Gal-1 colocalizes with sarcomeric actin but not myosin within skeletal muscle tissue. (A–C) Representative images of the immunofluorescence analysis of paraformaldehyde (PFA) fixed skeletal muscle using a Leica DM 6000 M microscope, as in Figure 4. (A) anti-myosin, (B) pαGal-1 and (C) merge of panels (A) and (B), or (D) anti- sarcomeric actin, (E) pαGal-1 and (F) merge of panels (D) and (E). Bar = 25 µm. (G) This is a schematic of striated muscle showing the predicted location of Gal-1 in the I-band region, as depicted by the green symbols
Discussion
Although many studies demonstrate critical roles for Gal-1 in immune regulation and muscle regeneration/development, the expression and localization of Gal-1 in adult tissue and primary leukocytes has been unclear. The results of the present study demonstrate that Gal-1 is found in all tissues examined and exhibits wide cytosolic expression in many cells. However, in contrast with several types of tissue including the cells of the vascular system, human peripheral leukocytes do not express Gal-1. Unexpectedly, we also found limited nuclear localization and an unprecedented expression pattern in striated muscles.
Our results are consistent with several previous studies showing that Gal-1 protein is expressed in multiple tissues (Caron et al. 1990; Wasano et al. 1990; Camby et al. 2006). For example, several studies demonstrate high expression of Gal-1 in endothelial cells (Baum et al. 1995; Norling et al. 2008), where Gal-1 may regulate many aspects of leukocyte behavior, including activation and extravasation (La et al. 2003; Cooper et al. 2008; Norling et al. 2008). The high expression in endothelial cells also suggested that broad expression of Gal-1 may reflect the uniform presence of endothelial cells in every tissue, consistent with the nearly ubiquitous presence of Gal-1 transcript (Lohr et al. 2007). However, previous studies also documented high levels of Gal-1 expression in various non-endothelial derived neoplastic lesions (Liu and Rabinovich 2005), suggesting that other types of cells can also express Gal-1. The present results corroborate the high levels of expression in endothelial cells and demonstrate that nontransformed parenchymal tissue also expresses significant Gal-1.
Interestingly, the results show that Gal-1 is significantly expressed in the nucleus as well as the cytosol of HUVEC cells, as well as red pulp of spleen. Regarding nuclear expression in HUVECS, this is consistent with previous results suggesting that Gal-1 participates in intracellular and nuclear events such as RNA splicing and spliceosome assembly (Patterson et al. 2004; Voss et al. 2008). In addition, in some cells tumor progression has been associated with a shift in the localization of Gal-1 from the nucleus to the cytoplasm (Saussez et al. 2008). Also, high expression of Gal-1 in endothelial cells and the localization of Gal-1 on the luminal surface of enterocytes (Figure 4) may be important for orchestrating innate immune responses associated with the previously reported activity of Gal-1 in recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (Sato et al. 2009). It was previously reported that Gal-1 can be found in the epithelial compartment and the lamina propria in the small intestine, where it also may be involved in PAMP-associated activity (Mercer et al. 2009). In Figure 4, we show a similar result, and the findings suggest that Gal-1 could also be involved in cellular homeostasis and modulation of mucosal tolerance and inflammation, as predicted (Mercer et al. 2009).
The present data also indicate a significant amount of Gal-1 in the nuclei and cytoplasm of the cells in splenic red pulp. The nuclear localization could implicate Gal-1 in the regulation of various intracellular functions as suggested for HUVEC cells. However, it was recently reported that changes in the intestinal flora promote an increase of Gal-1 expression in the spleen (Romond et al. 2009). Since the spleen red pulp is involved in removal of particulate matter, aged and abnormal red cells and platelets from circulation, an activity associated with macrophages found in the spleen, which express Gal-1 (Sato et al. 2009), it is possible that Gal-1 is involved in splenic functions such as removing particulate matter from the blood.
Gal-1 also demonstrates differential expression during embryogenesis, which originally suggested a critical role for Gal-1 in development (Poirier et al. 1992).Recent studies show that knock-down of the galectin-1-like protein in the zebrafish D. rerio results in defects in muscle development and disorganized muscle fibers (Ahmed et al. 2009). However, studies of Gal-1-null mice failed to demonstrate robust phenotypic requirements for Gal-1 in normal embryogenesis (Poirier and Robertson 1993), suggesting that Gal-1 may play more subtle roles in mammalian development. Consistent with this, recent studies show that Gal-1 has a significant role in the regeneration of muscle (Chan et al. 2006; Blois et al. 2007; Georgiadis et al. 2007; Shao et al. 2009). Previous findings suggest that Gal-1 may partially regulate muscle development and regeneration through extracellular signaling pathways (Goldring et al. 2002); however, the highly organized localization of the intracellular pool of Gal-1 suggests that it may also be involved in important muscle function.
Gal-1 colocalizes with sarcomeric actin at the I band of the sarcomere. This is interesting in light of previous reports that Gal-1 interacts with neural actin derived from human brain (Joubert et al. 1992). We also detected molecular interaction between Gal-1 and actin from porcine heart (data not shown), and we are currently investigating the molecular nature of this interaction. Interestingly, while our results demonstrated unique cytosolic localization of Gal-1 to the I band of the sarcomere in striated muscle, immunostaining of Gal-1 in injured striated muscle shows that Gal-1 is localized around myofibers and could present a cytosolic diffuse and punctate pattern (Cerri et al. 2008) It is possible that the localization of Gal-1 at the sarcomeric I band could be related to some aspects of muscle contraction as described for lectin on smooth muscles (Wasano et al. 1990; Case et al. 2007), while the altered localization in injured muscle is related to a function in muscle regeneration. Finally, it is clear that Gal-1 and sarcomeric actin are generally colocalized in porcine cardiac muscle, but there are areas lacking colocalization, as seen in Figure 9F. This may be a consequence of muscle contraction that could promote intermolecular complex formation involving Gal-1, actin and other molecules present on the I bands, such as titin and nebulin. Clearly, further studies are necessary to understand the roles of Gal-1 in muscle biology and potential changes in Gal-1 localization during muscle contraction. Interestingly, in human Trypanosoma cruzi infections, patients with acute and chronic Chagas’ disease develop anti-galectin-1 antibodies, and the immunoreactivity was correlated with the severity of cardiac damage (Giordanengo et al. 2001). It was also found that chronic disease caused increases in Gal-1 expression. Thus, it is possible that Gal-1 overexpression in the heart as part of the inflammatory response to the disease and heart injury could cause release of Gal-1 and induction of autoantibodies. It would be interesting in future studies to explore the subcellular localization and expression of Gal-1 in human heart tissue in association with T. cruzi infection.
Gal-1 may regulate leukocyte function by inducing the turnover of specific leukocyte subpopulations, regulating cytokine secretion and altering trafficking. Gal-1-null mice display TH1 skewed adaptive immune responses and exhibit impaired maternal–fetal tolerance (Blois et al. 2007; Toscano et al. 2007). TNF family members also display similar activities in the regulation of leukocyte function (Simon 2003a,b). However, unlike Gal-1, TNF family members, such as Fas, TNFα and TRAIL display restricted expression on specific subsets of leukocyte populations (Wong and Choi 1997). The wide expression and cytosolic localization of Gal-1 appears to be unique among factors that regulate leukocyte turnover and may reflect a mechanism whereby leukocytes involved in innate immunity, such as neutrophils, demarcate necrotic from viable tissue. Consistent with this, Gal-1 inhibits the chemotaxis and induces the turnover of neutrophils (Dias-Baruffi et al. 2003; La et al. 2003). Neutrophils often damage viable tissue during acute inflammatory episodes resulting in loss of intracellular contents (Nathan 2006). Thus, release of Gal-1 during leukocyte-mediated injury may allow Gal-1 to engage leukocytes and therefore be uniquely poised to inhibit their chemotaxis and induce their turnover (Dias-Baruffi et al. 2003; Cooper et al. 2008; Stowell, Arthur, Mehta, et al. 2008; Stowell, Arthur, Slanina, et al. 2008; Stowell, Qian, et al. 2008).
Gal-1 was not detectable in peripheral leukocytes at the protein level. However, previous studies indicate that Gal-1 is expressed in some leukocytes such as activated T cells, macrophages, activated B cells, follicular dendritic cells and regulatory T cells (Camby et al. 2006; Sato et al. 2009). It is therefore possible that Gal-1 expression in leukocytes is dependent on the type, activation level and the microenvironment of the leukocyte. Further studies will be needed using the specific approaches we have shown here to define Gal-1 expression relative to other galectins in leukocyte populations upon activation and other factors.
Materials and methods
Expression and isolation of recombinant galectin family members
The expression of recombinant forms of human galectin-1, galectin-2, galectin-3, galectin-4 and galectin-7 was accomplished using established procedures (Gitt et al. 1992; Ideo et al. 2002; Stowell et al. 2004). Briefly, a 1-L culture of transformant-positive Escherichia coli of each galectin was grown until the absorbance reached 600 nm and the optical density reached approximately 0.5, at which point cultures were induced with 1.17 mM isopropyl β-D-thiogalactopyranoside (IPTG) for 4 h at 37°C, except that Gal-2 transformant-positive E. coli were induced at 0.25–0.3 optical density at 600 nm with 0.5 mM IPTG for 1–1.25 h at 30°C. Cultures were then centrifuged at 4200 × g for 30 min, followed by discarding the supernatant and freezing the pellets overnight at −80°C. Cell pellets were then thawed on ice and resuspended in 10 mL lysis buffer 2-mercaptoethanol (2-ME)-containing phosphate-buffered saline (MEPBS) containing 0.01 M Na2HPO4, 0.01 M Na2HPO4, 0.85% NaCl, pH 7.4,14 mM 2-ME, 1 Complete Mini EDTA-Free Protease Inhibitor Cocktail Tablet (Roche), 1 mg lysozyme, 10 mg DNase and 10 mg RNase and allowed to incubate at room temperature for 30 min. Cells were further lysed by sonication (Branson Cell Disruptor 185), and debris was pelleted by centrifugation at 13,000 × g for 30 min. Supernatant was applied to a 30-mL lactosyl-Sepharose column pre-equilibrated in MEPBS. The column was then washed extensively with MEPBS. Each bound galectin was eluted with MEPBS containing 0.1 M lactose. To confirm that the galectin quantitatively retained carbohydrate-binding activity, the protein was rechromatographed on lactosyl-Sepharose. The elution profile of each repurified galectin revealed a single peak that was quantitatively eluted with lactose (data not shown). Both lactose and 2-ME were removed from galectin samples prior to use using gel filtration chromatography (Stowell et al. 2004). Gal-1 was biotinylated according to the manufacturer’s protocol followed by removal of excess biotin using a PD-10 gel filtration column as outlined previously (Stowell, Arthur, Mehta, et al. 2008; Stowell, Arthur, Slanina, et al. 2008; Stowell, Qian, et al. 2008).
Generation of monoclonal antibody against human Gal-1
The development of monoclonal antibodies was accomplished using a protocol approved by the University of Oklahoma Health Sciences Center. BALB/c mice were given initial intraperitoneal injections with 20 μg of recombinant human Gal-1 emulsified in complete Freund’s adjuvant. At 2 and 4 weeks later, the animals were given booster injections intraperitoneally with 10 μg of the same antigen emulsified with Freund’s incomplete adjuvant. A final injection with 20 μg recombinant human Gal-1 (without adjuvant) was given intraperitoneally and intravenously 3 days before spleens were removed. The spleen cells were fused with SP2/O mouse myeloma cells according to standard protocols (Harlow and Lane 1988). Fused cells were plated into eight 96-well plates and maintained in Iscove’s medium containing 20% fetal bovine serum, 10 ng/mL recombinant interleukin-6, 1× HAT and OPI for 14 days. After 14 days, the media was changed to Iscove’s medium containing HT instead of HAT. Hybridomas secreting antibodies to recombinant Gal-1 were selected on day 14 by enzyme-linked immunosorbent assay (ELISA) using 50 µL of culture supernatants, and single-cell clones secreting anti-Gal-1 antibodies were generated by limited dilution. Positive clones were further grown in Iscove’s medium containing 20% fetal bovine serum and further screened for specificity toward Gal-1 as well as other galectin family members using ELISA. Through single-cell cloning, a single clone was identified, C1B11A12, that was found to express the specific antibody, and this antibody was designated αhGal-1. Affinity-purified polyclonal anti-Gal-1 antibody to Gal-1 (pαGal-1) was prepared as outlined previously (Cho and Cummings 1995).
Solid-phase peptide synthesis and epitope mapping
Briefly, maximally overlapping octapeptides of human Gal-1 (accession #PO9382) were constructed on the ends of polyethylene pins using Fmoc solid-phase peptide chemistry as previously described (James and Harley 1996; James et al. 1999; McClain et al. 2005). The murine monoclonal αhGal-1 was tested at two different concentrations. Briefly after blocking in 3% low-fat milk/phosphate-buffered saline (PBS), individual solid-phase peptides were incubated with αhGal-1 for 2 h at room temperature. Each pin block was washed and incubated with anti-mouse IgG whole molecule specific alkaline phosphatase conjugate or with anti-human IgG alkaline phosphatase conjugate for the positive controls (Jackson Immunoresearch Laboratories), overnight at 4°C. Pin blocks were washed and then incubated at 37°C with para-nitrophenyl phosphate disodium substrate (Sigma-Aldrich, St. Louis, MO) until positive control wells had absorbance readings of 1.0 at 405 nm. A well-characterized human positive control serum was used to normalize the results among multiple plates. Solid-phase epitope mapping results were considered positive if the absorbance was at least four standard deviations above the control mean of background binding of two different isotype control monoclonal antibodies directed against irrelevant antigens.
Detection of Gal-1 using αhGal-1 in microtiter wells
Fifty-microliter aliquots of a 0.5-mg/mL solution of either galectin-1, galectin-2, galectin-3, galectin-4 or galectin-7 in PBS (0.01 M Na2HPO4, 0.01 M Na2HPO4, 0.85% NaCl, pH 7.4) were used to coat each well followed by blocking with a solution of 5% bovine serum albumin (BSA) in PBS. Triplicate analyses of each coating density were preformed. Antibody dilutions were carried out in PBS-Tween 20 (0.3% Tween 20 in PBS) containing 1% BSA. A 50-μL volume of αhGal-1 at the indicated concentrations was allowed to incubate in each respective well for 1 h, followed by five PBS-Tween 20 washes, incubation with ALEXA 488-labeled goat anti-mouse secondary antibody (Molecular Probes), 5× wash and fluorescence detection using the Perkin Elmer Victor fluorimeter. Alternatively, galectin-1 was biotinylated by using EZ-LinkTM sulfo-NHS-Biotin (Pierce) as outlined by the manufacturer’s protocol. ELISAs were preformed in order to detect biotinylated galectin-1 when bound to laminin. This was accomplished by coating microtiter wells with 50 μL of a 1-mg/mL laminin (Roche) solution in PBS followed by blocking with 5% BSA in PBS. Biotinylated galectin-1 (10 μg/mL) pre-incubated in the presence or absence of the indicated concentrations of αhGal-1, 20 mM lactose or 20 mM maltose for 1 h, incubated in a total volume of 50 μL, washed three times with PBS-Tween 20. Bound biotinylated galectin-1 was then detected using peroxidase-labeled streptavidin diluted 1:5000 in PBS, followed by a 3× PBS-Tween 20 wash. To detect bound streptavidin, each well was incubated with 100 mL of ABTS/peroxidase substrate for 10 or 20 min, followed by absorbance detection at 405 nm using a microtiter plate reader (Molecular Devices). Each assay was performed in triplicate.
Cells
HL60 cells were obtained from the American Type Culture Collection (ATCC-Manassas, VA) and maintained in RPMI medium supplemented with 10% fetal bovine serum. Primary human umbilical vein endothelial cells (HUVECs) was kindly provided by Dr. Dimas Tadeu Covas (Regional Blood Center and Faculty of Medicine-USP, Ribeirao Preto, Sao Paulo, Brazil). Human peripheral leukocytes (polymorphonuclear and mononuclear cells) were obtained from 12 mL of heparinized peripheral blood of six normal donors, according to local human subjects ethical committee guidelines, as outlined previously (Dias-Baruffi et al. 1995).
Western blot analysis of recombinant galectins, tissue and cells
Equal wet weights of each tissue were minced in MEPBS with protease inhibitors (Roche) followed by further homogenization using a Dounce homogenizer. Homogenized tissue extracts were then directly subjected to SDS denaturation at 100°C for 1 h. To determine the protein concentration, tissue homogenate treated with SDS and 2-ME was diluted 1:20 with dH20, followed by protein precipitation using trichloroacetic acid (TCA). The TCA pellet was then washed with acetone and resuspended in modified Lowery reagent according to the manufacturer’s protocol (Pierce). BSA, treated under the same conditions, served as a standard. Tissue samples or recombinant galectins were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (4–20% Tris-glycine gels, Invitrogen) under reducing conditions and transferred to nitrocellulose membranes (Biorad). Pre-stained SDS-PAGE standards, broad range (Biorad), were used as molecular weight markers. Blots were blocked with 5% nonfat dried milk in Tris-buffered saline (TBS) (20 mM Tris–HCl, pH 7.5, 0.3 M NaCl) overnight and incubated with 10 μg/mL of αhGal-1 as indicated. After washing three times with TBS, the blots were incubated with peroxidase-labeled secondary antibody (goat anti-mouse diluted 1:5000) in TBS, with 1% BSA for 1 h at room temperature. After washing three times in TBS, immunoreactive bands were detected by enhanced chemiluminescence on BioMax film. To estimate the detection level of Gal-1 (in nanograms per microgram crude protein extract), we generated a standard curve using human recombinant Gal-1 in several concentrations ranging from 7.5 to 480 ng that was blotted on a nitrocellulose membrane and probed with αhGal-1. For Gal-1 detection, HUVEC, HL-60 and human leukocytes were submitted to the same procedures described to porcine tissues. Thirty micrograms per lane of the different protein cell extracts were loaded onto the gel.
Real-time polymerase chain reaction
Total RNA from peripheral leukocytes (six donors) or HL60 cells or HUVEC cells was extracted by using the RNeasy Plus Mini Kit (QIAGEN, Germany). Real-time polymerase chain reaction (PCR) analysis was performed in triplicate using SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA) in EP realplex Mastercycler thermocycler (Eppendorf, Germany). The relative expression of the Gal-1 gene was normalized against the expression of the β-actin housekeeping gene (forward primer: 5′ATTGCCGACAGGATGCAGAA 3′; reverse primer: 5′ACAGCGAGGCCAAGGATGGA3′). The Gal-1 primer pair used in the real-time PCR was 5′CCTGGAGAGTGCCTTCGAGT3′ (forward) and 5′CACACGATGGTGTTGGCGTC3′ (reverse).
Confocal, deconvolution and immunofluorescence analyses
Immunofluorescence analysis was accomplished using previously established procedures (Laszik et al. 1996; Crawley et al. 2002; Celes et al. 2007). Briefly, 5 µm sections of frozen tissue were incubated with 10 μg/mL αhGal-1 or pαGal-1 for 60 min at room temperature, followed by blocking with 5% goat serum and detection using an ALEXA 488-labeled goat anti-mouse secondary antibody, (Molecular Probes) alone, or isotype primary control for 30 min followed by secondary antibody as indicated. For detection of Gal-1 using pαGal-1, tissues were incubated for 60 min or overnight with pαGal-1, followed by blocking with 5% goat serum and detection using an ALEXA 488-labeled goat anti-rabbit secondary antibody. Frozen tissue sections were fixed using acetone or 4% paraformaldehyde. pαGal-1 was pre-incubated with Gal-1 or Gal-4 as indicated for 30 min. Confocal analysis was accomplished using a Leica TCS SP5 confocal microscope. Deconvolution analysis of the images was performed by a Leica DM 6000 M microscope using the Leica LAS AF software (version: 1.8.0 build 1346). αhGal-1 (10 μg/mL) was used for detection of galectin-1 in monolayers of activated HUVEC cells as described to porcine tissue. Colocalization experiments were performed in frozen sections of porcine cardiac muscle using primary antibodies to cardiac myosin heavy chain (Abcam, Cambridge, MA, USA) and pαGal-1 or α-sarcomeric actin (Vector Laboratories, Burlingame, CA, USA) and pαGal-1 (10 μg/mL). The cells and tissues were fixed with cold acetone (100%), and the DNA was stained with 4′,6′-diamidino-2-phenylindole (300nM) (Sigma-Aldrich).
Acknowledgments
This work was supported by US National Institutes of Health Grant HL085607 to RPM and RDC and Fundacao Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) Grant 23038.039425-42/2008 to MDB. We thank Vani M. A. Corrêa and Mônica A. de Abreu for their excellent assistance with histological processing.
Glossary
- αhGal-1,
murine monoclonal anti-human galectin-1
- BSA,
bovine serum albumin
- Gal-1
galectin-1
- HUVEC
human umbilical vein endothelial cells
- IPTG
isopropyl β-D-thiogalactopyranoside
- 2-ME
2-mercaptoethanol
- MEPBS
2-ME-containing phosphate-buffered saline
- pαGal-1
rabbit affinity-purified polyclonal anti-human galectin-1
- PAMP
pathogen-associated molecular patterns
- TBS
Tris-buffered saline
- TCA
trichloroacetic acid
- TNF
tumor necrosis factor
- SDS
sodium dodecyl sulfate
References
- Ahmed H, Du SJ, Vasta GR. Knockdown of a galectin-1-like protein in zebrafish (Danio rerio) causes defects in skeletal muscle development. Glycoconj J. 2009;26:277–283. doi: 10.1007/s10719-008-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H, Fink NE, Pohl J., Vasta GR. Galectin-1 from bovine spleen: biochemical characterization, carbohydrate specificity and tissue-specific isoform profiles. J Biochem. (Tokyo) 1996;120:1007–1019. doi: 10.1093/oxfordjournals.jbchem.a021493. [DOI] [PubMed] [Google Scholar]
- Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8 T-cell memory. Nat Rev Immunol. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- Baum LG, Seilhamer JJ, Pang M, Levine WB, Beynon D, Berliner JA. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj J. 1995;12:63–68. doi: 10.1007/BF00731870. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- Caron M, Bladier D, Joubert R. Soluble galactoside-binding vertebrate lectins: a protein family with common properties. Int J Biochem. 1990;22:1379–1385. doi: 10.1016/0020-711x(90)90226-s. [DOI] [PubMed] [Google Scholar]
- Case D, Irwin D, Ivester C, Harral J, Morris K, Imamura M, Roedersheimer M, Patterson A, Carr M, Hagen M, Saavedra M, Crossno J, Jr, Young KA, Dempsey EC, Poirier F, West J, Majka S. Mice deficient in galectin-1 exhibit attenuated physiological responses to chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L154–164. doi: 10.1152/ajplung.00192.2006. [DOI] [PubMed] [Google Scholar]
- Castronovo V, Luyten F, van den Brule F, Sobel ME. Identification of a 14-kDa laminin binding protein (HLBP14) in human melanoma cells that is identical to the 14-kDa galactoside binding lectin. Arch Biochem Biophys. 1992;297:132–138. doi: 10.1016/0003-9861(92)90650-l. [DOI] [PubMed] [Google Scholar]
- Celes MR, Torres-Duenas D, Alves-Filho JC, Duarte DB, Cunha FQ, Rossi MA. Reduction of gap and adherens junction proteins and intercalated disc structural remodeling in the hearts of mice submitted to severe cecal ligation and puncture sepsis. Crit Care Med. 2007;35:2176–2185. doi: 10.1097/01.ccm.0000281454.97901.01. [DOI] [PubMed] [Google Scholar]
- Cerri DG, Rodrigues LC, Stowell SR, Araujo DD, Coelho MC, Oliveira SR, Bizario JC, Cummings RD, Dias-Baruffi M, Costa MC. Degeneration of dystrophic or injured skeletal muscles induces high expression of galectin-1. Glycobiology. 2008;18:842–850. doi: 10.1093/glycob/cwn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, O'Donoghue K, Gavina M, Torrente Y, Kennea N, Mehmet H, Stewart H, Watt DJ, Morgan JE, Fisk NM. Galectin-1 induces skeletal muscle differentiation in human fetal mesenchymal stem cells and increases muscle regeneration. Stem Cells. 2006;24:1879–1891. doi: 10.1634/stemcells.2005-0564. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270:5207–5212. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- Cooper D, Norling LV, Perretti M. Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J Leukoc Biol. 2008;83:1459–1466. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- Cooper DN. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Cooper DN, Massa SM, Barondes SH. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol. 1991;115:1437–1448. doi: 10.1083/jcb.115.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couraud PO, Casentini-Borocz D, Bringman TS, Griffith J, McGrogan M, Nedwin GE. Molecular cloning, characterization, and expression of a human 14-kDa lectin. J Biol Chem. 1989;264:1310–1316. [PubMed] [Google Scholar]
- Crawley JT, Goulding DA, Ferreira V, Severs NJ, Lupu F. Expression and localization of tissue factor pathway inhibitor-2 in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2002;22:218–224. doi: 10.1161/hq0102.101842. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dias-Baruffi M, Roque-Barreira MC, Cunha FQ, Ferreira SH. Biological characterization of purified macrophage-derived neutrophil chemotactic factor. Mediators Inflamm. 1995;4:263–269. doi: 10.1155/S0962935195000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278:41282–41293. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- Garin MI, Chu CC, Golshayan D., Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD3//Q T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- Georgiadis V, Stewart HJ, Pollard HJ, Tavsanoglu Y, Prasad R, Horwood J, Deltour L, Goldring K, Poirier F, Lawrence-Watt DJ. Lack of galectin-1 results in defects in myoblast fusion and muscle regeneration. Dev Dyn. 2007;236:1014–1024. doi: 10.1002/dvdy.21123. [DOI] [PubMed] [Google Scholar]
- Giordanengo L, Gea S, Barbieri G, Rabinovich GA. Anti-galectin-1 autoantibodies in human Trypanosoma cruzi infection: differential expression of this beta-galactoside-binding protein in cardiac Chagas’ disease. Clin Exp Immunol. 2001;124:266–273. doi: 10.1046/j.1365-2249.2001.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitt MA, Massa SM, Leffler H, Barondes SH. Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J Biol Chem. 1992;267:10601–10606. [PubMed] [Google Scholar]
- Goldring K, Jones GE, Thiagarajah R, Watt DJ. The effect of galectin-1 on the differentiation of fibroblasts and myoblasts in vitro. J Cell Sci. 2002;115:355–366. doi: 10.1242/jcs.115.2.355. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Ideo H, Seko A, Ohkura T, Matta K.L., Yamashita K. High-affinity binding of recombinant human galectin-4 to SO(3)(-)– > 3Galbeta1– > 3GalNAc pyranoside. Glycobiology. 2002;12:199–208. doi: 10.1093/glycob/12.3.199. [DOI] [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- James JA, Harley JB. Human lupus anti-spliceosome A protein autoantibodies bind contiguous surface structures and segregate into two sequential epitope binding patterns. J Immunol. 1996;156:4018–4026. [PubMed] [Google Scholar]
- James JA, McClain MT, Koelsch G, Williams DG, Harley JB. Side-chain specificities and molecular modelling of peptide determinants for two anti-Sm B/B' autoantibodies. J Autoimmun. 1999;12:43–49. doi: 10.1006/jaut.1998.0252. [DOI] [PubMed] [Google Scholar]
- Joubert R, Caron M, Avellana-Adalid V, Mornet D, Bladier D. Human brain lectin: a soluble lectin that binds actin. J Neurochem. 1992;58:200–203. doi: 10.1111/j.1471-4159.1992.tb09296.x. [DOI] [PubMed] [Google Scholar]
- La M, Cao TV, Cerchiaro G, Chilton K, Hirabayashi J, Kasai K., Oliani SM, Chernajovsky Y, Perretti M. A novel biological activity for galectin-1: inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am J Pathol. 2003;163:1505–1515. doi: 10.1016/s0002-9440(10)63507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. 1996;88:3010–3021. [PubMed] [Google Scholar]
- Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric Galectin-1 Binds with High Affinity to {alpha}2, 3-Sialylated and Non-sialylated Terminal N-Acetyllactosamine Units on Surface-bound Extended Glycans. J Biol Chem. 2005;280:5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- Liu FT. Galectins: a new family of regulators of inflammation. Clin Immunol. 2000;97:79–88. doi: 10.1006/clim.2000.4912. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Lohr M, Lensch M, Andre S, Kaltner H., Siebert HC, Smetana K, Jr, Sinowatz F, Gabius HJ. Murine homodimeric adhesion/growth-regulatory galectins-1, -2 and -7: comparative profiling of gene/ promoter sequences by database mining, of expression by RT-PCR/immunohistochemistry and of contact sites for carbohydrate ligands by computational chemistry. Folia Biol. (Praha) 2007;53:109–128. [PubMed] [Google Scholar]
- Lopez-Lucendo MF, Solis D, Andre S, Hirabayashi J, Kasai K, Kaltner H, Gabius HJ, Romero A. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004;343:957–970. doi: 10.1016/j.jmb.2004.08.078. [DOI] [PubMed] [Google Scholar]
- McClain MT, Heinlen L.D., Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- Mercer N, Guzman L, Cueto Rua E, Drut R, Ahmed H, Vasta GR, Toscano MA, Rabinovich GA, Docena GH. Duodenal intraepithelial lymphocytes of children with cow milk allergy preferentially bind the glycan-binding protein galectin-3. Int J Immunopathol Pharmacol. 2009;22:207–217. doi: 10.1177/039463200902200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle RK, Zhou Q, Schultz TK, Harper WB, Cummings RD. Characterization of an S-type lectin purified from porcine heart. Arch Biochem Biophys. 1989;274:404–416. doi: 10.1016/0003-9861(89)90453-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Norling LV, Sampaio AL, Cooper D, Perretti M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. Faseb J. 2008;22:682–690. doi: 10.1096/fj.07-9268com. [DOI] [PubMed] [Google Scholar]
- Patterson RJ, Wang W, Wang JL. Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconj J. 2004;19:499–506. doi: 10.1023/B:GLYC.0000014079.87862.c7. [DOI] [PubMed] [Google Scholar]
- Poirier F, Robertson EJ. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development. 1993;119:1229–1236. doi: 10.1242/dev.119.4.1229. [DOI] [PubMed] [Google Scholar]
- Poirier F, Timmons PM, Chan CT, Guenet JL, Rigby PW. Expression of the L14 lectin during mouse embryogenesis suggests multiple roles during pre- and post-implantation development. Development. 1992;115:143–155. doi: 10.1242/dev.115.1.143. [DOI] [PubMed] [Google Scholar]
- Qiu H, Zhao S, Yu M, Fan B, Liu B. Expression patterns and subcellular localization of porcine (Sus Scrofa) lectin, galactose-binding, soluble 1 gene. Acta Biochim Biophys Sin. (Shanghai) 2008;40:85–90. doi: 10.1111/j.1745-7270.2008.00373.x. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Romond MB, Mullie C, Colavizza M, Revillion F, Peyrat JP, Izard D. Intestinal colonization with bifidobacteria affects the expression of galectins in extraintestinal organs. FEMS Immunol Med Microbiol. 2009;55:85–92. doi: 10.1111/j.1574-695X.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Saussez S, Decaestecker C, Lorfevre F, Chevalier D, Mortuaire G, Kaltner H, Andre S, Toubeau G, Gabius HJ, Leroy X. Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology. 2008;52:483–493. doi: 10.1111/j.1365-2559.2008.02973.x. [DOI] [PubMed] [Google Scholar]
- Shao H, Chen B, Tao M. Skeletal myogenesis by human primordial germ cell-derived progenitors. Biochem Biophys Res Commun. 2009;378:750–754. doi: 10.1016/j.bbrc.2008.11.134. [DOI] [PubMed] [Google Scholar]
- Shoji H, Deltour L, Nakamura T, Tajbakhsh S, Poirier F. Expression pattern and role of galectin-1 during early mouse myogenesis. Dev Growth Differ. 2009;51:607–615. doi: 10.1111/j.1440-169X.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003a;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Simon HU. Targeting apoptosis in the control of inflammation. Eur Respir J Suppl. 2003b;44:20s–21s. doi: 10.1183/09031936.03.00000603b. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology. 2004;14:157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectins-1, -2 and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD. Dimeric galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008;283:20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Toscano MA, Ilarregui JM, Bianco GA, Campagna L, Croci DO, Salatino M, Rabinovich GA. Dissecting the pathophysiologic role of endogenous lectins: glycan-binding proteins with cytokine-like activity? Cytokine Growth Factor Rev. 2007;18:57–71. doi: 10.1016/j.cytogfr.2007.01.006. [DOI] [PubMed] [Google Scholar]
- van der Leij J, van den Berg A, Blokzijl T, Harms G, van Goor H, Zwiers P, van Weeghel R, Poppema S, Visser L. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004;204:511–518. doi: 10.1002/path.1671. [DOI] [PubMed] [Google Scholar]
- Voss PG, Gray RM, Dickey SW, Wang W, Park JW, Kasai K, Hirabayashi J, Patterson RJ, Wang JL. Dissociation of the carbohydrate-binding and splicing activities of galectin-1. Arch Biochem Biophys. 2008;478:18–25. doi: 10.1016/j.abb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasano K, Hirakawa Y, Yamamoto T. Immunohistochemical localization of 14 kDa beta-galactoside-binding lectin in various organs of rat. Cell Tissue Res. 1990;259:43–49. doi: 10.1007/BF00571428. [DOI] [PubMed] [Google Scholar]
- Watt DJ, Jones GE, Goldring K. The involvement of galectin-1 in skeletal muscle determination, differentiation and regeneration. Glycoconj J. 2004;19:615–619. doi: 10.1023/B:GLYC.0000014093.23509.92. [DOI] [PubMed] [Google Scholar]
- Wells V, Mallucci L. Identification of an autocrine negative growth factor: mouse beta-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell. 1991;64:91–97. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- Wong B, Choi Y. Pathways leading to cell death in T cells. Curr Opin Immunol. 1997;9:358–364. doi: 10.1016/s0952-7915(97)80082-9. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Cummings RD. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch Biochem Biophys. 1993;300:6–17. doi: 10.1006/abbi.1993.1002. [DOI] [PubMed] [Google Scholar]