Abstract
One-pot three-component coupling of o-alkynylheteroaryl carbonyl derivatives with Fischer carbene complexes and dienophiles leading to the synthesis of quinoxaline and phenazine ring systems has been investigated. This involves the generation of furo[3,4-b]pyrazine and furo[3,4-b]quinoxaline as transient intermediates, which were trapped with Diels–Alder dienophiles. This is the first report on furo[3,4-b]pyrazine intermediates.
Keywords: azaisobenzofuran, Diels–Alder, Fischer carbene complex, phenazine, quinoxaline
Introduction
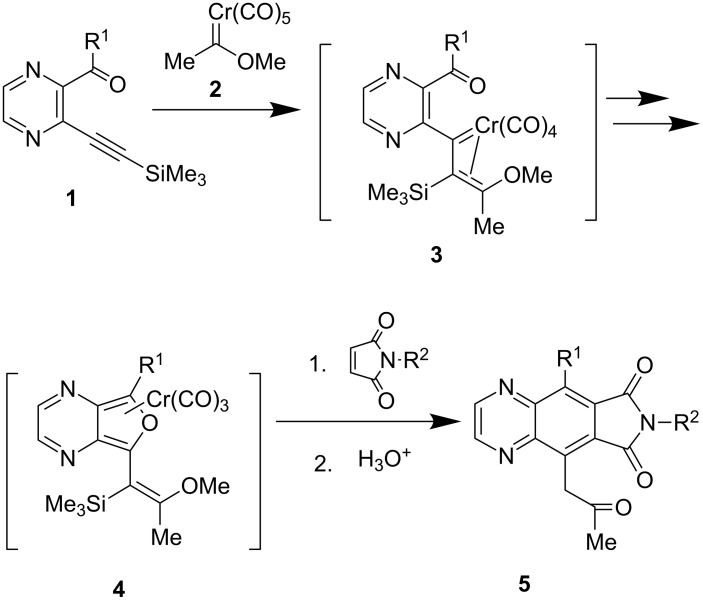
Nitrogen-containing heterocycles are abundant in nature and exhibit diverse and important biological properties [1]. Quinoxaline and phenazine derivatives are important classes of nitrogen containing heterocycles which exhibit a wide range of biological activities. Many phenazine compounds are found in nature and are produced by bacteria such as Pseudomonas spp., Streptomyces spp. and Pantoea agglomerans. These phenazine natural products have been implicated in the virulence and competitive fitness of the parent organisms [2–3]. These compounds show diverse biological activities such as antibacterial, antifungal, antiviral and antitumor properties [4–8]. While rarely found in nature, quinoxalines are well known in the pharmaceutical industry and have been shown to possess a broad spectrum of biological activity including antiviral and antibacterial properties and also act as kinase inhibitors [9–11]. These heterocyclic ring systems are most commonly assembled by the annulation of a heterocyclic ring onto a pre-existing benzene ring [12–21]. A less common approach to these ring systems is the annulation of benzene rings onto pre-existing heterocyclic rings [22]. This manuscript focuses on the successful execution of the latter transformation through a multicomponent reaction process to access these ring systems (Scheme 1). The synthetic approach involves a simultaneous one-pot construction of quinoxaline or phenazine rings which occurs in conjunction with the tandem generation and trapping of an azaisobenzofuran intermediate [23–26]. The synthesis of quinoxaline ring systems involves the coupling of Fischer carbene complexes [27–32] with 2-alkynyl-3-pyrazine carbonyl derivatives, followed by the generation of a hitherto unknown intermediate e.g. furo[3,4-b]pyrazine 4 and trapping of the latter with dienophiles. Phenazine derivatives can be synthesized using similar methodology from the coupling of 2-alkynyl-3-quinoxaline carbonyl derivative through the generation and trapping of furo[3,4-b]quinoxaline intermediates [22].
Scheme 1.
Synthetic plan towards quinoxaline derivatives.
Results and Discussion
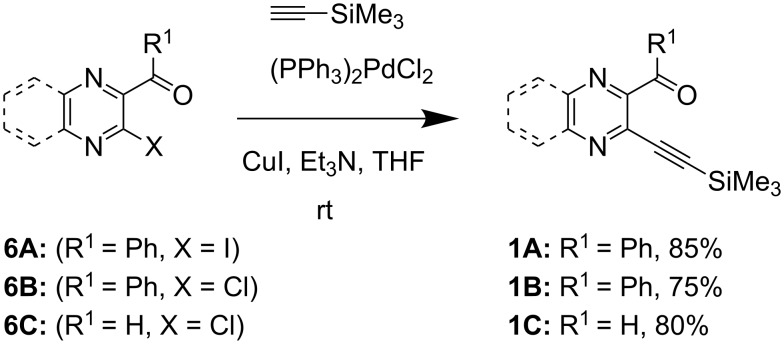
Our investigation commenced with the synthesis of o-alkynyl carbonyl derivatives 1, which were prepared in good yield from the iodoketone 6A or chloroketone 6B [33] or chloroaldehyde 6C [34] using palladium catalyzed Sonogashira coupling reactions as depicted in Scheme 2. Iodoketone 6A was prepared in 80% yield from (3-chloro-2-pyrazinyl)phenylmethanone [35] by halogen exchange with NaI in acetonitrile.
Scheme 2.
Preparation of o-alkynyl carbonyl derivatives 1. A: pyrazine series; B,C: quinoxaline series.
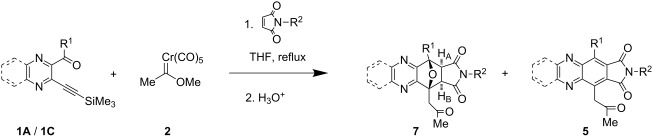
The three component coupling reaction of pyrazinyl ketone 1A, carbene complex 2 and N-phenylmaleimide (~ 1:1:1 ratio) in refluxing THF was initially investigated (Table 1, entry 1). This reaction led to a mixture of oxanorbornene derivative 7a and quinoxaline derivative 5a through the tandem generation and trapping of the furo[3,4-b]pyrazine intermediate 4 (R1 = Ph). Ring opening followed by extrusion of water by treatment of 7a with DBU in refluxing toluene, gave the quinoxaline derivative 5a [36]. The stereochemistry of the adduct 7a was assigned as exo based on the chemical shift of HA and HB (<4 ppm) [27]. A similar reaction process using N-methylmaleimide (entry 2) as dienophile led to the quinoxaline derivative 5b as the sole product after exposure to mild acid.
Table 1.
Synthesis of quinoxaline and phenazine derivatives.
 | |||||
| Entry | Carbonyl compounds | R1 | R2 | Products (yielda) | |
| 1 | 1A | Ph | Ph | 7a (42%) | 5a (30%) |
| 2 | 1A | Ph | Me | - | 5b (55%) |
| 3 | 1C | H | Ph | 7c (10%)b | 5c (52%) |
| 4 | 1C | H | Me | - | 5d (55%) |
aIsolated yield.
bContaminated with 5c.
The three component coupling reaction of o-alkynyl quinoxaline carbonyl derivative 1C, carbene complex 2 and N-phenylmaleimide/N-methylmaleimide was also examined (Table 1, entry 3 & 4). In these cases, tandem generation and trapping of the desired furo[3,4-b]quinoxaline intermediates proceeded smoothly to give the corresponding hetero-polyaromatic phenazine derivatives 5c/5d. Although the [4 + 2] oxa-bridged adduct 7c was isolated, but it was contaminated with 5c since 7c readily converts to 5c in chloroform at room temperature (Table 1, entry 3).
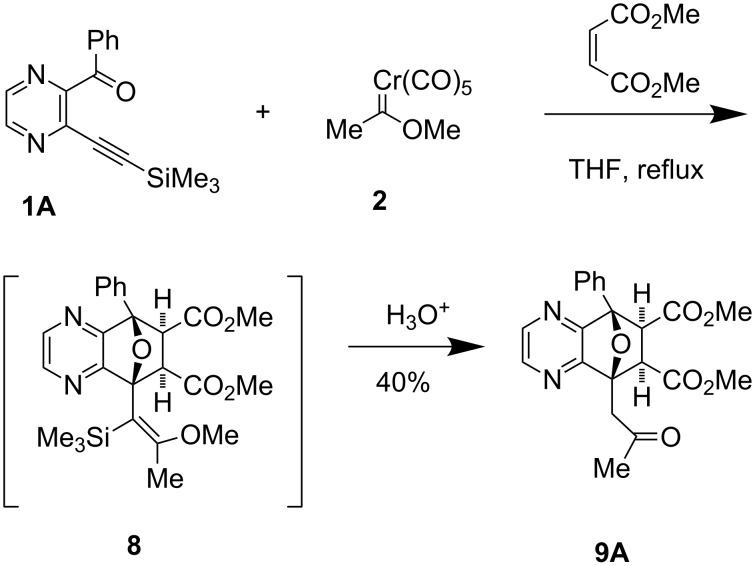
The reaction was also examined with dimethyl maleate as the dienophile (Scheme 3). The reaction of pyrazinyl ketone 1A, carbene complex 2 and dimethyl maleate under the same conditions as previously described afforded the three component coupling product 9A in 40% yield via the unstable enol ether 8. No aromatized product was isolated, even under mild acidic conditions.
Scheme 3.
Synthesis of quinoxaline derivative.
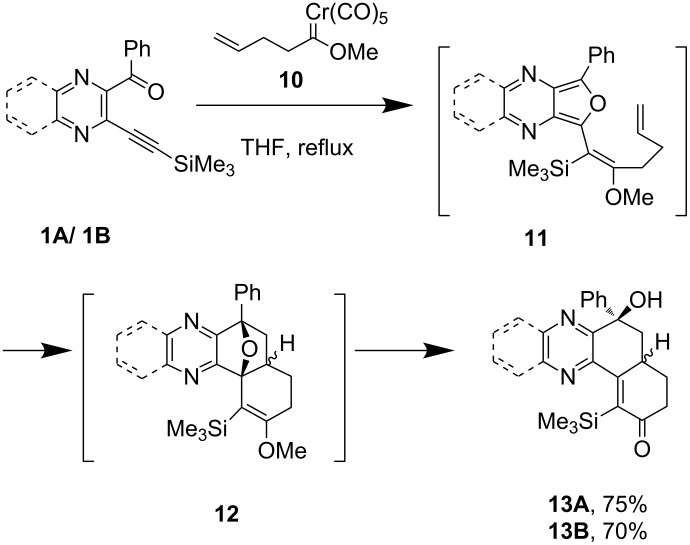
As part of a general effort to prepare aza-analogues of hydrophenanthrene natural products (including morphine alkaloids and abietanes) and tetracyclic triterpenes, the coupling of o-alkynyl pyrazine/quinoxaline carbonyl derivatives 1A/1B with simple γ,δ-unsaturated Fischer carbene complex 10 was investigated. This reaction proceeds via a tandem process involving the formation of azaisobenzofuran 11, followed by intramolecular Diels–Alder reaction, and ring opening of 12 to afford azahydrophenanthrone derivatives 13A/13B exclusively, in satisfactory yield (Scheme 4).
Scheme 4.
Synthesis of azahydrophenanthrone derivatives.
Conclusion
We have demonstrated a new route for the tandem generation of furo[3,4-b]pyrazine/ furo[3,4-b]quinoxaline intermediates by the coupling of o-alkynylheteroaryl carbonyl derivatives with Fischer carbene complexes. The intermediates can be trapped through Diels–Alder reaction with dienophiles leading to the synthesis of nitrogen containing heterocyclic analogues of quinoxaline and phenazine, respectively, in one-pot. This is the first report of in situ generation of furo[3,4-b]pyrazine intermediates.
Supporting Information
General procedure for the preparation of o-alkynyl carbonyl derivatives 1 and quinoxaline and phenazine derivatives and spectral data for selected compounds.
Acknowledgments
Financial support from CSIR [No.01 (2215)/08-EMR-II], Government of India is gratefully acknowledged. The CSIR, New Delhi, is also thanked for the award of a Senior Research Fellowship to P.R.
References
- 1.Porter A E A. In: Comprehensive Heterocyclic Chemistry: the structure, reactions, synthesis, and uses of heterocyclic compounds. Katritzky A R, Rees C W, editors. Oxford, U.K.: Pergamon Press; 1984. pp. 157–197. [Google Scholar]
- 2.Turner J M, Messenger A J. Occurrence, Biochemistry and Physiology of Phenazine Pigment Production. In: Rose A H, Tempest D W, editors. Vol. 27. Elsevier Ltd; 1986. pp. 211–275. ((Advances in Microbial Physiology)). [DOI] [PubMed] [Google Scholar]
- 3.McDonald M, Mavrodi D V, Thomashow L S, Floss H G. J Am Chem Soc. 2001;123:9459–9460. doi: 10.1021/ja011243+. [DOI] [PubMed] [Google Scholar]
- 4.Whistler C A, Pierson L S., III J Bacteriol. 2003;185:3718–3725. doi: 10.1128/JB.185.13.3718-3725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller P K, Krohn K, Muhlradt P F. Infect Immun. 1989;57:2591–2596. doi: 10.1128/iai.57.9.2591-2596.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald J C. Phenazines. In: Gottlieb D, Shaw P D, editors. Antibiotics. Vol. 2. New York: Springer; 1967. pp. 52–65. [Google Scholar]
- 7.Turner J M, Messenger A J. Occurrence, Biochemistry and Physiology of Phenazine Pigment Production. In: Rose A H, Tempest D W, editors. Vol. 27. Elsevier Ltd; 1986. pp. 211–275. ((Advances in Microbial Physiology)). [DOI] [PubMed] [Google Scholar]
- 8.Budzikiewicz H. FEMS Microbiol Lett. 1993;104:209–228. doi: 10.1111/j.1574-6968.1993.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 9.Ali M M, Ismail M M F, El-Gaby M S A, Zahran M A, Ammar Y A. Molecules. 2000;5:864–873. doi: 10.3390/50600864. [DOI] [Google Scholar]
- 10.He W, Meyers M R, Hanney B, Spada A P, Bilder G, Galzcinski H, Amin D, Needle S, Page K, Jayyosi Z, et al. Bioorg Med Chem Lett. 2003;13:3097–3100. doi: 10.1016/S0960-894X(03)00655-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y B, Kim Y H, Park J Y, Kim S K. Bioorg Med Chem Lett. 2004;14:541–544. doi: 10.1016/j.bmcl.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 12.Yadav J S, Reddy B V S, Rao Y G, Narsaiah A V. Chem Lett. 2008;37:348–349. doi: 10.1246/cl.2008.348. [DOI] [Google Scholar]
- 13.Brown D J. Quinoxalines: Supplement II. In: Taylor E C, Wipf P, editors. The Chemistry of Heterocyclic Compounds: A Series of Monographs. Vol. 61. New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 14.Huang T K, Shi L, Wang R, Guo X Z, Lu X X. Chin Chem Lett. 2009;20:161–164. doi: 10.1016/j.cclet.2008.10.048. [DOI] [Google Scholar]
- 15.Zhao Z, Wisnoski D D, Wolkenberg S E, Leister W H, Wang Y, Lindsley C W. Tetrahedron Lett. 2004;45:4873–4876. doi: 10.1016/j.tetlet.2004.04.144. [DOI] [PubMed] [Google Scholar]
- 16.Bhosale R S, Sarda S R, Ardhapure S S, Jadhav W N, Bhusare S R, Pawar R P. Tetrahedron Lett. 2005;46:7183–7186. doi: 10.1016/j.tetlet.2005.08.080. [DOI] [Google Scholar]
- 17.Boully L, Darabantu M, Turck A, Ple N. J Heterocycl Chem. 2005;42:1423–1428. doi: 10.1002/jhet.5570420726. [DOI] [Google Scholar]
- 18.Neochoritis C, Stephanidou-Stephanatou J, Tsoleridis C A. Synlett. 2009;2:302–305. doi: 10.1055/s-0028-1087518. [DOI] [Google Scholar]
- 19.Beifuss U, Tietze M. Methanophenazine and Other Natural Biologically Active Phenazines. In: Mulzer J, editor. Topics in Current Chemistry. Vol. 244. Berlin, Germany: Springer; 2005. pp. 77–113. [DOI] [Google Scholar]
- 20.Davarani S S H, Fakhari A R, Shaabani A, Ahmar H, Maleki A, Fumani N S. Tetrahedron Lett. 2008;49:5622–5624. doi: 10.1016/j.tetlet.2008.07.063. [DOI] [Google Scholar]
- 21.Pachter J, Kloetzel M C. J Am Chem Soc. 1951;73:4958–4961. doi: 10.1021/ja01154a144. [DOI] [Google Scholar]
- 22.Haddadin M J, Yavrouian A, Issidorides C H. Tetrahedron Lett. 1970;11:1409–1410. doi: 10.1016/S0040-4039(01)97982-1. [DOI] [Google Scholar]
- 23.Basak S, Ghosh S K, Sarkar T K J. Indian Inst Sci. 2001;81:431–452. [Google Scholar]
- 24.Jana G P, Ghorai B K. Tetrahedron. 2007;63:12015–12025. doi: 10.1016/j.tet.2007.09.007. [DOI] [Google Scholar]
- 25.Jana G P, Ghorai B K. Lett Org Chem. 2009;6:372–376. [Google Scholar]
- 26.Mukherjee S, Jana G P, Ghorai B K. J Organomet Chem. 2009;694:4100–4106. doi: 10.1016/j.jorganchem.2009.08.019. [DOI] [Google Scholar]
- 27.Jiang D, Herndon J W. Org Lett. 2000;2:1267–1269. doi: 10.1021/ol005691i. [DOI] [PubMed] [Google Scholar]
- 28.Ghorai B K, Herndon J W, Lam Y-F. Org Lett. 2001;3:3535–3538. doi: 10.1021/ol0166404. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Herndon J W, Cervantes-Lee F. J Am Chem Soc. 2003;125:12720–12721. doi: 10.1021/ja037500n. [DOI] [PubMed] [Google Scholar]
- 30.Ghorai B K, Herndon J W. Organometallics. 2003;22:3951–3957. doi: 10.1021/om030383k. [DOI] [Google Scholar]
- 31.Camacho-Davila A, Herndon J W. J Org Chem. 2006;71:6682–6685. doi: 10.1021/jo061053n. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Ye S, Jiao L, Liang Y, Sinha-Mahapatra D K, Herndon J W, Yu Z-X. J Am Chem Soc. 2007;129:10773–10784. doi: 10.1021/ja072203u. [DOI] [PubMed] [Google Scholar]
- 33.Turck A, Ple N, Tallon V, Queguiner G. J Heterocycl Chem. 1993;30:1491–1496. doi: 10.1002/jhet.5570300605. [DOI] [Google Scholar]
- 34.Yoshida K, Otomasu H. Chem Pharm Bull. 1984;32:3361–3365. [Google Scholar]
- 35.Turck A, Mojovic L, Queguiner G. Synthesis. 1988:881–884. doi: 10.1055/s-1988-27736. [DOI] [Google Scholar]
- 36.Sarkar T K, Panda N, Basak S. J Org Chem. 2003;68:6919–6927. doi: 10.1021/jo0344081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General procedure for the preparation of o-alkynyl carbonyl derivatives 1 and quinoxaline and phenazine derivatives and spectral data for selected compounds.