Abstract
The nucleotide sugar UDP-galactose (UDP-Gal) is essential for the biosynthesis of several abundant glycoconjugates forming the surface glycocalyx of the protozoan parasite Leishmania major. Current data suggest that UDP-Gal could arise de novo by epimerization of UDP-glucose (UDP-Glc) or by a salvage pathway involving phosphorylation of Gal and the action of UDP-glucose:α-d-galactose-1-phosphate uridylyltransferase as described by Leloir. Since both pathways require UDP-Glc, inactivation of the UDP-glucose pyrophosphorylase (UGP) catalyzing activation of glucose-1 phosphate to UDP-Glc was expected to deprive parasites of UDP-Gal required for Leishmania glycocalyx formation. Targeted deletion of the gene encoding UGP, however, only partially affected the synthesis of the Gal-rich phosphoglycans. Moreover, no alteration in the abundant Gal-containing glycoinositolphospholipids was found in the deletion mutant. Consistent with these findings, the virulence of the UGP-deficient mutant was only modestly affected. These data suggest that Leishmania elaborates a UDP-Glc independent salvage pathway for UDP-Gal biosynthesis.
Keywords: nucleotide sugars metabolism, trypanosomatids, UDP-galactose, UDP-glucose
Introduction
Leishmania parasites are responsible for a group of diseases collectively known as Leishmaniases ranging from self-healing ulcerative skin lesions to lethal visceral infections. They alternate between flagellated procyclic promastigotes colonizing the midgut of the sandfly vector, metacylic promastigotes residing in the foregut and transmitted to the mammalian host via a bite and nonflagellated amastigotes proliferating in the macrophage of the mammalian host. The promastigotes are coated with a thick glycocalyx rich in molecules of the glycosylphosphatidylinositol (GPI) family (Figure S1). GPIs are based on the conserved backbone structure Manα1,4GlcNα1,6-phosphatidylinositol and, in Leishmania, anchor proteins such as the proteophosphoglycans (PPGs) or a polysaccharide called lipophosphoglycan (LPG). They can also be free and are then termed glycoinositolphospholipids (GIPLs) (McConville and Ferguson 1993; Ferguson 1999; Guha-Niyogi et al. 2001; Mendonca-Previato et al. 2005). Leishmania glycocalyx is particularly rich in galactose (Gal) since LPG, the most abundant glycoconjugate of promastigotes, and protein-linked phosphoglycans (PGs) are comprised of linear chains of 6Galβ1,4Manα1-P repeating units (Figure S1) (Turco and Descoteaux 1992; Ilg 2000). Moreover, in L. major, Gal residues substitute the backbone structure of LPG, PPGs and GIPLs (McConville et al. 1990; Turco and Descoteaux 1992; Ilg 2000). While in protein-linked PGs only the pyranic form of Gal exists, LPG and GIPLs contain in addition galactofuranose (Galf), an unusual conformer absent from vertebrate species but commonly expressed in eukaryotic and prokaryotic pathogens (McConville et al. 1990; Turco and Descoteaux 1992; Ilg 2000; Bakker et al. 2005; Beverley et al. 2005).
Consistent with the glycocalyx composition, the main nucleotide sugars detected in L. major promastigotes are UDP-glucose (UDP-Glc), UDP-galactose (UDP-Gal), UDP-N-acetylglucosamine and GDP-mannose (Turnock and Ferguson 2007). The pool of UDP-Glc synthesized from the abundant metabolites UTP and glucose-1-phosphate by the UDP-glucose pyrophosphorylase (UGP) (also designated UTP:α-d-glucose-1-phosphate uridylyltransferase) is relatively large and can be use to produce UDP-Gal by epimerization (Lamerz et al. 2006; Turnock and Ferguson 2007) (Figure 1). Moreover, UDP-Gal may be generated by activation of galactose-1-phosphate. Leishmania parasites, in contrast to the trypanosomatids Trypanosoma cruzi and Trypanosoma brucei, indeed can take up Gal from the environment and utilize it for the biosynthesis of glycoconjugates, although the enzymes involved in Gal activation are still unknown (Turco et al. 1984). In most organisms, the UDP-glucose:α-d-galactose-1-phosphate uridylyltransferase that catalyzes synthesis of UDP-Gal from galactose-1-phosphate and UDP-Glc is classically involved in salvage of Gal (Leloir 1951) (Figure 1). This enzyme delineates the Leloir pathway which like the de novo pathway for UDP-Gal biosynthesis depends on UDP-Glc biosynthesis (Figure 1). UDP-Glc was thus expected to be an important metabolite for the biosynthesis of Leishmania glycocalyx.
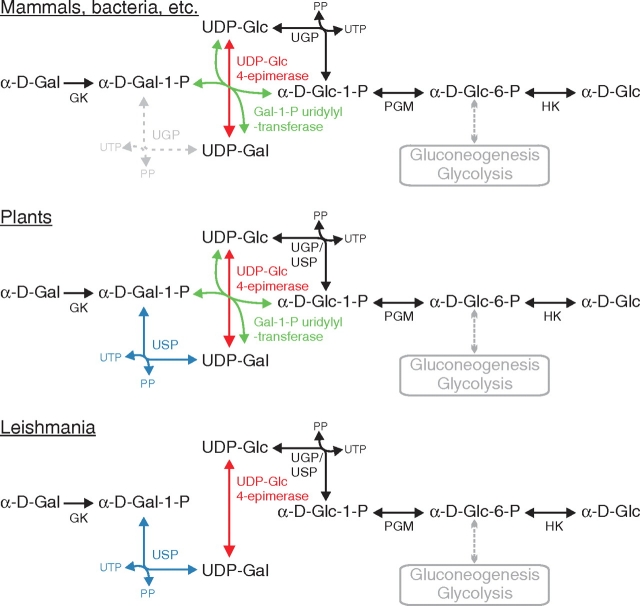
Fig. 1.
Biosynthesis of UDP-α-d-galactose in various organisms. UDP-α-d-galactose (UDP-Gal) is synthesized de novo by epimerization of UDP-α-d-glucose (UDP-Glc) by the UDP-glucose 4-epimerase (UDP-Glc 4-epimerase, EC:5.1.3.2). In addition, α-d-galactose-1-phosphate (α-d-Gal-1-P) produced from α-d-galactose (α-d-Gal) by the galactokinase (GK, EC:2.7.1.6) is activated by the UDP-glucose:α-d-galactose-1-phosphate uridylyltransferase (Gal-1-P uridylyltransferase, EC:2.7.7.12). These reactions depend on UDP-Glc production from α-d-glucose-1-phosphate (α-d-Glc-1-P) by the UTP:α-d-glucose-1-phosphate uridylyltransferase also named UDP-glucose pyrophosphorylase (UGP, EC:2.7.7.9). The phosphoglucomutase (PGM, EC:5.4.2.2) mediating the interconversion of α-d-Glc-1-P and α-d-glucose-6-P (α-d-Glc-6-P) connects the galactose metabolism to gluconeogenesis and glycolysis. α-d-Glc-6-P may also originate from phosphorylation of free glucose (α-d-Glc) by the glucokinase (EC:2.7.1.1) or hexokinase (HK, EC:2.7.1.2). The conversion of α-d-Gal-1-P into UDP-Gal described in mammals by Isselbacher is thought to be due to a weak UTP:α-d-galactose-1-phosphate uridylyltransferase activity (EC:2.7.7.10) of UGP. In plants, a third pathway for UDP-Gal biosynthesis is mediated by an unspecific UDP-sugar pyrophosphorylase (USP, EC:2.7.7.64). The pathways proposed for Leishmania parasites are based on analysis of the genome and the existence of a UDP-glucose independent pathway for UDP-Gal biosynthesis demonstrated in this work. Activation of α-d-Glc-1-P and α-d-Gal-1-P by USP would explain the production of UDP-Glc and UDP-Gal in the L. major ugp− mutant
The relevance of the glycocalyx for Leishmania survival and infectivity was demonstrated by targeted deletion of individual genes involved in the biosynthesis of surface glycoconjugates (Naderer et al. 2004). In particular, the contribution of LPG was unambiguously determined with a mutant exclusively deficient in this polysaccharide generated by targeted gene replacement of the putative galactofuranosyltransferase LPG1 (Späth et al. 2000). In L. major, LPG is clearly essential for survival in the insect vector and promastigote infectivity in the mammalian host but is not required for amastigote survival (Späth et al. 2000; Naderer et al. 2004). In a mouse model of cutaneous leishmaniasis, the LPG1-deficient mutant induces lesion formation after a pronounced delay in the establishment of infection (Späth et al. 2000). Similar delayed lesion appearance was observed with several other LPG-deficient mutants obtained by genetic deletion of, for instance, the UDP-galactopyranose mutase or alkyldihydroxyacetonephosphate synthase involved in UDP-Galf or ether phospholipid biosynthesis, respectively (Zufferey et al. 2003; Kleczka et al. 2007). Besides corroborating the role of LPG in infectivity, the study of these mutants suggested that despite their abundance in amastigotes, GIPLs are not crucial for survival of this parasitic stage (Zufferey et al. 2003; Kleczka et al. 2007). Intriguingly, absence of LPG and other PGs induced by replacement of the LPG2 gene encoding the Golgi GDP-Man transporter resulted in avirulence, whereas a mutant defective in UDP-Gal transport across the Golgi and essentially devoid of PGs only caused a modest delay in lesion appearance (Späth et al. 2003; Capul, Hickerson, et al. 2007). One hypothesis advanced for these findings was the possibility of an undiscovered molecule requiring the LPG2 GDP-Man transporter for its biosynthesis (Capul, Hickerson, et al. 2007).
To interfere with the biosynthesis of galactosylated molecules and eventually shed light on their role in parasite virulence, we targeted UGP in the hope of blocking not only the de novo synthesis of UDP-Gal but also its salvage pathway. Our data demonstrate, however, that the UDP-Gal salvage pathway is independent from UDP-Glc biosynthesis and able to sustain the biosynthesis of most of the glycocalyx.
Results
Targeted replacement of L. major UGP
The full length L. major UGP has been cloned previously and the enzyme partially characterized (Lamerz et al. 2006). L. major genome (Ivens et al. 2005) exhibits a single copy of UGP gene located on chromosome 18 (LmjF18.0990) and does not display any highly homologous gene. Prior to the generation of a null mutant, that was achieved by consecutive replacement of the two UGP alleles with genes encoding the selection markers hygromycin phosphotransferase (HYG) and phleomycin binding protein (BLE), the gene copy number was confirmed by Southern blot analysis of genomic DNA (Figure S2). The successful generation of ugp− mutant was confirmed by Southern blotting (Figure 2). After SacI digest, the UGP gene could be detected in wild type and in the heterozygous mutant but no signal was obtained in the ugp− mutant (Figure 2A). Moreover, integration of the resistance markers into the correct gene locus was demonstrated with a probe hybridizing outside the region used for homologous recombination after BlpI digest (Figure 2B). Multiple and/or random insertions of the resistant markers were excluded by additional Southern blots using probes specific for HYG or BLE (data not shown). Mutant parasites were morphologically identical to the parental strain and grew at similar rates and density under standard culture conditions.
Fig. 2.
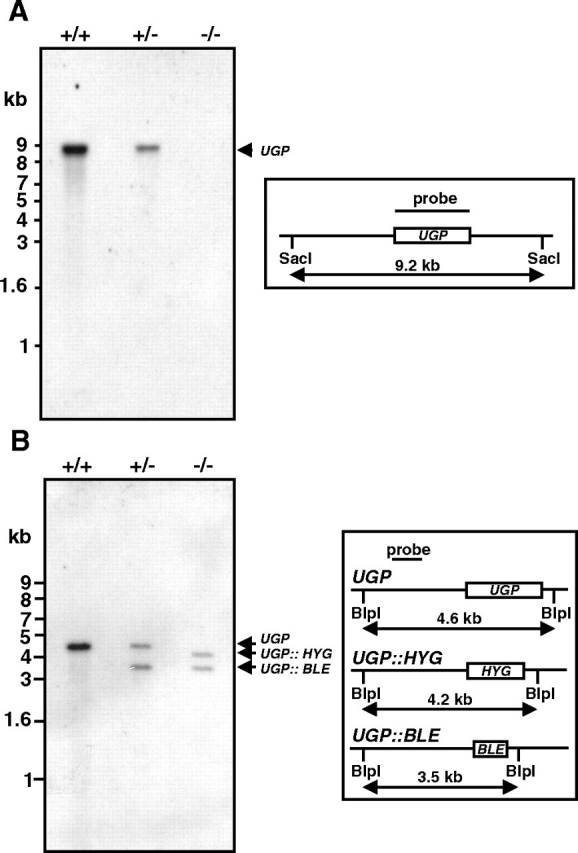
Targeted gene replacements of UGP alleles. Southern blot analysis of genomic DNA from wild type (+-/+-), heterozygous UGP/Δugp::BLE (+-/−) and homozygous ugp− mutant (−/−). DNA digested by SacI (A) or BlpI (B) was separated on agarose gel, transferred to nylon membrane and hybridized with a digoxigenin-labeled UGP probe or a digoxigenin-labeled 5′-flanking probe, respectively. The size of expected fragments is outlined in the right panel
The ugp− mutant exhibits residual UGP activity
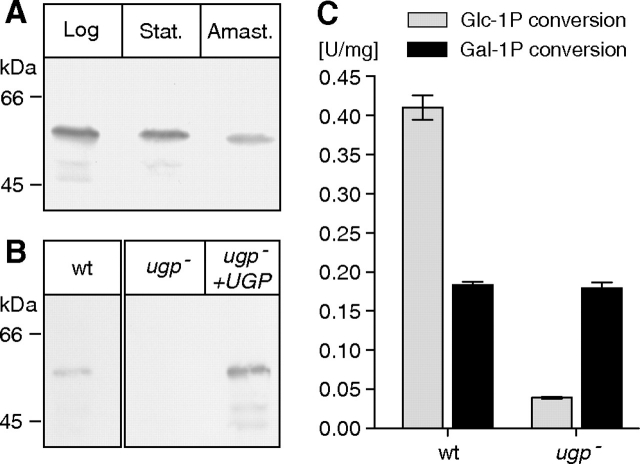
Western blotting of total cell lysates detected with an anti-UGP serum (Lamerz et al. 2006) demonstrates the expression of UGP in the logarithmic and stationary growth phase of promastigotes as well as in amastigotes (Figure 3A). Equal protein loading and transfer efficiency was assessed by reversible staining in Ponceau S-solution (data not shown). This expression through the parasite life cycle is consistent with the need of activated nucleotide sugars for glycoconjugate biosynthesis in both parasitic life stages. The lower amount detected in amastigotes is in agreement with previous reports, indicating lower expression of enzymes involved in the glycolytic pathway at this parasitic stage (McConville et al. 2007; Rosenzweig et al. 2008; Naderer and McConville 2008). As expected, UGP was totally absent from the ugp− mutant and its expression restored by reintroduction of UGP (ugp−/UGP strain) (Figure 3B). Despite the complete absence of UGP (Figure 3B), a residual UDP-Glc pyrophosphorylase activity was detected in the deletion mutant (Figure 3C). UDP-Glc formation from glucose-1-phosphate was measured in total cell lysates by a coupled enzymatic assay measuring reduction of NAD in presence of UDP-Glc dehydrogenase. In wild-type cells, activity of 0.41 U/mg (+-/−0.027 U/mg) was measured whereas the ugp− mutant showed a weak activity of 0.04 U/mg (+-/−0.002 U/mg). This value is clearly above the background level of 4 × 10−4 measured in controls without glucose-1-phosphate or without UDP-Glc dehydrogenase. These data suggest the existence of a second UDP-Glc forming activity in L. major contributing about ∼10% of the total activity. In addition, the conversion of galactose-1-phosphate into UDP-Gal was measured in a similar assay using galactose-1-phosphate as substrate instead of glucose-1-phosphate and containing in addition the UDP-Glc 4-epimerase for conversion of UDP-Gal into UDP-Glc, without the addition of exogenous UDP-Glc. The measured activity was identical in wild type and the UGP-deficient mutant (Figure 3C).
Fig. 3.
L. major ugp− mutant present residual UDP-glucose pyrophosphorylase activity despite absence of UGP. (A and B) Whole cell lysates (12 µg/lane) of logarithmic and stationary phase wild-type promastigotes, wild-type amastigotes isolated from mice lesions and ugp− and ugp−/UGP promastigotes were subjected to SDS–PAGE and western blotting with anti-UGP serum. (C) Conversion of glucose-1-phosphate (gray) or galactose-1-phosphate (black) into UDP-Glc or UDP-Gal measured in total cell lysates from L. major wild type or the ugp− mutant by a coupled enzymatic assay measuring reduction of NAD by the UDP-Glc dehydrogenase. UDP-glucose 4-epimerase converting UDP-Gal into UDP-Glc was added in assays measuring the activation of galactose-1-phosphate. Each value represents the average of three independent experiments in which measurements were carried out in triplicates
Galactosylation is reduced but not eliminated in the ugp− mutant
L. major mutant LPG and protein-linked PGs are made up of linear chains of 6Galβ1,4Manα1-P repeating units, where the 3 position of the Gal may be substituted by side chains rich in Gal and arabinose (Turco and Descoteaux 1992; Ilg 2000). Their synthesis requires thus the availability of UDP-Gal.
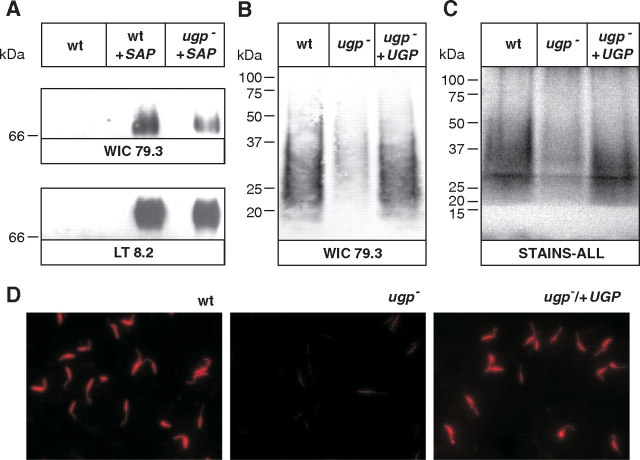
The effect of UGP deletion on protein-linked PGs was addressed first. We used a convenient PG reporter developed previously, expressing a secreted acid phosphatase (SAP) which is extensively phosphoglycosylated (Wiese et al. 1999; Späth et al. 2000). L. mexicana SAP1 was heterologously expressed (Wiese et al. 1999) in wild type and the ugp− mutant. After immunoprecipitation and western blotting with the anti-SAP mAb LT8.2 (Ilg et al. 1993), a specific signal of about 70 kDa could be detected in the stacking gel area indicating phosphoglycosylation of the Ser/Thr-rich repetitive motifs of the protein (Figure 4A, lower panel) (Wiese et al. 1995). The similar size of SAP expressed either in wild type or in the ugp− mutant suggests that the protein is properly phosphoglycosylated in the mutant (Figure 4A, lower panel). Immunoblotting with mAb WIC 79.3, recognizing the Gal side chains decorating the PG backbone (de Ibarra et al. 1982), revealed however a decrease of Gal-modified repeating units in the ugp− mutant (Figure 4A, top panel). These Gal-modified repeating units were estimated to be 70% from the intensity of the signal (Figure 4A, upper panel) after correction for loading (Figure 4A, lower panel). These results suggest that against expectations the ugp− mutant must be able to produce significant levels of UDP-Gal, consistent with the enzymatic data showing significant residual activity in the ugp− mutant.
Fig. 4.
Phosphoglycosylation of reporter secreted acid phosphatase and LPG in the ugp− mutant. (A) SAP was expressed in wild type and ugp− mutant, immunoprecipitated with mAb LT8.2 and subjected to western blot analysis with mAb WIC 79.3 (top panel). Loading was checked using mAb LT8.2 (lower panel). Untransfected wild-type cells served as negative control. (B) Cell extracts of wild type, ugp− and ugp−/UGP parasites were analyzed by western blotting with mAb WIC79.3. (C) Cell extracts of wild type, ugp− and ugp−/UGP parasites were digested with proteinase K and analyzed by western blotting with the Stains-all dye. (D) LPG expression of wild type, ugp− and ugp−/UGP parasites was analyzed by indirect immunofluorescence microscopy. Promastigotes were fixed, permeabilized and stained with mAb WIC79.3
The presence of UDP-Gal in the ugp− mutant was supported by analysis of LPG (Figure 4). Western blotting of LPG in whole cell lysates using the monoclonal antibody WIC 79.3 for detection revealed a strong decrease of Gal-modified repeating units in the ugp− mutant (Figure 4B), estimated to be 15–25% of WT (Figure 4B). By immunofluorescence microscopy, the reactivity of ugp− with WIC79.3 was hardly discernable (Figure 4D). The decrease of WIC79.3 binding may reflect a decrease of Gal side chain addition or/and a decrease in repeat unit number that could be due to a shortening of LPG molecules or/and a decrease of LPG copies number. Analysis of lysates by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) after digestion with DNAse I, RNAse A and proteinase K and visualization with the Stains-all dye supported the latter hypothesis (Figure 4C). Digested lysate from the ugp− mutant exhibits a much fainter signal indicative of reduced carbohydrate content (Figure 4C). Absence of nucleic acids and proteins from the digested lysates was confirmed in parallel by SDS–PAGE stained either with ethidium bromide or Coomassie blue (data not shown). Importantly, the reduction of signal intensity observed in the ugp− mutant with WIC79.3 or Stains-all labeling is comparable (Figure 4B and C). Furthermore in these experiments, the mobility of LPG is similar in all strains suggesting that the length of LPG is not greatly affected by UGP deficiency.
Together the analysis of PGs demonstrates significant, albeit reduced, production of UDP-Gal in the ugp− mutant.
The structural composition of GIPL is not affected in the ugp− mutant
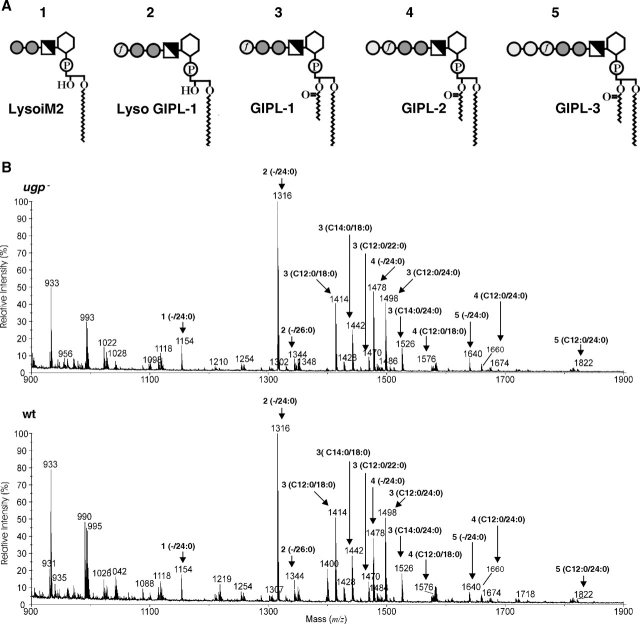
L. major synthesizes three different type-2 GIPLs containing the common glycan core Galfα1-3Manα1-3Manα1-4GlcN-phosphatidylinositol termed GIPL-1 that can be elongated by one (GIPL-2) or two (GIPL-3) terminal Gal residues (Galα1-3Galfα1-3Manα1-3Manα1-4GlcN-phosphatidylinositol and Galα1-6Galα1-3Galfα1-3Manα1-3Manα1-4GlcN-phosphatidylinositol, respectively) (McConville et al. 1990). To highlight eventual structural differences between wild type and ugp− GIPLs, the glycolipids were extracted, purified and analyzed by negative-ion matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (Figure 5). The ions at m/z 1414, 1498 and 1576, 1660 represent GIPL-1 and GIPL-2 species containing C12:0 acyl and C18:0 or C24 alkyl chains, respectively (Kleczka et al. 2007). Ions at m/z 1316, 1478 and 1640 (Figure 5) represent lyso-structures with C24:0 alkyl chains corresponding to each of the three GIPL types. Furthermore, the ions at m/z 1442, 1470 and 1526 represent GIPL-1 structures with C12:0 or C14:0 acyl and C20:0/C22:0/C24:0 alkyl chains, respectively. A very similar spectrum was obtained with the GIPL fraction from the ugp− mutant (Figure 5B) which indicates that the absence of UGP did not result in an increase of truncated GIPLs or precursor structures. Interestingly, the GIPL spectrum from wild-type parasites contained an ion of m/z 1400 that is not present in the spectrum from ugp− mutants (Figure 5). Preliminary data obtained by tandem mass spectrometry suggest that this peak represents a GIPL-1 species with C17:0 alkyl and C12:0 acyl chains (data not shown).
Fig. 5.
GIPLs structures are unaffected in the ugp− mutant. (A) Schematic representation of Leishmania GIPLs. Light shaded circles, Galp; light shaded circles with f, Galf; dark shaded circles, Man; half shaded squares, GlcN; hexagons, myoinositol; and P, phosphate. (B) Negative-ion MALDI spectra of GIPLs isolated from ugp− mutant (top panel) and wild-type (lower panel) parasites. The identities of the major ions are indicated by the schematics in A and can be inferred from the structure of GIPL3, which is Galα1-6Galα1-3Galfβ1-3Manα1-3Manα1-4GlcNα1-6myo-inositol-1-HPO4-3(sn-1-alkyl-2-acylglycerol). The numbers of C atoms and of C═C double bonds in the acyl and alkyl chains, respectively, are indicated in brackets above each peak
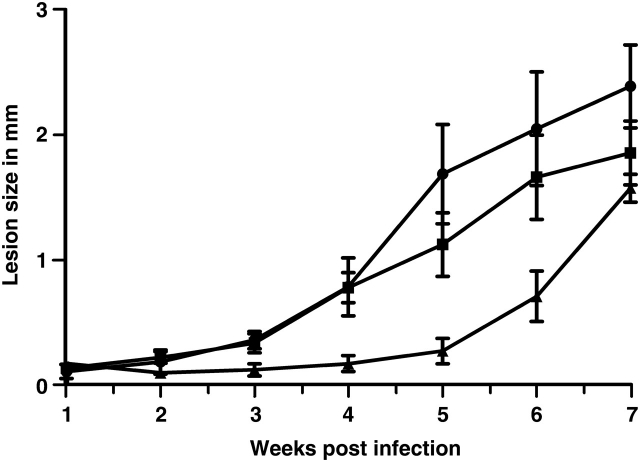
Delayed lesion formation in mice infected with ugp− mutant
The effect of UGP deletion on infectivity was determined by infection with stationary phase promastigotes of susceptible Balb/c mice. Lesion formation of the wild-type strain occurred 3 weeks after inoculation and progressed steadily (Figure 6). In contrast, the ugp− mutant showed a slight delay in lesion formation and swelling occurred 5 weeks after inoculation. Thereafter, the lesions developed as progressively as in mice infected with wild-type cells and the lesions size correlated with parasite burden. As expected, the ugp−/+-UGP cell line induced infections similar to wild type. The slight difference observed might be due to overexpression of UGP in the ugp−/+-UGP (Figure 3B). To exclude contaminants, amastigotes were recovered from infected animals and differentiated back into promastigotes. The identity of the reisolated cell lines was confirmed by western blotting using the anti-UGP serum (data not shown).
Fig. 6.
Delayed lesion formation of mice infected with the ugp− mutant. Female Balb/c mice were inoculated in the footpad with 2 × 106 wild type (square), ugp− mutant (triangle) or ugp−/+-UGP (circle), and lesion formation was measured once a week
Human peritoneal macrophage infections were performed with stationary phase promastigote parasites opsonized with C5-deficient mouse serum. The time course of the infection rate for the wild-type and knockout groups was observed in three independent double blind experiments (Figure 7A). The initial infection rate with wild-type parasites (85%) is slightly higher than the one with ugp− mutant (76%). After 25 h, the mean infection rate falls to 12% for wild type and 8% for the knockout and stays significantly lower with ugp− parasites. After 117 h, the difference in the mean infection rate between the two groups is too small to be significant. Additionally, the number of parasites per 30 macrophages was determined (Figure 7B). The initial uptake into macrophages is approximately two times higher with wild type than ugp− parasites. Within 2 days of infection about 80% and 90% of wild type and ugp− parasites perished, respectively. The data indicate a similar clearance of wild type and ugp− parasites and suggest that the delayed lesion formation observed in mice infected with the ugp− mutant might be due to a lower initial uptake of parasites into macrophages.
Fig. 7.
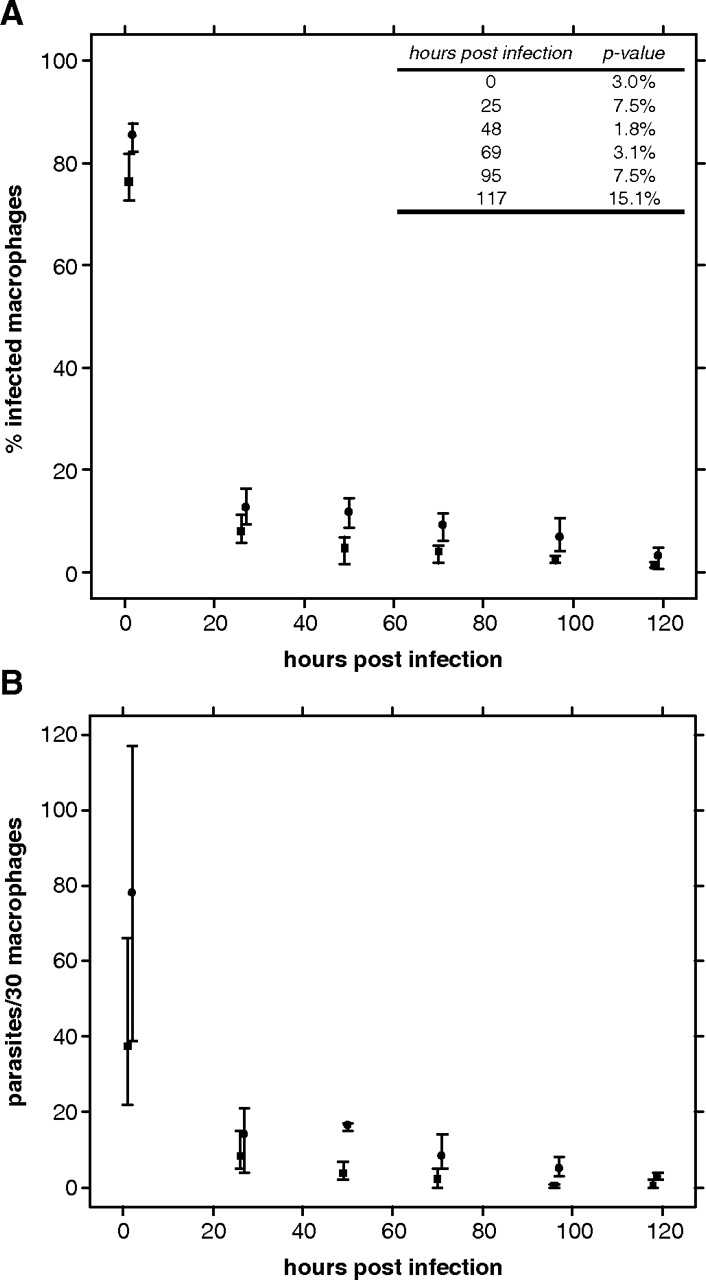
In vitro invasion of human peritoneal macrophages with the ugp− mutant. In vitro macrophage infection with C3-opsonized stationary phase wild type (circle) and ugp− (square) promastigotes. The percentage of infected macrophages and the survival of parasite/30 macrophages as a function of time are shown in (A and B) respectively. The data represent the mean, minimum and maximum from three independent experiments. The inlay in A displays the P-value of the Welch two sample t-test for each time point
Discussion
A salvage pathway for UDP-Gal synthesis is known to occur in Leishmania parasites (Turco et al. 1984). Gal is generally phosphorylated by a galactokinase before being converted to UDP-Gal by the UDP-glucose:α-d-galactose-1-phosphate uridylyltransferase (encoded by GALT) as described by Leloir (Leloir 1951). If the genome of L. major contains a putative galactokinase, no obvious GALT homologue was found. An alternative pathway, initially described by Isselbacher in mammals (Isselbacher 1958; Leslie et al. 2005), has been associated with the weak UDP-Gal pyrophosphorylase activity of UGP (Knop and Hansen 1970; Lai and Elsas 2000). UGP occupies thus a central position in Gal metabolism and was expected to control L. major cell surface molecules biosynthesis and affect virulence as previously observed in several gram-negative and gram-positive.
Surprisingly, targeted gene replacement of UGP in L. major showed only modest effects on the synthesis of several key molecules of the glycocalyx. Whereas the biosynthesis of LPG appears markedly reduced, the influence on protein-linked PGs seemed more limited. These effects were consistent with the partial reduction in UGP activity seen in the ugp− mutant. In contrast, the structure of GIPLs present in the ugp− mutant was totally unaffected. This could arise if enzymes required for GIPL synthesis have a lower Km than those involved in PG synthesis, and thus are less sensitive to partial UDP-Gal levels. Interestingly, deletion of the UDP-Gal transporter LPG5A, which also be expected to limit the amount of UDP-Gal, resulted in a decrease of LPG size and side chain galactosylation (Capul, Barron, et al. 2007). The ugp− phenotype is thus clearly distinct from the phenotype of the L. major lpg5A− mutant. This distinct phenotype potentially could arise through a requirement for UDP-Glc in the synthesis of the Glc-phosphate modification found in the LPG anchor but not protein-linked PGs or GIPLs, in addition to decreased levels of UDP-Gal. Consistent with the limited alteration of its surface glycocalyx components, the L. major ugp− mutant only induced a modest delay in lesion formation in susceptible Balb/c mice. Such delay in lesion emergence was previously observed with various LPG-deficient mutants (Späth et al. 2000; Zufferey et al. 2003; Kleczka et al. 2007; Capul, Hickerson, et al. 2007).
The characterization of the ugp− mutant thus suggests synthesis of a substantial, albeit reduced, UDP-Gal pool in the absence of UGP. Consistent with the observation made in this study, MacRae and collaborators showed that in presence of reduced amount of UDP-Gal due to deletion of one allele of the UDP-Glc 4-epimerase (TcGALE+-/− mutant), T. cruzi preserved its Galf-containing GIPLs, while the galactopyranose-rich mucins were more severely affected (MacRae et al. 2006). These data led to the assumption that GIPLs are of major importance for basic parasite survival in culture. In Leishmania parasites, however, a mutant expressing agalactosylated GIPLs was generated by targeting Galf metabolism and did not display any in vitro growth or morphological anomalies (Kleczka et al. 2007). Similarly, deletion of UGP in L. major did not induce morphological abnormality or growth defect, whereas the T. cruzi UDP-Glc 4-epimerase heterozygote mutant exhibited severe changes in cell surface molecular architecture and aberrant morphology (MacRae et al. 2006). This suggests that the UDP-Gal pool is larger in the L. major ugp− mutant than in the so called TcGALE+-/−. It should be mentioned that in T. cruzi and T. brucei, epimerization of UDP-Glc seems to be the exclusive path for UDP-Gal synthesis since the hexose transporters of these two parasites are unable to transport Gal (Tetaud et al. 1997; Barrett et al. 1998). Consequently, deletion of the UDP-Glc 4-epimerase is lethal in these two trypanosomatids (MacRae et al. 2006; Urbaniak, Turnock, et al. 2006), which makes the enzyme an attractive drug target (Urbaniak, Tabudravu, et al. 2006). While we had expected in these studies to be a similar test of the importance of UDP-Gal in Leishmania, our data showed surprisingly that Leishmania has another pathway of UDP-Gal synthesis bypassing the de novo and Leloir salvage pathways.
A remaining question is how is UDP-Gal synthesized in the ugp− mutant? Intriguingly, the deletion mutant still exhibited a 10% UGP residual activity. In yeast, a greater than 95% reduction in UGP activity obtained in a UGP antisense mutant did not lead to any obvious phenotype (Daran et al. 1997), although UGP activity is essential for survival of this organism (Daran et al. 1995). The 10% residual enzymatic activity for UDP-Glc synthesis detected in the ugp− mutant might thus be sufficient to maintain the biosynthesis of most surface glycoconjugates. Since absence of the UGP gene and protein was confirmed in the ugp− mutant, however, this residual activity can be clearly attributed to a different enzyme. Recently, it has become apparent that several organisms may contain isozymes of UGP encoded by different genes. For instance, deletion of a gene encoding UGP (udpgp1) in Dictyostelium discodeum pointed toward the importance of a second UGP involved in differentiation and development of the slime mold (Bishop et al. 2002). Plants also often express different UGPs (Chen et al. 2007; Meng et al. 2007; Meng et al. 2008). Arabidopsis, for instance, contains homologous UGP genes encoding two enzymes located in the cytoplasm and a chloroplastic UGP involved in sulfolipid biosynthesis (Meng et al. 2008; Okazaki et al. 2009). However, the genome of Leishmania major does not contain any close UGP homologues. Conversely, a leishmanial homologue of the recently described plant UDP-sugar pyrophosphorylase (USP) was found and characterized in L. major (Damerow et al. 2010). USP is an enzyme that can nonspecifically utilize UTP and glucose-1-phosphate or galactose-1-phosphate to produce UDP-Glc or UDP-Gal and pyrophosphate (Kotake et al. 2004; Litterer et al. 2006; Kotake et al. 2007; Damerow et al. 2010). Such an enzyme would be able to fuel the UDP-Gal pool by direct activation of galactose-1-phosphate and be responsible for the limited UDP-Glc pyrophosphorylase activity observed in the ugp− mutant. Deletion of the two Arabidopsis cytoplasmic UGPs had no effect on cell wall composition and resulted in a 15–25% residual activity. This outcome was at least partially due to USP overexpression (Meng et al. 2009). In Leishmania, however, such compensation mechanism does not seem to take place since the galactose-1-phosphate pyrophosphorylase activity is not increased in the ugp− mutant. Altogether, this work demonstrates that the UDP-Gal salvage pathway of Leishmania does not proceed via the Leloir pathway and is able to contribute significantly to the biosynthesis of the glycocalyx.
Materials and methods
Parasite culture and transfection
L. major MHOM/SU/73/5ASKH was grown at 27°C in M199 media (Invitrogen, Karlsruhe, Germany) containing 10% fetal calf serum (FCS), 40 mM Hepes pH 7.5, 0.1 mM adenine, 0.0005% hemin, 0.0002% biotin and 50 U/mL penicillin/streptomycin. Parasites were transfected by electroporation (Robinson and Beverley 2003) and allowed to grow in 1× M199 medium for 24 h before transferring to semi-solid media containing 1% Noble agar (Becton Dickinson, Heidelberg, Germany) and appropriate antibiotics. Individual colonies were picked and grown in selective M199 liquid media. The antibiotics phleomycin, hygromycin B and puromycin were obtained from InvivoGen (Toulouse, France) and G418 from Sigma (Munich, Germany).
Generation of L. major UGP deletion mutants and add back lines
For gene replacement by homologous recombination, the resistance markers hygromycin B phosphotransferase (HYG) and the phleomycin binding protein (PHLEO) were cloned between the 5′ and 3′ regions directly flanking the UGP gene. Therefore, sequences 1.5 kb upstream and downstream of the ugp locus were amplified by polymerase chain reaction (PCR) from genomic DNA using the primers CTG ATC TAG AAA CGA AGA CGA GCT ACA GCG CAT G / TAA AGG ATC CCC ATG GCT TCA CCT CCG TGA CAG C and GAA AGG ATC CGC TAG CTA GGG GTC ACA AGC TGC TGA / ATA CGG TAC CCC GCC GTC ATC TGT CGA TTG CAC AC, respectively, and ligated into the XbaI, BamHI and BamHI and KpnI restriction sites of the pcDNA3 vector, respectively. The above primers contained additional BspHI and NheI restriction sites at the 3′ and 5′ ends of the 5′ and 3′ flanking regions, respectively, which allowed cloning of the selection markers amplified from the vectors pCR2.1hyg and pCR2.1phleo (M. Wiese, unpublished data) between the flanking regions. The resulting UGP::HYG and UGP::PHLEO targeting constructs were digested with BsaAI and the corresponding fragments purified by gel extraction and subsequent ethanol precipitation. The deletion mutant was generated by two consecutive rounds of homologous recombination using the UGP::PHLEO fragment in the first and the UGP::HYG in the second round. Southern blotting techniques were used to confirm the precise gene replacement. The obtained homozygous mutant was named ugp−.
For episomal expression of UGP in the ugp− background, the construct pXG-UGP was transfected into several clones, referred to as ugp−/+-UGP. The plasmid was generated by PCR amplification of the UGP open reading frame with the primer pair AGT ACC CGG GAT GGA AAA CGA CAT GAA GTC C / AGT AGG ATC CCT ACT TGT TGG TCG ACT GCT G. After XmaI/BamHI restriction digest, the fragment was ligated into pXG-PAC (Freedman and Beverley 1993).
In vitro determination of UGP activity
For the in vitro testing of UGP activity, lysates obtained from promastigotes were assessed by a coupled enzymatic assay. The assay which measures the forward reaction of the enzyme has been previously described in detail (Lamerz et al. 2006). Whole cell lysates were used as enzyme source. Galactose-1-phosphate conversion was tested in a preparation supplemented with 2 mM NAD, 2 mM galactose-1-phosphate, 1 mM UTP, 0.12 units/mL UDP-Glc 4-epimerase (Streptococcus thermophilus, Calbiochem, Darmstadt, Germany) and 0.08 U/mL UDP-Glc dehydrogenase (bovine liver, Calbiochem) similar to the procedure previously described (Lamerz et al. 2006).
Western blot analysis
Whole cell lysates from exponentially growing and stationary phase L. major promastigote cultures as well as from amastigotes isolated from mice were separated by SDS–PAGE and transferred onto nitrocellulose membranes (Whatman Schleicher & Schüll, Dassel, Germany). Protein concentration was measured in triplicate by Bradford protein assay (Biorad, Munich, Germany) to ensure equal loading and checked by reversible Ponceau S staining. L. major UGP was detected using a 1:60,000 dilution of a recently prepared antiserum (Lamerz et al. 2006) and AP-conjugated goat-antirabbit antibody (1:2000, Dianova, Hamburg, Germany).
For LPG analyses, L. major cell lysates were separated on a 12% gel by SDS–PAGE, transferred onto polyvinylidene difluoride (Millipore) membranes and detected on infrared Li-Cor Odyssey Imager after incubation with WIC79.3 ascites fluid (de Ibarra et al. 1982) and goat antimouse IgG IR Dye 800 CW (Li-Cor Biosciences) at dilutions of 1:4000 and 1:20,000, respectively. In addition, in-gel staining with Stains-all (Sigma) was performed according to Bahr et al. (1993). The cell lysates were previously digested with a mixture of DNase I (0.5 mg mL−1, Roche)/RNase A (1 mg mL−1, Qiagen, Hilden, Germany) for 2 h at 37°C and subsequently treated with proteinase K (0.5 mg mL-1, Merck, Darmstadt, Germany) for 2 h at 55°C. Probes corresponding to 1 × 108 cells (0.6 mg proteins) were evaporated to dryness, dissolved in 10 µL SDS–PAGE sample buffer and subjected to SDS–PAGE before staining with Stains-all (Bahr et al. 1993). Absence of nucleic acids and proteins was verified on parallel blots stained with ethidium bromide or Coomassie blue.
Expression and analysis of L. mexicana SAP
Proteophosphoglycosylation was analyzed by heterologous expression of the L. mexicana SAP1 (Wiese et al. 1999). Therefore, the plasmid pXG-Lmex-SAP1 (Späth et al. 2000) was transfected into wild type and ugp− L. major cells. Recombinant proteins were immunoprecipitated from the cell culture supernatants with the anti-SAP mAb LT8.2 (Ilg et al. 1993). Both expression of SAP and phosphoglycosylation were monitored by western blotting using the mAbs LT8.2 and WIC 79.3 and displayed with the Super Signal West Femto ECL substrate (Pierce, Bonn, Germany).
GIPL analysis
GIPLs were extracted in chloroform/methanol/water (1:2:0.8), purified over a C18/SepPak® Plus column (Waters) and dried under a stream of nitrogen as described previously (Kleczka et al. 2007). MALDI-TOF-MS analyses of lipid extracts were performed in the negative-ion mode with delayed extraction on a Voyager DE STR time-of-flight mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a 337 nm nitrogen laser. Analyses were performed in reflector mode over the m/z range 800–3000 with an accelerating voltage of 20 kV and a delay of 300 ns. The instrument was externally calibrated. A low-mass gate value of m/z 500 was selected to avoid saturation of the detector. α-Cyano-4-hydroxycinnamic acid (10 µg µL−1 in 60% ACN–0.1% TFA) was used as a matrix. Final mass spectra represented an average of 5–10 spectra, each of which is acquired from 200 laser shots. For structural assignment of GIPLs, linear ion trap (LIT) MSn spectra (n = 2, 3, 4) were obtained as previously described (AA Capul, F-F Hsu and SM Beverley, unpublished; AA Capul, PhD thesis, Washington University 2005). Briefly, [M–H]− ions were generated by electrospray ionization (ESI) and subjected to low energy CAD on a Thermo Finnigan (San Jose, CA) LTQ LIT mass spectrometer (MS) operated with Xcalibur software. Methanolic GIPL solutions were continuously infused into the ESI source with a syringe pump at a flow rate of 2 μL/min. The automatic gain control of the ion trap was set to 5 × 104 and the maximum injection time 100 ms. Helium was used as buffer and collision gas at a pressure of 1 × 10−3 mbar (0.75 mTorr). The relative collision energy ranged from 20 to 30%, and an activation time of 30 ms and an activation q value of 0.25 were used, which resulted in a residual precursor ion abundance of about 20%. The mass resolution of the instrument was tuned to 0.6 Da at half peak height.
Infection of human peritoneal macrophages
Human peritoneal monocytes were isolated as previously described (van Zandbergen et al. 2002). Briefly, freshly isolated human buffy coats were diluted and monocytes were isolated by Histopaque 1077 (Sigma) gradient centrifugation. Collected cells were subjected to magnetic cell sorting using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Isolated monocytes were cultured in Roswell Park Memorial Institute (RPMI) 1660 media supplemented with 10% FCS, L-glutamin and human macrophage colony stimulating factor (Tebu, Offenbach, Germany) for 7 days. Before use in infection studies, parasites were 30 min incubated with 4% complement factor 5-deficient human serum (C5-deficient serum; Sigma) in RPMI. Parasites were then allowed to invade macrophages for 2 h at 37°C in a parasite to macrophage ratio of 10:1. Measured infection rates were normalized to values obtained with wild-type L. major after a 2-h infection step.
Mouse infection
Promastigotes passed through BALB/c mice (Charles River, Sulzfeld, Germany) were grown to stationary phase, and 2 × 106 parasites were injected subcutaneously into the footpad of female Balb/c mice (Charles River). Each experimental group consisted of five individuals. Lesion formation was monitored once a week by measuring the infected and the noninfected footpad using a Vernier caliper. The median size difference (+-−MAD) of the infected and noninfected footpad was plotted against the weeks postinfection. Mice were sacrificed when necrosis appeared in the group, and lesion-derived parasites were enumerated in limiting dilution assays. In addition, amastigotes were isolated from lesions and either used directly or after differentiation into promastigotes used for further analyses.
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported by the impact-based institutional funding (Leistungsorientiere Mittelvergabe, LOM) established at Medical School Hannover, the Deutsche Forschungsgemeinschaft (DFG) via the Junior Research Group “Glycomics” established under the roof of the DFG cluster of Excellence REBIRTH. Support from the National Institute of Diabetes and Digestive and Kidney Diseases (grant number P30DK056341 to F.-F.H. and J.T.), National Institutes of Health (grant number AI31078 to S.M.B.) and United States Public Health Service (grant numbers P41-RR00954, P60-DK20579 and P30-DK56341 to J.T.) is acknowledged.
Acknowledgments
We thank Rita Gerardy-Schahn for her continuous support, Phillip Key for assistance with MS samples and John LeBowitz for technical advices.
Abbreviations
- ESI
electrospray ionization
- FCS
fetal calf serum
- Gal
galactose
- Galf
galactofuranose
- Glc
glucose
- GlcN
glucosamine
- MALDI-TOF-MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- Man
Mannose
- MS
mass spectrometer
- GIPL
glycoinositolphospholipid
- GPI
glycosylphosphatidylinositol
- LPG
lipophosphoglycan
- PCR
polymerase chain reaction
- PG
phosphoglycan
- PPG
proteophosphoglycan
- RPMI
Roswell Park Memorial Institute
- SAP
secreted acid phosphatase
- SDS–PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- UGP
UDP-glucose pyrophosphorylase
- USP
UDP-sugar pyrophosphorylase
Conflict of interest statement
None declared.
References
- Bahr V, Stierhof YD, Ilg T, Demar M, Quinten M, Overath P. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993;58(1):107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- Bakker H, Kleczka B, Gerardy-Schahn R, Routier FH. Identification and partial characterization of two eukaryotic UDP-galactopyranose mutases. Biol Chem. 2005;386(7):657–661. doi: 10.1515/BC.2005.076. [DOI] [PubMed] [Google Scholar]
- Barrett MP, Tetaud E, Seyfang A, Bringaud F, Baltz T. Trypanosome glucose transporters. Mol Biochem Parasitol. 1998;91(1):195–205. doi: 10.1016/s0166-6851(97)00192-8. [DOI] [PubMed] [Google Scholar]
- Beverley SM, Owens KL, Showalter M, Griffith CL, Doering TL, Jones VC, McNeil MR. Eukaryotic UDP-galactopyranose mutase (GLF gene) in microbial and metazoal pathogens. Eukaryot Cell. 2005;4(6):1147–1154. doi: 10.1128/EC.4.6.1147-1154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JD, Moon BC, Harrow F, Ratner D, Gomer RH, Dottin RP, Brazill DT. A second UDP-glucose pyrophosphorylase is required for differentiation and development in Dictyostelium discoideum. J Biol Chem. 2002;277(36):32430–32437. doi: 10.1074/jbc.M204245200. [DOI] [PubMed] [Google Scholar]
- Capul AA, Barron T, Dobson DE, Turco SJ, Beverley SM. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J Biol Chem. 2007;282(19):14006–14017. doi: 10.1074/jbc.M610869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capul AA, Hickerson S, Barron T, Turco SJ, Beverley SM. Comparisons of mutants lacking the Golgi UDP-galactose or GDP-mannose transporters establish that phosphoglycans are important for promastigote but not amastigote virulence in Leishmania major. Infect Immun. 2007;75(9):4629–4637. doi: 10.1128/IAI.00735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Zhao X, Shao Z, Wei Z, Wang YY, Zhu LL, Zhao J, Sun MX, He RF, He GC. Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell. 2007;19(3):847–861. doi: 10.1105/tpc.106.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerow S, Lamerz AC, Haselhorst T, Fuhring J, Zarnovican P, von Itzstein M, Routier FH. Leishmania UDP-sugar pyrophosphorylase: The missing link in galactose salvage? J Biol Chem. 2010;285(2):878–887. doi: 10.1074/jbc.M109.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daran JM, Bell W, Francois J. Physiological and morphological effects of genetic alterations leading to a reduced synthesis of UDP-glucose in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1997;153(1):89–96. doi: 10.1111/j.1574-6968.1997.tb10468.x. [DOI] [PubMed] [Google Scholar]
- Daran JM, Dallies N, Thinessempoux D, Paquet V, Francois J. Genetic and biochemical-characterization of the UGP1 gene Encoding the UDP-glucose pyrophosphorylase from Saccharomyces cerevisiae. Eur J Biochem. 1995;233(2):520–530. doi: 10.1111/j.1432-1033.1995.520_2.x. [DOI] [PubMed] [Google Scholar]
- de Ibarra AA, Howard JG, Snary D. Monoclonal antibodies to Leishmania tropica major: Specificities and antigen location. Parasitology. 1982;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112(Pt 17):2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Beverley SM. Two more independent selectable markers for stable transfection of Leishmania. Mol Biochem Parasitol. 1993;62(1):37–44. doi: 10.1016/0166-6851(93)90175-w. [DOI] [PubMed] [Google Scholar]
- Guha-Niyogi A, Sullivan DR, Turco SJ. Glycoconjugate structures of parasitic protozoa. Glycobiology. 2001;11(4):45R–59R. doi: 10.1093/glycob/11.4.45r. [DOI] [PubMed] [Google Scholar]
- Ilg T. Proteophosphoglycans of Leishmania. Parasitol Today. 2000;16(11):489–497. doi: 10.1016/s0169-4758(00)01791-9. [DOI] [PubMed] [Google Scholar]
- Ilg T, Harbecke D, Wiese M, Overath P. Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur J Biochem. 1993;217(2):603–615. doi: 10.1111/j.1432-1033.1993.tb18283.x. [DOI] [PubMed] [Google Scholar]
- Isselbacher KJ. A mammalian uridinediphosphate galactose pyrophosphorylase. J Biol Chem. 1958;232(1):429–444. [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309(5733):436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczka B, Lamerz AC, van Zandbergen G, Wenzel A, Gerardy-Schahn R, Wiese M, Routier FH. Targeted gene deletion of Leishmania major UDP-galactopyranose mutase leads to attenuated virulence. J Biol Chem. 2007;282(14):10498–10505. doi: 10.1074/jbc.M700023200. [DOI] [PubMed] [Google Scholar]
- Knop JK, Hansen RG. Uridine diphosphate glucose pyrophosphorylase. IV. Crystallization and properties of the enzyme from human liver. J Biol Chem. 1970;245(10):2499–2504. [PubMed] [Google Scholar]
- Kotake T, Hojo S, Yamaguchi D, Aohara T, Konishi T, Tsumuraya Y. Properties and physiological functions of UDP-sugar pyrophosphorylase in Arabidopsis. Biosci Biotechnol Biochem. 2007;71(3):761–771. doi: 10.1271/bbb.60605. [DOI] [PubMed] [Google Scholar]
- Kotake T, Yamaguchi D, Ohzono H, Hojo S, Kaneko S, Ishida HK, Tsumuraya Y. UDP-sugar pyrophosphorylase with broad substrate specificity toward various monosaccharide 1-phosphates from pea sprouts. J Biol Chem. 2004;279(44):45728–45736. doi: 10.1074/jbc.M408716200. [DOI] [PubMed] [Google Scholar]
- Lai K, Elsas LJ. Overexpression of human UDP-glucose pyrophosphorylase rescues galactose-1-phosphate uridyltransferase-deficient yeast. Biochem Biophys Res Commun. 2000;271(2):392–400. doi: 10.1006/bbrc.2000.2629. [DOI] [PubMed] [Google Scholar]
- Lamerz AC, Haselhorst T, Bergfeld AK, von Itzstein M, Gerardy-Schahn R. Molecular cloning of the Leishmania major UDP-glucose pyrophosphorylase, functional characterization, and ligand binding analyses using NMR spectroscopy. J Biol Chem. 2006;281(24):16314–16322. doi: 10.1074/jbc.M600076200. [DOI] [PubMed] [Google Scholar]
- Leloir LF. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem. 1951;33(2):186–190. doi: 10.1016/0003-9861(51)90096-3. [DOI] [PubMed] [Google Scholar]
- Leslie N, Yager C, Reynolds R, Segal S. UDP-galactose pyrophosphorylase in mice with galactose-1-phosphate uridyltransferase deficiency. Mol Genet Metab. 2005;85(1):21–27. doi: 10.1016/j.ymgme.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Litterer LA, Schnurr JA, Plaisance KL, Storey KK, Gronwald JW, Somers DA. Characterization and expression of Arabidopsis UDP-sugar pyrophosphorylase. Plant Physiol Biochem. 2006;44(4):171–180. doi: 10.1016/j.plaphy.2006.04.004. [DOI] [PubMed] [Google Scholar]
- MacRae JI, Obado SO, Turnock DC, Roper JR, Kierans M, Kelly JM, Ferguson MAJ. The suppression of galactose metabolism in Trypanosoma cruzi epimastigotes causes changes in cell surface molecular architecture and cell morphology. Mol Biochem Parasitol. 2006;147(1):126–136. doi: 10.1016/j.molbiopara.2006.02.011. [DOI] [PubMed] [Google Scholar]
- McConville MJ, de Souza D, Saunders E, Likic VA, Naderer T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23(8):368–375. doi: 10.1016/j.pt.2007.06.009. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Ferguson MA. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Homans SW, Thomas-Oates JE, Dell A, Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990;265(13):7385–7394. [PubMed] [Google Scholar]
- Mendonca-Previato L, Todeschini AR, Heise N, Previato JO. Protozoan parasite-specific carbohydrate structures. Curr Opin Struct Biol. 2005;15(5):499–505. doi: 10.1016/j.sbi.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Meng M, Geisler M, Johansson H, Harholt J, Scheller HV, Mellerowicz EJ, Kleczkowski LA. UDP-glucose pyrophosphorylase is not rate limiting, but is essential in Arabidopsis. Plant Cell Physiol. 2009;50(5):998–1011. doi: 10.1093/pcp/pcp052. [DOI] [PubMed] [Google Scholar]
- Meng M, Geisler M, Johansson H, Mellerowicz EJ, Karpinski S, Kleczkowski LA. Differential tissue/organ-dependent expression of two sucrose- and cold-responsive genes for UDP-glucose pyrophosphorylase in Populus. Gene. 2007;389(2):186–195. doi: 10.1016/j.gene.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Meng M, Wilczynska M, Kleczkowski LA. Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from Arabidopsis. Biochim Biophys Acta. 2008;1784(6):967–972. doi: 10.1016/j.bbapap.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Naderer T, McConville MJ. The Leishmania-macrophage interaction: A metabolic perspective. Cell Microbiol. 2008;10(2):301–308. doi: 10.1111/j.1462-5822.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- Naderer T, Vince JE, McConville MJ. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr Mol Med. 2004;4(6):649–665. doi: 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Shimojima M, Sawada Y, Toyooka K, Narisawa T, Mochida K, Tanaka H, Matsuda F, Hirai A, Hirai MY, et al. A chloroplastic UDP-glucose pyrophosphorylase from Arabidopsis is the committed enzyme for the first step of sulfolipid biosynthesis. Plant Cell. 2009;21(3):892–909. doi: 10.1105/tpc.108.063925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128(2):217–228. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Rosenzweig D, Smith D, Opperdoes F, Stern S, Olafson RW, Zilberstein D. Retooling Leishmania metabolism: From sand fly gut to human macrophage. FASEB J. 2008;22(2):590–602. doi: 10.1096/fj.07-9254com. [DOI] [PubMed] [Google Scholar]
- Späth GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 2000;97(16):9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späth GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003;301(5637):1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- Tetaud E, Barrett MP, Bringaud F, Baltz T. Kinetoplastid glucose transporters. Biochem J. 1997;325(Pt 3):569–580. doi: 10.1042/bj3250569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Turco SJ, Wilkerson MA, Clawson DR. Expression of an unusual acidic glycoconjugate in Leishmania donovani. J Biol Chem. 1984;259(6):3883–3889. [PubMed] [Google Scholar]
- Turnock DC, Ferguson MAJ. Sugar nucleotide pools of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Eukaryot Cell. 2007;6(8):1450–1463. doi: 10.1128/EC.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak MD, Tabudravu JN, Msaki A, Matera KM, Brenk R, Jaspars M, Ferguson MA. Identification of novel inhibitors of UDP-Glc 4′-epimerase, a validated drug target for African sleeping sickness. Bioorg Med Chem Lett. 2006;16(22):5744–5747. doi: 10.1016/j.bmcl.2006.08.091. [DOI] [PubMed] [Google Scholar]
- Urbaniak MD, Turnock DC, Ferguson MAJ. Galactose starvation in a bloodstream form Trypanosoma brucei UDP-glucose 4′-epimerase conditional null mutant. Eukaryot Cell. 2006;5(11):1906–1913. doi: 10.1128/EC.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zandbergen G, Hermann N, Laufs H, Solbach W, Laskay T. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect Immun. 2002;70(8):4177–4184. doi: 10.1128/IAI.70.8.4177-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M, Gorcke I, Overath P. Expression and species-specific glycosylation of Leishmania mexicana secreted acid phosphatase in Leishmania major. Mol Biochem Parasitol. 1999;102(2):325–329. doi: 10.1016/s0166-6851(99)00095-x. [DOI] [PubMed] [Google Scholar]
- Wiese M, Ilg T, Lottspeich F, Overath P. Ser/Thr-rich repetitive motifs as targets for phosphoglycan modifications in Leishmania mexicana secreted acid phosphatase. EMBO J. 1995;14(6):1067–1074. doi: 10.1002/j.1460-2075.1995.tb07089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, Smith DF, Turco SJ, Ferguson MA, Beverley SM. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278(45):44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]