Abstract
Trypanosoma cruzi, the agent of the American trypanosomiasis or Chagas disease, bypasses its lack of de novo synthesis of sialic acids by expressing a surface-anchored trans-sialidase. This enzyme transfers sialic acid residues from the host's sialylglycoconjugates to the parasite's galactosylglycoconjugates. In addition to carrying out a pivotal role in parasite persistence/replication within the infected mammal, the trans-sialidase is shed into the bloodstream and induces alterations in the host immune system by modifying the sialylation of the immune cells. A major obstacle to understand these events is the difficulty to identify the transferred sialic acid among all those naturally occurring on the cell surface. Here, we report the use of azido-modified unnatural sialic acid to identify those molecules that act as cell surface acceptors of the sialyl residue in the trans-sialidase-catalyzed reaction, which might then be involved in the immune alterations induced. In living parasites, we readily observed the transfer of azido-sialic acid to surface mucins. When evaluating mouse thymocytes and splenocytes as acceptors of the azido-sugar, a complex pattern of efficiently tagged glycoproteins was revealed. In both leukocyte populations, the main proteins labeled were identified as different CD45 isoforms. Disruption of the cell architecture increased the number and the molecular weight distribution of azido-sialic acid tagged proteins. Nevertheless, CD45 remained to be the main acceptor. Mass spectrometry assays allowed us to identify other acceptors, mainly integrins. The findings reported here provide a molecular basis to understand the abnormalities induced in the immune system by the trans-sialidase during T. cruzi infection.
Keywords: azido-sugars, CD45, integrins, parasite–host interactions, sialylation
Introduction
Trypanosoma cruzi is the protozoan parasite agent of the American trypanosomiasis or Chagas disease, a chronic illness that represents a major health and economic problem in Latin America with an estimated total of 100 million people at risk and about 20 million already infected. The infection is naturally transmitted by triatomine vectors, the “kissing bugs”, that can be found from the southern USA to the southern part of South America. However, the infection through contaminated blood together with migration processes extends the transmission risk to developed countries out of the endemic region. Infected women can also transmit the parasite in utero leading to the congenitally acquired disease. The parasite requires surface-bound sialic acids to infect mammalian hosts, but is unable to synthesize these residues de novo. Rather, this need is fulfilled by expression of a trans-sialidase by the parasite. This surface-located, GPI-anchored enzyme is able to transfer α2-3-linked sialyl residues from glycoconjugates (Ferrero-Garcia et al. 1993) and allows the acquisition of the sugar to the mucins that cover the parasite (Acosta-Serrano et al. 2001; Buscaglia et al. 2006). Ultimately, the sialylated mucins aid in the invasion of mammalian host cells (Schenkman et al. 1991) and the protection against lysis by serum factors (Acosta-Serrano et al. 2001; Pereira-Chioccola et al. 2000; Tomlinson et al. 1994).
The trans-sialidase expressed by the infective trypomastigote is also shed to the milieu and can be detected in blood during the acute stage of Chagas disease, both in patients and in experimental infections (de Titto and Araujo 1988; Leguizamón et al. 1994; Risso et al. 2004). The enzyme shed by the parasite persists in the blood due to a repetitive motif located at its C-terminus (Alvarez et al. 2004; Buscaglia et al. 1999). The trans-sialidase exerts its effect in the bloodstream during the acute phase of the disease, precisely when some hallmarks of the T. cruzi infection, such as immune suppression (Kierszenbaum and Sztein 1990) and polyclonal activation (Minoprio et al. 1989), are observed. The intravenous administration of trans-sialidase in mice reproduces several crucial pathogenic findings observed during the infection, such as alterations in the thymus via thymocyte apoptosis inside the “nurse cell complex” (Mucci et al. 2002), apoptosis in the cellular components of the spleen and ganglia (Leguizamón et al. 1999) and the thrombocytopenia observed early in acute infections (Tribulatti et al. 2005). These abnormalities are prevented by the passive administration of trans-sialidase-neutralizing antibodies of either polyclonal (Mucci et al. 2002) or monoclonal origin (Risso et al. 2007; Tribulatti et al. 2005). In agreement, a recombinant trans-sialidase molecule lacking enzymatic activity but retaining substrate specific lectin-like binding (Cremona et al. 1999) does not induce in vivo alterations (Leguizamón et al. 1999; Mucci et al. 2002). Depending both on the availability of suitable acceptors and enzyme concentration, the enzyme is able to either hydrolyze or transfer the sialyl residue to the acceptor oligosaccharide (Schenkman et al. 1992). In vivo, this property induces two virulence activities on different cellular components. Desialylation of platelets and red cells leads to erythropenia and thrombocytopenia (de Titto and Araujo 1988; Pereira 1983; Tribulatti et al. 2005) and becomes evident during the acute phase of the disease when high enzymatic activity is present in blood (de Titto and Araujo 1988; Leguizamón et al. 1994; Risso et al. 2004). On the other hand, the transferase activity is able to induce apoptosis in organs of the immune system even when the enzyme is present in minute amounts (Mucci et al. 2006). All these reports have identified the trans-sialidase enzyme from T. cruzi as a systemically distributed virulence factor, which acts far from the infection site/s inducing damage in the immune system by mobilizing sialyl residues on cell surfaces. However, a complete inventory/global profile of the points of attachment of those residues is lacking.
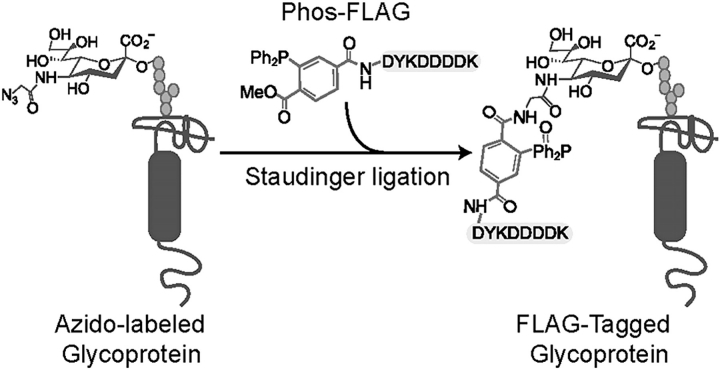
In mammals, sialyl residues decorate several molecules with important cell functions such as lymphocyte homing, intrathymic routing, rolling and intercellular interactions (Daniels et al. 2002; Hernandez and Baum 2002; Lowe 2001). Its privileged occupancy on the periphery of cells regulates the interaction of the cell with its environment and with several lectins (siglecs, selectins and galectins). Therefore, the attack to sialyl residues by microbial virulence factors might result in strong disturbances in the immune system, as is observed with the trans-sialidase from T. cruzi (Leguizamón et al. 1999; Mucci et al. 2002; Mucci et al. 2005). Because the apoptosis of lymphoid cells induced by this virulence factor is associated with the transfer of the sialyl residue (Mucci et al. 2006), the identification of the cell acceptors is of strong interest to understand the underlying mechanisms involved. The ideal situation is to identify the sialyl residues transferred by the trans-sialidase among all the sialyl residues naturally occurring on the cell surface. To address this issue, we utilized unnatural sugars equipped with chemical handles to identify the glycoproteins involved (see Figure 1 and (Dube and Bertozzi 2003) for a review). This methodology offers a useful means to characterize the molecules involved as sialic acid acceptors in the trans-sialidase reaction.
Fig. 1.
Scheme displaying the tagging of the azido-sugar with a FLAG epitope (DYKDDDDK), see also (Dube and Bertozzi 2003) for a review.
Results
The trans-sialidase from T.cruzi accepts azido-modified sialic acids as substrate
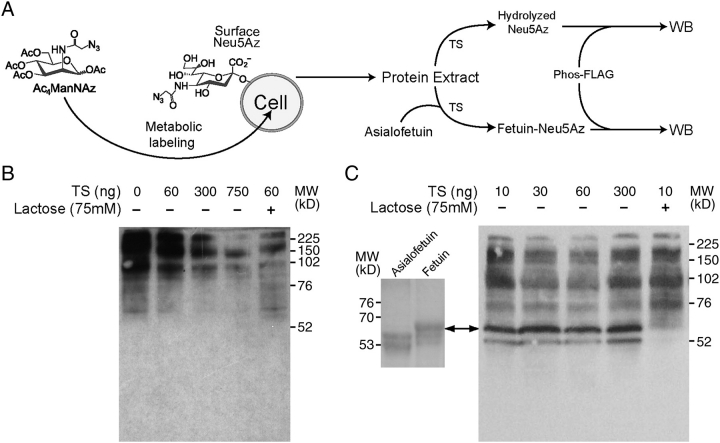
Labeling of cell surface glycans with unnatural azido-sugars followed by chemical tagging and retrieval with phosphine probes may be a useful approach to elucidate how the host's immune system is manipulated by T. cruzi through trans-sialidase activity. To assay the ability of this enzyme to accept the modified substrate N-azidoacetyl neuraminic acid (Neu5Az) (Prescher et al. 2004), Jurkat cells were fed with peracetylated N-azidoacetylmannosamine (Ac4ManNAz), which is an azido-modified derivative of the precursor N-acetylmannosamine (ManNAc) for N-acetylneuraminic acid in the nutrition-salvage pathway (Prescher et al. 2004). The metabolically incorporated Neu5Az present in the glycoconjugates of these cells can be detected by the reaction of the azido group with a phosphine-tagged FLAG (Phos-FLAG) (Prescher et al. 2004) to covalently associate the Neu5Az residue with the FLAG epitope (see Figures 1 and 2A). Thus, the Neu5Az present in the glycoproteins of the extract can be detected by western blot analysis using an anti-FLAG monoclonal antibody (mAb). When trans-sialidase was tested in the absence of added suitable sialyl acceptor substrates, it efficiently hydrolyzed the Neu5Az residue in the glycoconjugates that are present in the lysate of Jurkat cells fed with Ac4ManNAz (Figure 2B). Moreover, when lactose was included as an acceptor enabling the enzyme to work as a transferase, a stronger depletion of Neu5Az was observed at the lowest enzyme concentration tested (Figure 2B, lanes 2 vs. 5). This result indicates that the modified residue was efficiently transferred from glycoconjugates to lactose and supports that the trans-sialidase acts preferentially as a transferase rather than as a sialidase in the presence of suitable acceptor substrates (Schenkman et al. 1992). It is important to recall that the trans-sialidase can only cleave α2-3-linked sialic acid residues (Ferrero-Garcia et al. 1993), therefore the remaining label probably corresponds mainly to α2-6-linked residues.
Fig. 2.
Trans-sialidase accepts Neu5Az as substrate. (A) Scheme of the experimental design. Jurkat cells were fed with Ac4ManNAz so they carry Neu5Az in their glycoconjugates. Protein extracts were treated with trans-sialidase (TS) in the presence or absence of asialofetuin as a model of complex sialyl residue acceptor and then reacted with Phos-FLAG. The presence of Neu5Az is then detected in western blots with anti-FLAG mAb. (B) Cell extracts were treated with trans-sialidase in the presence or absence of lactose as sialyl residue acceptor. (C) Asialofetuin (10 µg) was added together with trans-sialidase. The arrow indicates the position of fetuin as determined in the left panel (SDS–PAGE, Coomassie Blue-stained) where asialofetuin and fetuin were run as standards. Note the increase in MW of the asialofetuin due to the acquisition of sialyl residues. In the last lane, 75 mM lactose was added as an acceptor competitor.
The transfer of Neu5Az to acceptors of similar complexity to glycoproteins found on the cell surfaces was assayed by including asialofetuin as a model acceptor protein substrate. The trans-sialidase-mediated transference of the modified sialyl residues from the Jurkat protein extract to asialofetuin increased its molecular weight (MW) to values comparable to control fetuin (Figure 2C). This transference reaction was efficiently competed by the inclusion of lactose as an acceptor (Figure 2C, last lane). Therefore, trans-sialidase was able to accept the Neu5Az residue and to both hydrolyze and transfer it among glycoproteins.
Trans-sialidase-mediated transfer of sialyl residues to the parasite cell surface
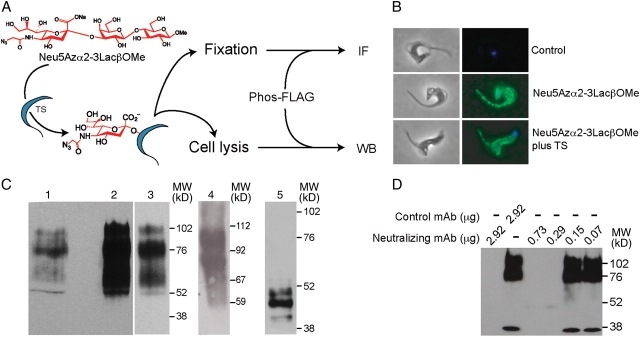
The sialylation of parasite surface mucins by trans-sialidase is a critical step for cell invasion (Schenkman et al. 1991) and evasion of lysis by serum factors (Acosta-Serrano et al. 2001; Pereira-Chioccola et al. 2000; Tomlinson et al. 1994). To further test the biological mimicry of the modified residue in the transference, we assayed the ability of the endogenous enzyme to sialylate the parasite surface molecules. As an alternative substrate to Jurkat lysates, we assayed Neu5Azα2-3LacßOMe (Yu et al. 2005), a defined small reagent easily removable from the medium by washing cells after trans-sialidase treatment. This substrate was tested in standard trans-sialidase-mediated sialyl residue transference to [14C]-lactose reaction, and kinetic results were similar to those obtained using α2-3 sialyllactose as donor (Neu5Azα2-3LacßOMe vs. α2-3 sialyllactose; Kmapp: 9.4 vs. 11.6 mM and Vmax: 0.09 vs. 0.23 nmol min−1). As summarized in Figure 3A, live trypomastigotes derived from infected cell cultures were incubated with Neu5Azα2-3LacßOMe, with or without trans-sialidase addition. Parasites were then washed, fixed and reacted with Phos-FLAG. The presence of the FLAG epitope, indicative of the presence of the transferred Neu5Az, was analyzed by immunofluorescence. The parasites became labeled, thus indicating the incorporation of the residue at the cell surface mediated by both the endogenously and the exogenously added trans-sialidase (Figure 3B). To determine the acceptors of the sialyl residue, western blot assays were performed with lysates of these parasites (Figure 3C, lanes 1–3). The addition of recombinant trans-sialidase rendered the same bands, although showing stronger labeling, thus suggesting the absence of other acceptors. Since T. cruzi surface mucins are the main acceptors of the sialyl residues, mucins purified from cell culture-derived trypomastigotes were used as control in the Neu5Az transfer mediated by the trans-sialidase (Figure 3C, lane 4). The major labeled bands presented the same MW as that of those obtained from the transfer to the whole parasite cells, thus indicating that in intact parasites the Neu5Az was transferred to the expected mucin acceptors. Metacyclic trypomastigotes derived from axenic cultures, which reproduce the infective stage present in the vector, display differently sized mucins on the cell surface (Serrano et al. 1995). When these parasites became labeled with the Neu5Az, a pattern compatible with the MW of the expected mucins corresponding to this parasite stage was obtained (Figure 3C, lane 5). These findings indicate that the azido-sugar labeling method was a faithful reporter of the endogenous labeling even in the presence of an excess of enzyme. This approach was then used to test for the ability of a trans-sialidase-specific mAb (Risso et al. 2007; Tribulatti et al. 2005) to inhibit the enzyme expressed by the parasite. As shown in Figure 3D, as few as 290 ng of purified mAb was able to fully suppress the acquisition of the sialyl residue by 40 × 106 parasites.
Fig. 3.
Trypanosoma cruzi surface molecules are labeled with Neu5Az by trans-sialidase. (A) Scheme of the experimental design. Live trypomastigotes were incubated with Neu5Azα2-3LacßOMe as sialyl donor, washed and reacted with Phos-FLAG. The incorporation of Neu5Az was followed by immunofluorescence (IF) or western blot (WB) with anti FLAG mAb. (B) Immunofluorescence (right panels) and phase contrast (left panels) of parasites that acquired the Neu5Az residue. Tissue culture-derived RA strain parasites were incubated with Neu5Azα2-3LacßOMe (middle panels) and allowed to incorporate the residue by their endogenous trans-sialidase. In another set of assays, recombinant enzyme was added to allow possible overlabeling (lower panels). (C) Western blots of parasites treated as in B. Lane 1: total extract of parasites labeled with Neu5Az by the endogenous enzyme. Lane 2: total extract of parasites labeled with Neu5Az after incubation with exogenously added enzyme. Lane 3: lower exposure of lane 2 to show that the labeled pattern is in fact the same as in lane 1, although stronger. Lane 4: Purified mucins from tissue culture-derived trypomastigotes were labeled with Neu5Azα2-3LacßOMe plus trans-sialidase. Note that the main bands displayed the same MW as previous lanes. Lane 5: total extract of metacyclic parasites labeled with Neu5Az by exogenously added enzyme. Note the different pattern of labeled mucins that characterize this stage. (D) CL-Brener strain trypomastigotes were allowed to acquire Neu5Az as before but in the presence of a purified trans-sialidase-neutralizing or control mAbs. In all cases, blots were developed with an anti-FLAG mAb. TS, trans-sialidase. In color in the online version.
Identification of lymphocyte acceptors of the sialyl residue
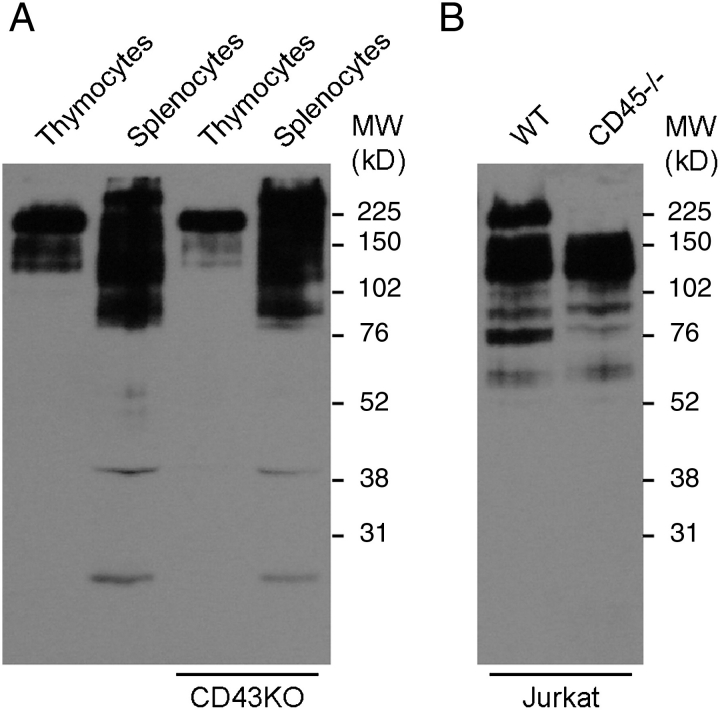
By the expression of trans-sialidase, T. cruzi is not only able to acquire sialic acids on the parasite surface but also utilizes this enzyme to alter the immune system modifying the lymphocyte glycosylation. Therefore, the identification of the acceptor molecules on the lymphocyte surface is a major goal in understanding the pathogenicity of Chagas disease. The Neu5Az transfer catalyzed by trans-sialidase to lymphocyte surface molecules was assayed on mouse thymocytes and splenocytes by using Neu5Azα2-3LacßOMe as donor of the sialyl residue. Trans-sialidase transferred Neu5Az to these cells and generated differential patterns among them (Figure 4A). CD43 mucin is a known acceptor (Mucci et al. 2006) both in vivo (Mucci et al. 2005) and in in vitro assays (Todeschini, Girard et al. 2002; Todeschini, Nunes et al. 2002), and then cells/tissues isolated from B6.CD43null mice were assayed here. No visible differences were found with the wild-type mice when cells from B6.CD43null mice were assayed, a result that might be ascribed to the complex pattern of bands that superimposes with the positions corresponding to the CD43 isoforms. Thymocytes and splenocytes showed a main band, although displaying a higher MW in splenocytes. Due to their MW, we speculate that these bands might correspond to the different isoforms of CD45 found between these cells. CD45 is a heavily glycosylated and abundant glycoprotein present in leukocytes that accounts for about 22% of the cell surface molecules. To test this hypothesis, mutant CD45−/− and wild-type Jurkat T cells were assayed for Neu5Az acceptors in the transfer catalyzed by the trans-sialidase. The absence of this mucin leaves a notorious blank space in the western blot pattern, thus pointing to CD45 as a major acceptor of the sialyl residue transferred by trans-sialidase to the lymphocyte surface (Figure 4B).
Fig. 4.
Lymphocyte labeling with Neu5Az by trans-sialidase. (A) Thymocytes and splenocytes from C57BL/6J and B6.CD43null (CD43KO) mice were treated with trans-sialidase using Neu5Azα2-3LacßOMe as Neu5Az donor and a western blot made with total extracts. (B) Human Jurkat T cells and their CD45−/− derivative were labeled with Neu5Az by the trans-sialidase as before. Western blots were developed with an anti-FLAG mAb.
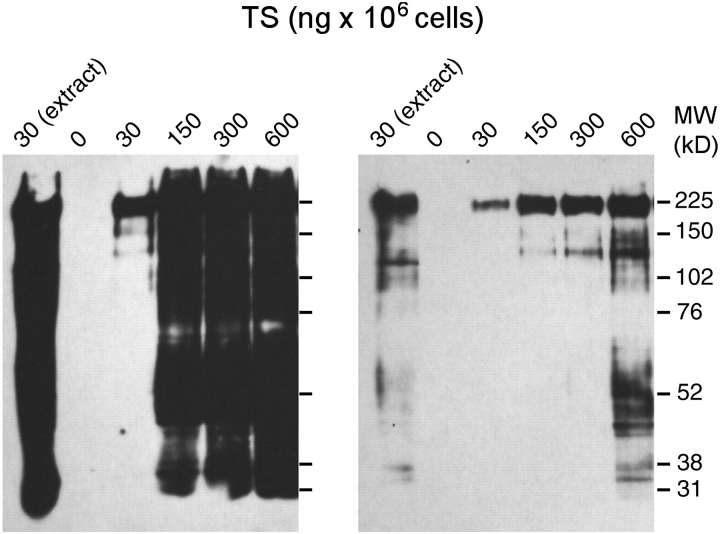
Taking into account the high number of putative acceptors that can be expected on the cell surface, the number of bands was notably small, especially at the lower MW range. One possible explanation for this is that larger proteins are more easily accessible because they protrude from the cell surface and that CD45 is also present at high density, thus hampering the access of trans-sialidase to the lower MW glycoproteins. To increase the chance of trans-sialidase to reach the smaller proteins, increasing amounts of enzyme were added to intact cells (30–600 ng/106 cells). At the highest trans-sialidase amounts assayed, the enzyme efficiently transferred Neu5Az to a broader glycoprotein panel and labeled even very low MW proteins (Figure 5, where two exposures are shown). To facilitate the access of the enzyme to cell substrates, we also assayed the transfer of the Neu5Az residue to thymocyte protein extracts. In this case, the lowest amount of enzyme previously tested (30 ng/106 cells) was enough to efficiently transfer the residue to glycoproteins of a broad MW range. Therefore, it seems that, despite the many putative surface acceptors, those with higher MW are favored targets of the enzyme, probably due to accessibility concerns. However, it is to be noted that even when the lysis allowed equal access to all putative substrates by disruption of the cell surface architecture, CD45 remained to be the major sialyl residue acceptor. CD8, whose biological activity depends in part on its sialylation status (Daniels et al. 2001; Moody et al. 2001; Moody et al. 2003), also emerges as a putative target because of the doublet of bands observed between 38 and 31 kDa, which seems to correspond to this marker (see Figure 5).
Fig. 5.
Steric hindrance of lymphocyte surface labeling by trans-sialidase. Thymocytes were transferred with Neu5Az with increased amounts of trans-sialidase (30–600 ng/106 cells). The first lane corresponds to a cell lysate that was treated with the lowest amount of enzyme. Left and right panels are two exposures of the same blot. Note that although other surface markers were transferred with Neu5Az at high enzyme amounts, CD45 remains to be the major acceptor even when the cells were disrupted prior to labeling.
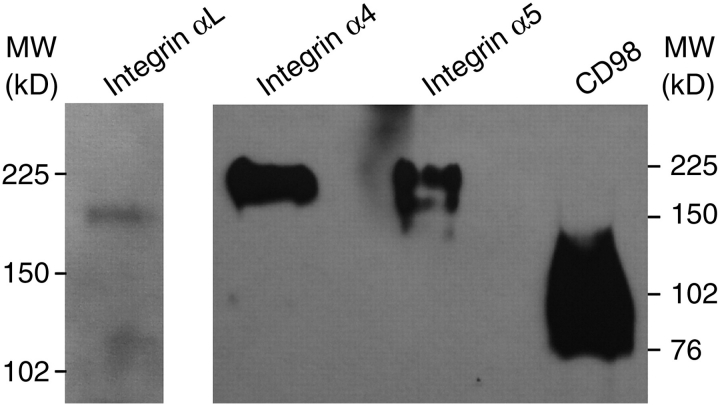
To favor the identification of other lymphocyte trans-sialidase acceptor molecules, Jurkat CD45−/− T cells were treated with trans-sialidase plus Neu5Azα2-3LacßOMe, and protein extracts were then subjected to pull-down assays with an anti-FLAG mAb. The material was resolved by SDS–PAGE and silver-stained to subject individual bands to mass spectrometry analysis. The results indicate that α-integrins are included among the targets, together with CD98, an early T cell activation marker, which is an amino acid transporter also associated with ß-integrin activation (Table I). Cytoskeleton proteins and HSP90AB1 chaperone are probably copurified due to the mild denaturing conditions used in cell lysis to allow the anti-FLAG antibody interaction. The sialylation of integrins might alter their ability to interact with other cells or with the extracellular matrix. To confirm these results, extracts from trans-sialidase-catalyzed Neu5Az-labeling of Jurkat cells were subjected to pull-down assays with anti-integrins α4, α5 and αL and anti-CD98 antibodies and then subjected to western blots that were developed with anti-FLAG antibodies. Results allowed the visualization of the corresponding bands then confirming the acquisition of Neu5Az by these molecules (Figure 6).
Table I.
Molecules identified by MALDI-TOF
| Access | Protein | MW empiric | MW in silico |
|---|---|---|---|
| AAB59613 | Integrin-α 4 | +AH4-150 | 116 |
| NP_002196 | Integrin-α 5 | +AH4-150 | 116 |
| NP_690621 | PTK7 | +AH4-150 | 114 |
| AAC31672 | Integrin-α L | +AH4-150 | 136 |
| NP_690621 | PTK7 | +AH4-135 | 114 |
| P08195 | CD98 | +AH4-120 | 58 |
| NP_031381 | HSP90AB1 | +AH4-90 | 84 |
| NP_006073 | α-Tubulin | +AH4-60 | 50 |
| AAH08633 | β-Actin | +AH4-42 | 42 |
Human Jurkat CD45−/− cells were treated with trans-sialidase and Neu5Azα2-3LacßOMe. Transferred azido-sialic acid was tagged with Phos-FLAG and acceptor molecules were pull-down with anti-FLAG mAb after lysis with Triton X-100, solved by SDS–PAGE and protein bands processed for MALDI-TOF assay.
Fig. 6.
Validation of identified sialyl acceptors. Antibodies anti-integrins α4, α5 and αL and anti-CD98hc were used to pull-down molecules from Jurkat CD45−/− cells transferred with Neu5Az by the trans-sialidase using Neu5Azα2-3LacßOMe as donor substrate. Precipitates were analyzed by western blots developed with anti-FLAG antibodies.
Discussion
Sialylation of proteins is known to have profound biological consequences. For instance, it is involved in several processes such as the regulation of the affinity of receptors and in modulation of transmembrane signaling and differentiation (Varki and Schauer 2009). T. cruzi alters the immune system of the mammalian host by modifying cell sialylation through the trans-sialidase (Leguizamón et al. 1999; Mucci et al. 2002). By the acquisition of the sialyl residue (Mucci et al. 2006), cells became prone to induction of apoptosis resulting in the depletion of thymocytes (Mucci et al. 2002; Mucci et al. 2005) and the prevention of secondary follicle formations in spleen and ganglia (Risso et al. 2007). Therefore, the characterization of the acceptor molecules involved might allow not only to understand some aspects of the pathogenesis of Chagas disease but also to deepen our knowledge on lymphocyte glycobiology. In this work, we utilized azido-modified sugars and bio-orthogonal chemistries approach that allowed us to determine for the first time the sialyl acceptors of the trans-sialidase from T. cruzi on the lymphocyte surface. The information obtained helps to understand the abnormalities induced by this virulence factor and to advance several hypotheses for future work.
CD45 isoforms were identified as the major sialyl acceptors on the cell surface. CD45 is a transmembrane phospho-tyrosine phosphatase whose main targets are the Src-kinases Lck and Fyn. Lck is a primary initiator of signal-transduction upon T cell receptor (TCR) engagement and then CD45 is crucial in T cell differentiation and activation (Zamoyska 2007; Zamoyska et al. 2003). In T lymphocytes, the CD45 glycosylation pattern is developmentally regulated and also varies with the functional stage of the mature cells (Earl and Baum 2008). These variations allow T cells to interact with the environment and, for instance, render T cells susceptible to death induced by CD45 binders such as galectin-1 and -3 (Earl and Baum 2008). Therefore, modifications catalyzed by the T. cruzi trans-sialidase in the glycosylation of CD45 isoforms might also alter their functionality by disturbing the interaction with several different counter-receptors and their subsequent signaling including galectin-3, a lectin whose genetic disruption prevents thymocyte depletion during T. cruzi infection (Silva-Monteiro et al. 2007). In this context, CD98, another detected trans-sialidase sialylation target, is also a known ligand of galectin-3 (Dalton et al. 2007; MacKinnon et al. 2008).
Several molecules other than CD45 were identified as sialyl acceptors by matrix assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Table I). Detection of α-integrins (α4/VLA4, α5/VLA5 and αL/LFA1) as targets of T. cruzi trans-sialidase takes biological relevance because these molecules are associated to the intrathymic routing of the maturating thymocytes (Crisa et al. 1996) and also costimulate T cells and thymocytes (Paessens et al. 2008; Starr et al. 2003). In this sense it has been observed that, during T. cruzi infection, immature CD4+-CD8+- (“double positive”, DP) thymocytes suffer apoptosis (Mucci et al. 2002; Mucci et al. 2005; Mucci et al. 2006) and are also prompted to abandon the thymus at an early stage (Savino 2006; Savino et al. 2004) processes that lead to the cell exhaustion of the organ known to take place during infection (Taliaferro and Pizzi 1955). VLA4 is highly expressed on most thymocytes, and in CD3loCD69lo DP cells is constitutively activated, being able to mediate firm attach to fibronectin (FN) (Salomon et al. 1994). As this subset matures to CD3int/hiCD69int, adherence to FN is lost even when the level of VLA4 remains unaltered, suggesting that its function and not its expression that is modulated. Crisa et al. (1996) demonstrated that VLA4 and VLA5 are involved in the different migration patterns occurring during thymocyte maturation: CD3hi thymocytes (either “single positive” or DP) migrate efficiently on FN whereas the CD3lo DP appears to be stationary. The authors also show that FN expression and density differ between thymic cortex and medulla, providing a mechanism for controlling thymocyte migration along these compartments. In the context of T. cruzi infection, thymuses from infected animals show a stronger FN network in the parenchyma and higher deposition in the capsular and septal basement membranes (Cotta-de-Almeida et al. 2003). These alterations are associated with a significant increase in VLA4hi thymocytes that exhibit higher density of VLA4/VLA5. Thymocytes display enhanced migratory properties in infected animals resulting in the abnormal presence of DP (VLAhi) cells in peripheral organs (Cotta-de-Almeida et al. 2003). Then DP cells are leaving the thymus even when FN deposition is stronger and FN receptors expression is higher, an event that might be related with the fact that unsialylated integrins are more adhesive to FN (Seales et al. 2005; Semel et al. 2002) and then the extra sialylation inserted by the trans-sialidase might reduce even further their binding abilities. Increased sialylation of VLA4 and LFA1 might also help to explain the DP thymocyte apoptosis in the infection. It is known that the adhesion receptors vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 costimulate T cells and thymocytes via these integrins (Paessens et al. 2008). The contribution of sialylation on T cell activation can be also observed in the formation of ordered synapses to low and high affinity peptide/major histocompatibility complex where mature T cells only achieve similar levels compared to thymocytes when they are desialylated (Starr et+//0Awv/9AJI-al. 2003). Taking into account these results, it might be suggested that over-sialylation of α4 and αL might interfere with TCR thymocyte sensibility during selection process increasing apoptosis in the nurse cell complex.
Materials and methods
Azido-modified sugars
Ac4ManNAz, Phos-FLAG and Neu5Azα2-3LacßOMe were synthesized as previously described (Laughlin et al. 2006; Yu et al. 2005). mAb M2 anti-FLAG were obtained from Sigma (St. Louis, MO).
Parasites
Trypomastigotes from T. cruzi RA and CL-Brener strains were obtained from infected Vero cells cultured in minimum essential medium (MEM) plus 5% fetal bovine serum (FBS) (both from Invitrogen, Carlsbad, CA). Parasites were collected from supernatants and exhaustively washed with MEM. For further purification from cell debris, pelleted parasites were allowed to+//0Awv/9AJI-swim from the bottom and collected from the supernatant. Metacyclic trypomastigotes were obtained from axenic epimastigote cultures grown in brain heart infusion medium plus 10% FBS and purified by adhesion to diethylaminoethyl cellulose.
Mice and cells
C57BL/6J breeding pairs were obtained from The Jackson Labs and bred in our facilities. B6.CD43null mice backcrossed 8× to C57BL/6 were gently provided by Dr. Anne I. Sperling (University of Chicago, USA). Animals were used when they were 60–90 days old. All animal experiments were approved by the Instituto de Investigaciones Biotecnológicas, Universidad Nacional de San Martin Review Committee. Human Jurkat T cells and their CD45−/− variant were obtained from the ATCC (Manassas, VI). Trans-sialidase-neutralizing mAb (Risso et al. 2007; Tribulatti et al. 2005) was obtained from culture supernatant of the producing hybridoma and subjected to protein A Sepharose and MonoQ (both from GE-Healthcare, Sweden) purification steps. Anti-E Tag mAb (GE-Healthcare) was dialyzed against 25 mM NaCl, 25 mM Tris–HCl pH 8.8 before using as control.
Trans-sialidase
Recombinant trans-sialidase was expressed in Escherichia coli DH5α (Campetella et al. 1994) induced with isopropyl ß-d-thiogalactopyranoside (Sigma) and purified to homogeneity by chromatography through Ni+-+- charged Hi-Trap Chelating followed by MonoQ columns (GE-Healthcare) as previously described (Buschiazzo et al. 1996).
Metabolic labeling of cells with ManNAc analogs
Jurkat cells were cultured in the presence of Ac4ManNAz as previously described (Laughlin et al. 2006). Briefly, cells were incubated for 3 days with Ac4ManNAz (30 μM final concentration) in Roswell Park Memorial Institute (1640 RPMI) medium supplemented with 10% FBS (Invitrogen), 10 μg/mL gentamicin (Sigma) at 37°C, in 5% CO2. Cells were washed twice with RPMI and resuspended at 1 × 108 cells/mL, in 150 mM NaCl, 50 mM Tris–HCl pH 7.6 (TBS) plus 1% Triton X-100, supplemented with proteinase inhibitors (pepstatin, E64 and PMSF) and subjected to five freeze/thaw cycles. Cell debris was pelleted by centrifugation (15 min at 4°C, 10,000 rpm) and stored at −70°C.
Neu5Az hydrolysis and transference assays
Metabolically labeled Jurkat cell protein extracts (2 μL) were treated for 30 min at room temperature (RT) with the indicated amount of trans-sialidase in a final volume of 20 μL of TBS with 0.1 mg/mL of bovine serum albumin (BSA). For trans-sialylation assays, 2.5 μg of asialofetuin (Sigma) was added to the reaction. After incubation, trans-sialidase was inactivated by heating at 65°C and extracts were labeled for 16 h at RT with 250 μM Phos-FLAG. The assay was analyzed by western blot, 4 μL of each reaction was diluted in SDS–PAGE cracking buffer and after heating at 100°C aliquots were analyzed on 7.5% gels and then electroblotted to polyvinylidene fluoride (PVDF) membranes (GE-Healthcare). Membranes were blocked in 5% nonfat dried milk in TBS plus 0.1% Tween 20, for 1 h, then incubated for 1 h with anti-FLAG M2 mAb (Sigma) diluted 1:5000 in 2.5% nonfat dried milk in TBS plus 0.1% Tween 20. After extensive washing with TBS plus 0.1% Tween 20, membranes were incubated for 1 h with horseradish peroxidase (HRP)-labeled anti-mouse immunoglobulins (Pierce, Rockford, IL) diluted 1:5000 in 2.5% nonfat dried milk in TBS plus 0.1% Tween 20, and after washings with TBS plus 0.1% Tween 20, revealed with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Neu5Az transference by trans-sialidase to cell surface acceptors
Jurkat cells and their CD45−/− mutant derivatives were cultured in RPMI medium with 10% FBS (Invitrogen), 10 μg/mL gentamicin (Sigma) at 37°C, in a 5% CO2 atmosphere. Cells were harvested, washed with RPMI medium and resuspended at 30 × 106 cells/mL with 10 mM 2-deoxyglucose before trans-sialidase treatment. T. cruzi trypomastigotes were obtained from infections in Vero cell cultures, washed three times with MEM and suspended in MEM with 10 mM 2-deoxyglucose (Sigma) at 400 × 106/mL. Thymuses and spleens were harvested, single cell suspensions prepared in RPMI and red cells lysed with ammonium chloride (Sigma). After washing with RPMI, cells were suspended at 300 × 106 cells/mL in RPMI with 10 mM 2-deoxyglucose. Neu5Azα2-3LacßOMe was included at 1 mM final concentration, and trans-sialidase was added at 50 ng/1 × 106 cells for thymocytes and splenocytes; 100 ng/1 × 106 cells (wt) or 200 ng/1 × 106 (CD45−/−) for Jurkat cells and 10 ng/1 × 106 cells for trypomastigotes, unless otherwise indicated. Reactions were allowed to proceed for 15 to 30 min at RT with gentle agitation and then cells were washed four times with medium. For western blots, cells were suspended in TBS plus 1% Triton X-100 and supplemented with proteinase inhibitors (pepstatin, E64 and PMSF, all from Sigma) at 1 × 108 cells/mL for thymocytes or splenocytes and 0.1 × 108 cells/mL for Jurkat cells and subjected to five freeze/thaw cycles. Trypomastigotes were suspended in 20 mM NaCl, 50 mM Tris–HCl pH7.6, 0.5% Triton X-100, with proteinase inhibitors at 5 × 108 cells/mL and subjected to five freeze/thaw cycles. Cell debris was separated by centrifugation, and protein extracts were labeled with 250 µM Phos-FLAG, for 16 h at RT. Western blots were prepared and processed as above. For trans-sialidase reaction in thymocytes and splenocytes lysates, protein extracts were prepared and assayed with trans-sialidase and 1 mM Neu5Azα2-3LacßOMe for 15 min at RT, heated at 65°C to inactivate the enzyme and labeled with 250 μM Phos-FLAG, for 16 h at RT. Western blots were prepared and processed as above. For trans-sialidase inhibition assay, 40 × 106 T. cruzi trypomastigotes were obtained as above and preincubated with the indicated amount of the purified neutralizing or control mAbs for 10 min at RT at 8 × 106 parasites/mL. Neu5Azα2-3LacßOMe was then added at 1 mM final concentration and incubated 30 min at RT with gentle agitation. Protein extracts, labeling and western blots were performed as above.
Purification and labeling of mucins from trypomastigotes
T. cruzi trypomastigote mucins were purified by organic solvents extraction as described by Almeida et al. (1994). Labeling was performed with 30 ng of trans-sialidase and 1 mM Neu5Azα2-3LacßOMe for 30 min at RT; the reaction was heated at 65°C to inactivate the enzyme and labeled with 250 μM Phos-FLAG, for 16 h at RT. Western blots were prepared and processed as above.
Mass spectrometry of Neu5Az/Phos-FLAG labeled glycoproteins
Jurkat CD45−/− cells (60 × 106) were treated with trans-sialidase as described above, resuspended in 1% FBS (Invitrogen), 250 μM Phos-FLAG, 10 mM 2-deoxyglucose (Sigma) in phosphate-buffered saline (PBS) at 30 × 106 cells/mL and incubated at RT with gentle agitation for 4.5 h. Then, cells were extensively washed with PBS and protein extracts were prepared as described above. The same procedure was followed to prepare trans-sialidase untreated control Jurkat CD45−/− cell extracts. For pull-down assays, trans-sialidase-treated and control extracts were diluted 1:1 in TBS and incubated for 16 h at 4°C with 20 μg of anti-FLAG M2 mAb (Sigma), and then 50 μL of Protein G-agarose slurry (Invitrogen) was added; after 3 h of incubation with gentle end-over-end agitation at 4°C, the resine was washed four times with TBS plus 0.5% Triton X-100. For elution, agarose was resuspended in 1.5 mg/mL of FLAG peptide (Sigma) in TBS plus 0.5% Triton X-100 and incubated for 3 h as above. After alkylation with iodoacetamide, samples were suspended in cracking buffer and resolved by 10% SDS–PAGE. After silver staining, discrete bands were cut out and prepared for in-gel digestion with trypsin as described (Hellman 2002) and subjected to peptide mass fingerprinting on a Bruker Ultraflex TOF/TOF (Bruker Daltonics, Bremen, Germany) MALDI mass spectrometer. The manufacturer's instructions were followed, and the resulting peptide mass lists were used to scan the latest NCBInr sequence database for protein identity using the search engine ProFound.
Pull-down of integrins α4, α5, αL and CD98
Jurkat CD45−/− cells were treated with trans-sialidase, Neu5Azα2-3LacßOMe and Phos-FLAG as described above, and extracts were incubated overnight with the corresponding antibodies (all from Santa Cruz, CA), then 50 µL of Protein G-agarose slurry (Invitrogen) was added. After 3 h of incubation with gentle end-over-end agitation at 4°C, the resine was washed 10 times with TBS plus 0.5% Triton X-100. The slurries were resuspended in cracking buffer, resolved by 7.5% SDS–PAGE and electroblotted to PVDF membranes (GE-Healthcare). Membranes were blocked in 5% BSA in TBS plus 0.1% Tween 20 for 1 h, then incubated for 1 h with anti-FLAG M2 mAb (Sigma) diluted 1:5000 in 2.5% BSA in TBS plus 0.1% Tween 20. After extensive washing with TBS plus 0.1% Tween 20, membranes were incubated for 1 h with HRP-labeled streptavidin (R&D Systems Minneapolis, MN) diluted 1:200 in 2.5% BSA in TBS plus 0.1% Tween 20, and after washings with TBS plus 0.1% Tween 20, revealed with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Acknowledgments
Authors are in debt to Dr. C. Buscaglia (IIB-UNSAM) for providing purified parasite mucins and to Dr. A. Sperling (University of Chicago) for the B6.CD43null mice.
Abbreviations
- Ac4ManNAz
peracetylated N-azidoacetylmannosamine
- BSA
bovine serum albumin
- DP thymocytes
CD4+-CD8+- double positive thymocytes
- FBS
fetal bovine serum
- FN
fibronectin
- HRP
horseradish peroxidase
- mAb
monoclonal antibody/ies
- MALDI
matrix assisted laser desorption ionization
- ManNAc
N-acetylmannosamine
- MEM
minimum essential medium
- MW
molecular weight
- Neu5Az
N-azidoacetyl neuraminic acid
- PBS
phosphate-buffered saline
- Phos-FLAG
phosphine-tagged FLAG
- PVDF
polyvinylidene fluoride
- RPMI
Roswell Park Memorial Institute
- RT
room temperature
- TBS
50 mM Tris–HCl pH 7.6, 150 mM NaCl
- TCR
T cell receptor
- TOF
time of flight
Funding
This work was supported by the Agencia Nacional de Promocion Cientifica y Tecnologica (Argentina) and National Institutes of Health (grant R01AI075589 to O.C.). R.P.M. was supported by a Fellowship from the Fogarty International Center (grant number D43TW007888). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health. O.C. is a Researcher from the Concejo Nacional de Investigaciones Cientificas y Tecnicas (Argentina).
Conflict of interest statement
None Declared.
References
- Acosta-Serrano A, Almeida IC, Freitas-Junior LH, Yoshida N, Schenkman S. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol Biochem Parasitol. 2001;114:143–150. doi: 10.1016/s0166-6851(01)00245-6. [DOI] [PubMed] [Google Scholar]
- Almeida IC, Ferguson MA, Schenkman S, Travassos LR. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J. 1994;304:793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Buscaglia CA, Campetella O. Improving protein pharmacokinetics by genetic fusion to simple amino acid sequences. J Biol Chem. 2004;279:3375–3381. doi: 10.1074/jbc.M311356200. [DOI] [PubMed] [Google Scholar]
- Buscaglia CA, Alfonso J, Campetella O, Frasch AC. Tandem amino acid repeats from Trypanosoma cruzi shed antigens increase the half-life of proteins in blood. Blood. 1999;93:2025–2032. [PubMed] [Google Scholar]
- Buscaglia CA, Campo VA, Frasch AC, Di Noia JM. Trypanosoma cruzi surface mucins: Host-dependent coat diversity. Nat Rev Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- Buschiazzo A, Frasch AC, Campetella O. Medium scale production and purification to homogeneity of a recombinant trans-sialidase from Trypanosoma cruzi. Cell Mol Biol (Noisy-le-grand) 1996;42:703–710. [PubMed] [Google Scholar]
- Campetella OE, Uttaro AD, Parodi AJ, Frasch AC. A recombinant Trypanosoma cruzi trans-sialidase lacking the amino acid repeats retains the enzymatic activity. Mol Biochem Parasitol. 1994;64:337–340. doi: 10.1016/0166-6851(94)00036-0. [DOI] [PubMed] [Google Scholar]
- Cotta-de-Almeida V, Bonomo A, Mendes-da-Cruz DA, Riederer I, De Meis J, Lima-Quaresma KR, Vieira-de-Abreu A, Villa-Verde DM, Savino W. Trypanosoma cruzi infection modulates intrathymic contents of extracellular matrix ligands and receptors and alters thymocyte migration. Eur J Immunol. 2003;33:2439–2448. doi: 10.1002/eji.200323860. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Campetella O, Sanchez DO, Frasch AC. Enzymically inactive members of the trans-sialidase family from Trypanosoma cruzi display beta-galactose binding activity. Glycobiology. 1999;9:581–587. doi: 10.1093/glycob/9.6.581. [DOI] [PubMed] [Google Scholar]
- Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA4, and VLA5. J Exp Med. 1996;184:215–228. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Christian HC, Redman CW, Sargent IL, Boyd CA. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells–implication for placental cell fusion. FEBS J. 2007;274:2715–2727. doi: 10.1111/j.1742-4658.2007.05806.x. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- de Titto EH, Araujo FG. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin Immunol Immunopathol. 1988;46:157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr Opin Chem Biol. 2003;7:616–625. doi: 10.1016/j.cbpa.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Earl LA, Baum LG. CD45 glycosylation controls T-cell life and death. Immunol Cell Biol. 2008;86:608–615. doi: 10.1038/icb.2008.46. [DOI] [PubMed] [Google Scholar]
- Ferrero-Garcia MA, Trombetta SE, Sanchez DO, Reglero A, Frasch AC, Parodi AJ. The action of Trypanosoma cruzi trans-sialidase on glycolipids and glycoproteins. Eur J Biochem. 1993;213:765–771. doi: 10.1111/j.1432-1033.1993.tb17818.x. [DOI] [PubMed] [Google Scholar]
- Hellman U. Peptide mapping using MALDI-TOFMS. In: Silberring J, Ekman R, editors. Mass Spectrometry and Hyphenated Techniques in Neuropeptide Research. New York: John Wiley & Sons, Inc; 2002. pp. 259–275. [Google Scholar]
- Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12:127R–136R. doi: 10.1093/glycob/cwf081. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F, Sztein MB. Mechanisms underlying immunosuppression induced by Trypanosoma cruzi. Parasitol Today. 1990;6:261–264. doi: 10.1016/0169-4758(90)90187-9. [DOI] [PubMed] [Google Scholar]
- Laughlin ST, Agard NJ, Baskin JM, Carrico IS, Chang PV, Ganguli AS, Hangauer MJ, Lo A, Prescher JA, Bertozzi CR. Metabolic labeling of glycans with azido sugars for visualization and glycoproteomics. Methods Enzymol. 2006;415:230–250. doi: 10.1016/S0076-6879(06)15015-6. [DOI] [PubMed] [Google Scholar]
- Leguizamón MS, Campetella OE, González Cappa SM, Frasch AC. Mice infected with Trypanosoma cruzi produce antibodies against the enzymatic domain of trans-sialidase that inhibit its activity. Infect Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguizamón MS, Mocetti E, Garcia Rivello H, Argibay P, Campetella O. Trans-sialidase from Trypanosoma cruzi induces apoptosis in cells from the immune system in vivo. J Infect Dis. 1999;180:1398–1402. doi: 10.1086/315001. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Glycosylation, immunity, and autoimmunity. Cell. 2001;104:809–812. doi: 10.1016/s0092-8674(01)00277-x. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- Minoprio P, Itohara S, Heusser C, Tonegawa S, Coutinho A. Immunobiology of murine T. cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol Rev. 1989;112:183–207. doi: 10.1111/j.1600-065x.1989.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Moody AM, Chui D, Reche PA, Priatel JJ, Marth JD, Reinherz EL. Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding. Cell. 2001;107:501–512. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- Moody AM, North SJ, Reinhold B, Van Dyken SJ, Rogers ME, Panico M, Dell A, Morris HR, Marth JD, Reinherz EL. Sialic acid capping of CD8b core 1-O-glycans controls thymocyte-major histocompatibility complex Class I interaction. J Biol Chem. 2003;278:7240–7246. doi: 10.1074/jbc.M210468200. [DOI] [PubMed] [Google Scholar]
- Mucci J, Hidalgo A, Mocetti E, Argibay PF, Leguizamón MS, Campetella O. Thymocyte depletion in Trypanosoma cruzi infection is mediated by trans-sialidase-induced apoptosis on nurse cells complex. Proc Natl Acad Sci USA. 2002;99:3896–3901. doi: 10.1073/pnas.052496399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci J, Mocetti E, Leguizamón MS, Campetella O. A sexual dimorphism in intrathymic sialylation survey is revealed by the trans-sialidase from Trypanosoma cruzi. J Immunol. 2005;174:4545–4550. doi: 10.4049/jimmunol.174.8.4545. [DOI] [PubMed] [Google Scholar]
- Mucci J, Risso MG, Leguizamón MS, Frasch AC, Campetella O. The trans-sialidase from Trypanosoma cruzi triggers apoptosis by target cell sialylation. Cell Microbiol. 2006;8:1086–1095. doi: 10.1111/j.1462-5822.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- Paessens LC, Singh SK, Fernandes RJ, van Kooyk Y. Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) provide co-stimulation in positive selection along with survival of selected thymocytes. Mol Immunol. 2008;45:42–48. doi: 10.1016/j.molimm.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Pereira ME. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983;219:1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira-Chioccola VL, Acosta-Serrano A, Correia de Almeida I, Ferguson MA, Souto-Padron T, Rodrigues MM, Travassos LR, Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J Cell Sci. 2000;113:1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- Prescher JA, Dube DH, Bertozzi CR. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- Risso MG, Garbarino GB, Mocetti E, Campetella O, González Cappa SM, Buscaglia CA, Leguizamón MS. Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J Infect Dis. 2004;189:2250–2259. doi: 10.1086/420831. [DOI] [PubMed] [Google Scholar]
- Risso MG, Pitcovsky TA, Caccuri RL, Campetella O, Leguizamón MS. Immune system pathogenesis is prevented by the neutralization of the systemic trans-sialidase from Trypanosoma cruzi during severe infections. Parasitology. 2007;134:503–510. doi: 10.1017/S0031182006001752. [DOI] [PubMed] [Google Scholar]
- Salomon DR, Mojcik CF, Chang AC, Wadsworth S, Adams DH, Coligan JE, Shevach EM. Constitutive activation of integrin alpha 4 beta 1 defines a unique stage of human thymocyte development. J Exp Med. 1994;179:1573–1584. doi: 10.1084/jem.179.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006;2:e62. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W, Mendes-Da-Cruz DA, Smaniotto S, Silva-Monteiro E, Villa-Verde DM. Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix. J Leukoc Biol. 2004;75:951–961. doi: 10.1189/jlb.1003455. [DOI] [PubMed] [Google Scholar]
- Schenkman S, Jiang MS, Hart GW, Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991;65:1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schenkman S, Pontes de Carvalho L, Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J Exp Med. 1992;175:567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seales EC, Shaikh FM, Woodard-Grice AV, Aggarwal P, McBrayer AC, Hennessy KM, Bellis SL. A protein kinase C/Ras/ERK signaling pathway activates myeloid fibronectin receptors by altering beta1 integrin sialylation. J Biol Chem. 2005;280:37610–37615. doi: 10.1074/jbc.M508476200. [DOI] [PubMed] [Google Scholar]
- Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL. Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J Biol Chem. 2002;277:32830–32836. doi: 10.1074/jbc.M202493200. [DOI] [PubMed] [Google Scholar]
- Serrano AA, Schenkman S, Yoshida N, Mehlert A, Richardson JM, Ferguson MA. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J Biol Chem. 1995;270:27244–27253. doi: 10.1074/jbc.270.45.27244. [DOI] [PubMed] [Google Scholar]
- Silva-Monteiro E, Reis Lorenzato L, Kenji Nihei O, Junqueira M, Rabinovich GA, Hsu DK, Liu FT, Savino W, Chammas R, Villa-Verde DM. Altered expression of galectin-3 induces cortical thymocyte depletion and premature exit of immature thymocytes during Trypanosoma cruzi infection. Am J Pathol. 2007;170:546–556. doi: 10.2353/ajpath.2007.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: A partial role for sialylation. J Immunol. 2003;171:4512–4520. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- Taliaferro WH, Pizzi T. Connective tissue reactions in normal and immunized mice to a reticulotropic strain of Trypanosoma cruzi. J Infect Dis. 1955;96:199–226. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- Todeschini AR, Girard MF, Wieruszeski JM, Nunes MP, DosReis GA, Mendonca-Previato L, Previato JO. Trans-Sialidase from Trypanosoma cruzi binds host T-lymphocytes in a lectin manner. J Biol Chem. 2002;277:45962–45968. doi: 10.1074/jbc.M203185200. [DOI] [PubMed] [Google Scholar]
- Todeschini AR, Nunes MP, Pires RS, Lopes MF, Previato JO, Mendonca-Previato L, DosReis GA. Costimulation of host T lymphocytes by a trypanosomal trans-sialidase: involvement of CD43 signaling. J Immunol. 2002;168:5192–5198. doi: 10.4049/jimmunol.168.10.5192. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Pontes de Carvalho LC, Vandekerckhove F, Nussenzweig V. Role of sialic acid in the resistance of Trypanosoma cruzi trypomastigotes to complement. J Immunol. 1994;153:3141–3147. [PubMed] [Google Scholar]
- Tribulatti MV, Mucci J, Van Rooijen N, Leguizamón MS, Campetella O. The trans-sialidase from Trypanosoma cruzi induces thrombocytopenia during acute Chagas' disease by reducing the platelet sialic acid contents. Infect Immun. 2005;73:201–207. doi: 10.1128/IAI.73.1.201-207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schauer R. Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 199–217. [PubMed] [Google Scholar]
- Yu H, Chokhawala H, Karpel R, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J Am Chem Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- Zamoyska R. Why is there so much CD45 on T cells? Immunity. 2007;27:421–423. doi: 10.1016/j.immuni.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B. The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol Rev. 2003;191:107–118. doi: 10.1034/j.1600-065x.2003.00015.x. [DOI] [PubMed] [Google Scholar]