Abstract
Background & Aims
Little is known about how endothelial cells respond to injury, regulate hepatocyte turnover and reconstitute the hepatic vasculature. We aimed to determine the effects of the vascular ectonucleotidase CD39 on sinusoidal endothelial cell responses following partial hepatectomy and to dissect purinergic and growth factor interactions in this model.
Methods
Parameters of liver injury and regeneration, as well as the kinetics of hepatocellular and sinusoidal endothelial cell proliferation, were assessed following partial hepatectomy in mice that do not express CD39, that do not express ATP/UTP receptor P2Y2, and in controls. The effects of extracellular ATP on vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and interleukin-6 responses were determined in vivo and in vitro. Phosphorylation of the endothelial VEGF receptor in response to extracellular nucleotides and growth factors was assessed in vitro.
Results
After partial hepatectomy, expression of the vascular ectonucleotidase CD39 increased on sinusoidal endothelial cells. Targeted disruption of CD39 impaired hepatocellular regeneration, reduced angiogenesis, and increased hepatic injury, resulting in pronounced vascular endothelial apoptosis, and decreased survival. Decreased HGF release by sinusoidal endothelial cells, despite high levels of VEGF, reduced paracrine stimulation of hepatocytes. Failure of VEGF receptor-2/KDR transactivation by extracellular nucleotides on CD39-null endothelial cells was associated with P2Y2 receptor desensitization.
Conclusions
Regulated phosphohydrolysis of extracellular nucleotides by CD39 coordinates both hepatocyte and endothelial cell proliferation following partial hepatectomy. Lack of CD39 activity is associated with decreased hepatic regeneration and failure of vascular reconstitution.
Differentiated hepatocytes have the capacity to proliferate in a highly regulated manner following partial hepatic resection, transplantation, or toxin exposure.1 Essential paracrine mechanisms involved in initiating, maintaining, and organizing cellular proliferation involve complex interactions between varieties of hepatic and sinusoidal cell types.1–3 Liver sinusoidal endothelial cells (LSEC), for example, secrete hepatocyte growth factor (HGF) and interleukin-6 (IL-6) in response to the release of vascular endothelial growth factor (VEGF) by injured hepatocytes to further boost hepatocellular proliferation in a paracrine manner.4 It is known that LSEC undergo delayed proliferation relative to hepatocytes during regeneration.2 However, the mechanisms and mediators that regulate the function and proliferation of these cells in the setting of liver regeneration are not fully elucidated.
Recent reports suggest that platelet and/or vasculature-derived factors, eg, serotonin may boost liver regeneration.5 Extracellular nucleotides are likewise released in a regulated manner by platelets and a variety of vascular and hepatic cells in response to inflammatory stress and cellular swelling or with exocytosis. Levels of extracellular nucleotides are in turn regulated by CD39 (ectonucleoside triphosphate diphosphohydrolase-1/ENTPD1), the dominant vascular ectonucleotidase. The major ectoenzymatic function of CD39 is that of phosphohydrolysis of extracellular adenosine 5′ triphosphate (ATP) to adenosine diphosphate and adenosine monophosphate, ultimately leading to adenosine production, generated in tandem by CD73/5′ ectonucleotidase. This cascade results in scavenging of extracellular nucleotides and the modulation of extracellular purinergic signaling via adenosine (P1) and P2 receptors.6
We have investigated regulation of purinergic signaling by CD39 in a classic model of liver regeneration. CD39 is expressed only on the luminal surface of proliferating or activated LSEC and not on hepatocytes.7,8 In hepatocytes, extracellular ATP is known to augment liver regeneration by the direct activation of purinergic P2Y2 receptors (P2Y2R) to promote entry into the cell cycle.5,7,9 CD39 on LSEC might therefore be expected to balance levels of extracellular nucleotides/nucleosides impacting both hepatocytes and LSEC and could therefore modulate proliferation in both angiogenesis-independent and angiogenesis-dependent manners.
Materials and Methods
Animal Studies
Pathogen-free, wild-type, CD39-null, and P2Y2R-null male mice aged 10–12 weeks were used in accordance with the guidelines from the American Association for Laboratory Animal Care. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved all research protocols. C57Bl/6 wild-type mice were purchased from Taconic (Germantown, NY). CD39-null mice were derived and backcrossed with C57Bl/6 mice, as described.7 P2Y2R mice were obtained from Dr B. Koller, derived as detailed in the original publication.10,11 All mice tested in our models had been backcrossed onto the C57/BL6 strain for a total of 10 generations and were maintained in both the Missouri-Columbia and BIDMC facilities. Mice had free access to a standard mouse chow.
For partial hepatectomy studies, animals aged 6 to 8 weeks were anesthetized using xylacin 10 mg/mL and ketamin 80 mg/kg and were subjected to oblique laparotomy with two thirds liver resection as described previously.12 At least 4 animals of each group were killed at each time point analyzed. For the measurements of 5-bromo-2-deoxyuridine (BrdU) uptake, mice received a single dose of BrdU (Sigma Chemical Co, St. Louis, MO) intraperitoneally 60 minutes before death (at a dose of 50-mg/kg animal weight). At the time of death, mice were anesthetized; blood was harvested from the inferior vena cava; and the remaining liver lobes were removed, weighed, and further processed.
Primary LSEC Culture, Assays of Proliferation, and Apoptosis
LSEC isolation from murine livers was modified to published methods.4 Briefly, livers were perfused through the infrahepatic vena cava first with Ca-free buffer (142 mmol/L NaCl, 6.7 mmol/L KCl, 10 mmol/L HEPES [pH 7.4]) and then with 20–25 mL 0.05% collagenase type I (Worthington Biochemicals, Lakewood, NJ) including 0.025% dispase in collagenase buffer (67 mmol/L NaCl, 6.7 mmol/L KCl, 100 mmol/L HEPES, 4.8 mmol/L CaCL2 H2O2) for 15 minutes. Livers were excised, and the filtrate was centrifuged at 50g for 10 minutes. The supernatant was collected and the pellet discarded. The nonparenchymal cell supernatant fraction was washed twice. Labeling with electromagnetic beads was performed according to the manufacturer’s protocol (Miltenyi Biotec Inc, Auburn, CA). After separation, cells were plated at a density of 105 per cm2 on plates coated with fibronectin 2 μg per cm2. Proliferation of LSEC was assessed with the BrdU cell proliferation assay from Calbiochem (EMD Chemicals, Gibbstown, NJ). Briefly, at day 1 in culture, cells were stimulated with ATP 100 μmol/L and VEGF 20 ng/mL and then incubated with BrdU (Sigma–Aldrich Co, St Louis, MO) at 37°C for 24 hours. Cells were then fixed and labeled according to the manufacturer’s protocol. For assessment of apoptosis, LSEC were cultured with 1% of fetal bovine serum (FBS), without additional growth factors for 48 hours. Apoptosis was induced by pretreatment with cycloheximide 2 μg/mL and recombinant mouse tumor necrosis factor (TNF) α 400 U/mL 120 minutes later (R&D Systems Inc, Minneapolis, MN). Cells were harvested 24 hours following TNF-α stimulation, and DNA content was analyzed on a FACScan using CELLQuest software (Beckton Dickson Immunocytometric Systems, San Jose, CA).
Immunohistochemistry
The tissue was fixed with 2% paraformaldehyde. For Ki67 and BrdU-treated tissue, heat treatment with citrate buffer, pH6, was performed. Sections were then incubated overnight with primary antibody for CD39, Ki67, BrdU, and CD31 at 4°C, then rinsed with phosphate-buffered saline (PBS), and incubated with 3% H2O2 for 5 minutes. After incubation with the AB-Complex (Dako, Glostrup, Denmark) for 30 minutes, staining was performed with the DAB elite kit (Vectorlab, CA). Sections were counterstained with hematoxylin. Total BrdU-labeled and Ki67-labeled hepatocytes were determined by counting positively stained hepatocyte nuclei in 10 high-power microscope fields (×40) per liver. Apoptotic cells in the liver were detected by staining for terminal deoxy-nucleotidyl transferase dUTP nick-end labeling (TUNEL). TUNEL assays were performed on paraffin-embedded and snap-frozen liver sections with counts as the number of apoptotic nuclei per high-power (×40) field. A positive cell was defined as a cell that had the associated nuclear changes along with a TUNEL-positive stain. A minimum of 5 fields for each liver section was counted with at least 500 cells visualized. DAPI, CD31, and TUNEL staining was used to localize these markers on LSEC by fluorescence immunohistochemistry and to confirm the distinct vascular sinusoidal cell morphology in contrast to that of hepatocytes.
Cytokine Measurements by Enzyme-Linked Immunosorbent Assay
Blood was taken under anesthesia by puncture of inferior vena cava after median laparotomy. After centrifugation, serum was taken and stored at −80°C. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used for determination of HGF (B-Bridge International Inc, Mountain View, CA), VEGF (R&D Systems), and IL-6 (eBioscience, San Diego, CA). Serum levels of circulating cytokines were assessed following the manufacturer’s instructions.
Primary Hepatocyte Cell Cultures and Proliferation Assays
Using a modified 2-step EDTA/collagenase protocol, primary hepatocytes were isolated from 8-week male CD39-null mice and sex- and age-matched wild-type mice. The cells were washed and suspended in Williams’ E medium (Invitrogen, Carlsbad, CA) supplemented with L-glutamine (2 mmol/L), 10% FBS, insulin (20 mU/mL), epidermal growth factor (20 ng/mL), dexamethazone at 10−9 mol/L, streptomycin (100 U/mL), and penicillin (100 U/mL). Twenty thousand viable cells were dispensed into a well of 24-well-dishes. The cells were maintained at 37°C in a humidified incubator under an atmosphere of 5% CO2-95% air. Confluent cells were starved with Williams’ E medium 1% FBS, supplemented with L-glutamine and streptomycin-penicillin for 24 hours. Next, cells were stimulated with ATP or HGF for 24 hours. Cells were pulsed with [3H]-thymidine (1 μCi/well; Perkin Elmer, Boston, MA) 16 hours before harvesting.
Immunoprecipitation and Western Blotting of VEGFR2
Cultured LSEC were washed with ice-cold PBS 3 times and lysed in modified RIPA buffer (50 mmol/L Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mmol/L NaCl) supplemented with Complete Proteinase Inhibitor Cocktails (Roche Diagnostics, Indianapolis, IN) and Phosphatase Inhibitor Cocktails (Sigma–Aldrich Co). One hundred micrograms of total cell lysate was precleared with 40 μL protein G-Sepharose 4 fast flow (Amersham Biosciences, Uppsala, Sweden) at 4°C for 30 minutes followed by centrifugation. Precleared cell lysate was incubated with 10 μg of antiphosphotyrosine monoclonal antibody (4G10; Upstate, Lake Placid, NY) with end-over-end rotation at 4°C for 3 hours. Fifty microliters of protein G-Sepharose 4 fast flow (Amersham Biosciences, Uppsala, Sweden) was added into each mixture and incubated at 4°C overnight. The mixtures were centrifuged at 4°C for 5 minutes at 2500 rpm, washed 3 times with RIPA buffer, eluted with 2X SDS reducing sample buffer, and resolved on a 4%–15% gradient SDS-PAGE gel, followed by Western blot analysis with anti-KDR/flk-1 polyclonal antibody (C-1158) (Santa Cruz Biotechnology Inc, Santa Cruz, CA).
Statistical Analyses
Results are expressed as median and range or as mean ± standard deviation. For statistical analysis Mann–Whitney test, Student t test, and analysis of variance (ANOVA) were used as indicated. Survival curves were computed by the Kaplan–Meier method and analyzed by the log-rank test. Significance was defined as P < .05.
Results
CD39 Expression and Outcomes Postpartial Hepatectomy
Under normal conditions, hepatic CD39 is expressed by endothelial cells of both muscularized venous and arterial vessels (Figure 1A, and Supplementary Figure 1; see Supplementary material online at www.gastrojournal.org). CD39 is also present on Kupffer cells and natural killer T cells within the hepatic sinusoids (Figure 1A).13 After partial hepatectomy, expression of CD39 is substantially and rapidly increased within the vascular sinusoids. This is noted to be an endothelial cell-specific manner. Within 6 hours postpartial hepatectomy, we observed heightened expression of CD39 on LSEC that further increases at day 2 (not shown) to day 5 (Figure 1B).
Figure 1.
Expression of CD39 in normal and regenerating liver postpartial hepatectomy. (A) In untreated mice, CD39 is expressed on Kupffer cells and chiefly the endothelium of muscularized vessels. (B) After partial hepatectomy, heightened expression on LSEC is found at day 2 (not shown) with further increases to day 5. (C) CD39-null mice exhibit acute and substantial defects in liver regeneration. Survival of mice null for CD39 is significantly decreased compared with wild-type mice (30/32 [94%] vs 21/32 [66%], respectively, P = .003). (D) ALT measurements of postpartial hepatectomy revealing significantly increased liver injury in mice null for CD39 at postoperative day 1. (E) Representative pictures of the liver 4 hours post-partial hepatectomy. Vascular injury is seen in CD39 null but not in wild-type mice. (F) Ratios of dry liver weight to total body weight at day 2 postpartial hepatectomy show significant decreases in mice null for CD39. Values are means ± standard deviation of at least 4 animals per time point. Levels of significance were assessed by unpaired t tests. P values are as indicated.
Outcomes of postpartial hepatectomy of wild-type mice can be compared with mutant mice null for CD39. In matched mice, overall survival is significantly decreased in CD39-null mice in comparison with wild-type mice (66% vs 94%, respectively, P = .003) by day 10 (Figure 1C). Heightened mortality of CD39-null mice is associated with liver injury, and heightened surrogate markers of this, as noted at 24 hours postpartial hepatectomy (Figure 1D and E). Decreased dry liver weights are noted in mice null for CD39 at day 2 (Figure 1F).
Disordered Proliferative Responses of Hepatocytes and LSEC
Proliferation of hepatocytes in mice null for CD39 is significantly impaired at day 2 postpartial hepatectomy when compared with wild-type mice (Figure 2A–C). In a similar manner, proliferation of LSEC is significantly decreased in mutant mice (Figure 2D). We also note decreased proliferation of CD39-null LSEC in response to VEGF in vitro (Supplementary Figure 2; see Supplementary material online at www.gastrojournal.org). After partial hepatectomy, expression of hepatocyte-expressed active Ras, as a putative consequence of c-Met ligation by HGF, is decreased in mice null for CD39 at days 1 and 2 postpartial hepatectomy (Figure 2E).
Figure 2.
CD39-null mice show abnormalities in hepatocyte proliferation with altered kinetics of LSEC proliferation in vivo and in vitro. (A and B) Representative immunohistochemistry images showing hepatocyte BrdU incorporation at day 2 postpartial hepatectomy in (A) wild-type and mice null for (B) CD39. (C) BrdU uptake of hepatocytes at various time points postpartial hepatectomy. At day 2 postpartial hepatectomy, numbers of BrdU-positive hepatocytes are significantly lower in mice null for CD39 (P = .02). (D) Proliferation of LSEC (defined by morphology and location) in vivo was significantly decreased in mutant mice compared with wild-type mice at day 3 and day 5 postpartial hepatectomy as measured by BrdU uptake. (E) After partial hepatectomy, expression of active Ras is relatively decreased in mice null for CD39, at days 1 and 2 post-surgery. Values are means ± standard deviation of at least 4 animals per time point. Levels of significance were assessed by unpaired t tests. P values are as indicated.
Abnormalities in proliferation responses are also associated with increased vascular endothelial cell apoptosis in mice null for CD39 (Figure 3A–D). TUNEL staining reveals significantly increased levels of apoptotic endothelial cells, specifically in the sinusoids of mutant mice at day 5 and day 7, when compared with wild-type mice.
Figure 3.
Regulation of endothelial cell apoptosis by CD39. (A and B) Heightened levels of apoptosis of CD39-null LSEC relative to wild-type are noted at day 7, postpartial hepatectomy. (C) Significant increases of numbers of apoptotic LSEC (TUNEL-positive cells with distinct morphology within the vascular sinusoids) occur at day 5 and day 7 post-partial hepatectomy. (D) Colocalization of TUNEL staining and CD31 expression in hepatic sinusoids (arrowheads) by fluorescence immunohistochemistry confirms the specific apoptosis induction impacting LSEC: DAPI staining nuclei in blue, CD31 as a marker of endothelial cells in green, and TUNEL in red. (E) In vitro apoptosis is induced after 48 hours of serum starvation with 1% FBS. Sequential administration of cycloheximide (2 μg/mL) and TNF-α (400 U/mL) resulted in significantly increased levels of apoptosis in LSEC null for CD39 (P = .01). Further administration of ATPγS (a nonhydrolysable ATP analog) results in increased apoptosis in wild-type and mutant LSEC. Values are expressed as means ± standard deviation. Levels of significance were assessed by unpaired t tests. P values are as indicated.
In vitro, LSEC null for CD39 show significantly (P = .01) increased levels of DNA fragmentation following stimulation with both cycloheximide and TNF-α (Figure 3E). After administration of ATPγS (a nonhydrolysable ATP analog; to mimic the relative lack of CD39-mediated phosphohydrolysis of nucleotides), induction of apoptosis in wild-type LSEC is substantially increased, to the comparable levels noted in CD39-null LSEC exposed to ATPγS.
CD39 Deficiency and Secondary P2Y2R Desensitization Lead to Failure of VEGF Signaling
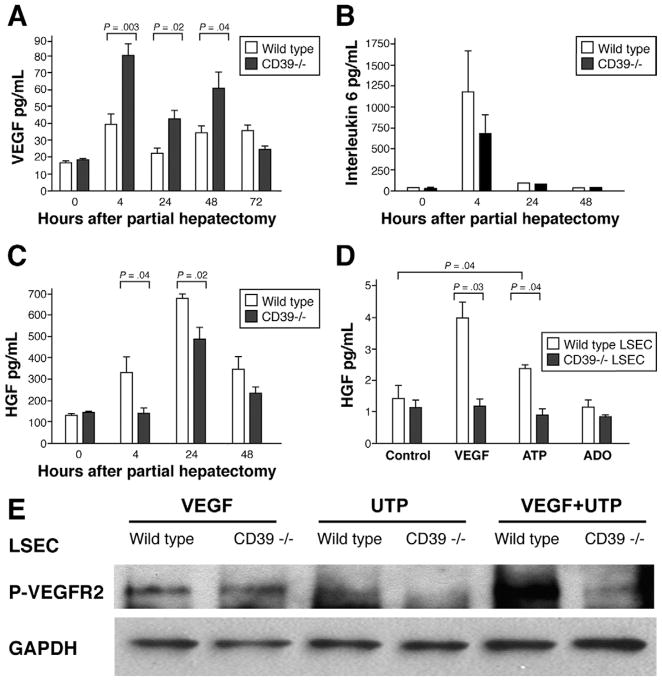
Serum levels of VEGF are substantially elevated at 4, 24, and 48 hours postpartial hepatectomy in mice null for CD39 when compared with wild-type mice (Figure 4A). Serum IL-6 levels do not differ significantly between wild-type and CD39-null mice (Figure 4B). However, basal and ATP-stimulated IL-6 release by CD39-null LSEC both increase in vitro (Supplementary Figure 3; see Supplementary material online at www.gastrojournal.org). Conversely, serum HGF levels are decreased significantly when compared with wild-type controls (Figure 4C). In vitro, CD39-null LSEC fail to secrete levels of HGF comparable with wild-type cells, in direct response to exogenous VEGF (Figure 4D).
Figure 4.
Deficiency of CD39 results in secondary VEGF resistance. (A) Serum levels of VEGF are increased in mice null for CD39 within first 48 hours post-partial hepatectomy. (B) Serum IL-6 levels in vivo are not significantly different between the 2 groups. (C) HGF levels in vivo are significantly decreased in mice null for CD39 at 4 and 24 hours post-partial hepatectomy. (D) In vitro, HGF levels are measured in the supernatants of cultured LSEC. Wild-type LSEC release HGF in response to stimulation with VEGF but not after stimulation with adenosine (Ado) alone. LSEC null for CD39 do not show these increases in HGF secretion in response to stimulation with these agonists. (E) Failure of P2Y(2)-mediated phosphorylation of VEGFR2. LSEC of wild-type and CD39-null mice were stimulated with UTP (100 μmol/L, 10 minutes) or VEGF (30 ng/mL, 3 minutes) in vitro. Stimulation with VEGF alone does not result in any significant differences. Stimulation with UTP results in heightened phosphorylation of the VEGFR2 in wild-type LSEC but not in CD39-null LSEC. The enhanced effect seen with VEGF and UTP co-stimulation is lost in CD39-null cells. Values are given as mean ± standard deviation. Level of significance was assessed by unpaired t tests. P values are as indicated.
Activation of VEGFR2 in Response to Uridine Triphosphate
Previous studies by Seye et al have revealed that stimulation of P2Y2R by uridine 5′ triphosphate (UTP) results in VEGFR2 transactivation.14 After stimulation with VEGF at 30 ng/mL, levels of VEGFR2 phosphorylation are comparable in mutant and wild-type LSEC (Figure 4E). However, UTP-induced phosphorylation of VEGFR2 is wholly abrogated in mice null for CD39, in keeping with P2Y desensitization events and disordered purinergic signaling responses.15 In wild-type LSEC, combinations of VEGF and UTP result in increased phosphorylation of VEGFR2 when compared with VEGF stimulation alone. In CD39-null LSEC, phosphorylation of VEGFR2 is decreased following joint stimulation with VEGF and UTP.
P2Y2R Regulates Hepatocellular Proliferation In Vivo and In Vitro
We then confirmed the specific role of P2Y2R during liver regeneration. We determined parameters of hepatocellular proliferation using P2Y2R-null mice and cells in vivo and in vitro. After partial hepatectomy, Ki67 uptake of hepatocytes is significantly lower in mice null for P2Y2R (Figure 5A). In cultured hepatocytes obtained from wild-type and P2Y2R-null animals, cellular proliferation is significantly decreased in hepatocytes null for P2Y2R under both basal and stimulated conditions (comparison with wild-type cells; Figure 5B). Extracellular ATP also induces hepatocellular proliferation when combined in cultures with submitogenic dose of HGF.
Figure 5.
P2Y2R is required for vascular coordination of liver regeneration. (A) Ki67 staining of hepatocytes is shown at various time points postpartial hepatectomy. At day 2 postpartial hepatectomy, the number of replicating hepatocytes is significantly lower in mice null for P2Y2R compared with wild-type (P = .003). (B) Proliferative responses ([3H]-thymidine) of hepatocytes to combinations of ATP (100 μmol/L) and/or a submitogenic dose of HGF (20 ng/mL) were studied. Synergistic effects of ATP and HGF in the boosting of proliferation of P2Y2R null hepatocytes are attenuated in a statistically significant manner when compared with wild-type hepatocytes. (C) Phosphorylation of VEGFR2 in LSEC was studied following P2Y2R costimulation with VEGF. In mice null for P2Y2R, phospho-VEGFR2 levels do not increase in response to UTP or to low concentrations of VEGF. Phosphorylation of VEGFR2 is substantively attenuated after optimal VEGF and UTP stimulation.(D)Hepatocellular proliferation postpartial hepatectomy in wild-type mice is increased by continuous infusion of apyrase (8.3 U/kg/h) (*P = .027). ATP (0.8 μmol/kg/h) and the A2A agonist ATL146e (600 ng/kg/h) had no significant effects on hepatocyte proliferation. (E) Images of Ki67 staining in wild-type livers postapyrase supplementation. Values are given as mean ± standard error. Levels of significance were assessed by unpaired t tests and ANOVA. P values are as indicated.
In P2Y2R-null LSEC, phosphorylation of VEGFR2 is completely absent after administration of UTP and/or VEGF (Figure 5C). Furthermore, long-term stimulation with UTP, followed by activation with VEGF, results in decreased phosphorylation of ERK in endothelial cells (not shown).
To explore possible therapeutic options further, we continuously infused wild-type mice between 1 day pre-operatively until postoperative day 2 with either ATP, soluble nucleoside-triphosphate diphosphohydrolase (apyrase), or ATL146e (agonist of adenosine receptor A2a; a kind gift of Dr Joel Linden, University of Virginia, Charlottesville, VA). In these experiments, hepatocellular proliferation was significantly boosted only in the group receiving a continuous injection of apyrase (P = .027) (Figure 5D and E).
Discussion
Absence of CD39 leads to a secondary imbalance between extracellular nucleotides and nucleosides.15 This disordered purinergic signaling results in increased immediate vascular injury, impaired hepatocyte turnover, and later increments in endothelial cell apoptosis in CD39 null mice, after partial hepatectomy. CD39 deletion in mice alters both the early paracrine stimulation of hepatocyte turnover by LSEC and the crucial later proliferative responses of LSEC during liver regeneration. There is also decreased survival in CD39-null mice that is associated with increased liver injury and heightened LSEC apoptosis.
The expression of CD39 in the normal liver is typically confined to Kupffer cells and endothelial cells of muscularized vessels. Interestingly, vascular and sinusoidal expression of CD39 postpartial hepatectomy is up-regulated. Ecto-NTPDase activity is already boosted on LSEC in wild-type mice, prior to the angiogenic responses noted at days 3–5. Such early growth responses of LSEC postpartial hepatectomy are significantly perturbed in mice null for CD39.
We also show that CD39 deletion can alter proliferation of hepatocytes that do not intrinsically express this ecto-enzyme. We suggest that CD39 expression by LSEC might further coordinate liver remodeling by initially facilitating VEGF-induced paracrine release of HGF to regulate hepatocellular proliferation and liver regeneration. Therefore, CD39 and extracellular nucleotide/nucleoside fluxes appear to be implicated in growth factor responses involving the signaling pathways induced by tyrosine receptor kinases in addition to their previously proven roles in angiogenesis.8,15
Postpartial hepatectomy, CD39 appears to boost angiogenesis directly by controlling pericellular nucleotide/nucleoside boluses that regulate endothelial cell proliferation and may also regulate endothelial cell apoptosis. Persistent elevations in pericellular ATP levels in mice null for CD39 also result in P2Y2R receptor desensitization, the receptor that has been previously implicated in the regulation of vascular growth responses.14 In contrast, continuous stimulation of P2X7R, a receptor that is resistant to desensitization, by ATP may differentially promote cell death.16 Similar to our findings in vivo, increased levels of apoptotic LSEC were found in vitro in response to continuous stimulation with extracellular nucleotides in addition to cycloheximide and TNF-α. We suggest that this is related to unopposed activation of the P2X7R, triggering cellular apoptosis.17–19 The presence of CD39 seems to be crucial in protecting from increased levels of extracellular nucleotides. These mediators are released during inflammatory or surgical stress, as seen in other models of immune liver injury.13
VEGF has been shown to impact liver regeneration in both angiogenesis-dependent and angiogenesis-independent manners, following on the coordinated activation of both VEGFR1 and VEGFR2.4,20 As VEGFR1 and VEGFR2 form heterodimers,21,22 concurrent VEGFR2 signaling by VEGFR1 (and flt-1) may be considered highly probable. In response to soluble flt-1, LSEC can be shown to modulate both HGF and IL-6 expression and functions on hepatocytes.4 In our study, failure of VEGF signaling by CD39-null LSEC is associated with decreased levels of HGF secretion (Figure 6). Transactivation of VEGFR2 signaling by extracellular nucleotides (UTP/ATP) via P2Y2R seems to impact on postoperative outcome post-partial hepatectomy.
Figure 6.
Impact of purinergic signaling upon interactions between LSEC and hepatocytes during early liver regeneration. (1) Absence of CD39 leads to elevated nucleotide levels. These changes in fluxes of extracellular nucleotides (ATP, UTP, and ADP) impact P2 receptor signaling in paracrine (and autocrine) manner(s) for hepatocytes (left) and LSEC (right). (2) Continuous stimulation of LSEC P2Y receptors results in preferential desensitization responses, here specifically of P2Y2R. P2Y2R and VEGFR2 colocalize on vascular cell membranes, and activation of the P2Y2R induces rapid tyrosine phosphorylation of VEGFR2 in endothelial cells. Inhibition of P2Y2R function is associated with failure of VEGFR2 transactivation and VEGF resistance (see text for details). (3) Failure of VEGFR2 signaling in turn results in decreased secretion of HGF and decreased hepatocyte proliferation. IL-6 pathways appear minimally impacted (Figure 4B, and Supplementary Figure 3). Unopposed P2X7R activation in this setting because of resistance to desensitization results in heightened LSEC apoptosis and failure of late angiogenesis (not shown here; see Figure 3).
This finding is in complete agreement with observations in other systems in that activation of VEGFR2 is tightly modulated by extracellular nucleotides.14,23 P2Y2R and VEGFR2 are also shown to colocalize on vascular cell membranes, and activation of the P2Y2R thereby induces rapid tyrosine phosphorylation of VEGFR2 in endothelial cells.14,23 Desensitization of P2Y2R can occur in response to heightened and prolonged levels of extracellular nucleotides, following on deletion of CD39.7 This process then leads to failure of VEGFR2 transactivation.8
We propose that the effects of disordered purinergic signaling in vivo are related to vascular expression of CD39 with secondary persistent impacts on P2 nucleotide receptor responses on LSEC and hepatocytes.7,8 Changes in fluxes of extracellular nucleotides impact P2Y receptor signaling (inclusive of desensitization responses) in nonvascular tissues,7 shown here to include liver. This hypothesis was further tested in mice null for P2Y2R. These mice had defects that are comparable with the CD39 null mice with respect to readouts of hepatocellular proliferation in vivo and in vitro and disordered phosphorylation of VEGFR2 in LSEC. Thus, as expected, mice null for P2Y2R also show altered cross talk between endothelial cells and hepatocytes.
Injecting apyrase, a soluble ENTPD, boosted levels of replicating hepatocytes postpartial hepatectomy. In previous studies, short-term bolus injection of ATP into normal livers was noted to result in entry of hepatocytes into cell cycle and increased replication.9 In our study, however, injection was continuous and nucleotide infusion was employed, further perturbing the elevated levels expected with tissue injury secondary to obligatory surgical injury. In this setting, extracellular nucleotide levels are likely to be increased over normal levels. Therefore, the importance of tight regulation including clearance of increased levels of extracellular nucleotides seems to be crucial in this setting.
We conclude that CD39 modulates liver regeneration in both angiogenesis-independent and -dependent manners. We have identified extracellular nucleotides and purinergic pathways as potential targets for therapeutic intervention in liver injury and regeneration. Pharmacologic strategies to enhance liver regeneration with the infusion of soluble CD39 derivatives might result in better clinical outcomes in liver failure and after surgical resection.
Supplementary Material
Acknowledgments
The authors disclose the following: Supported by the Swiss National Research Foundation (PBBEB-112764 and PASMA-115700, to G.B.) and the National Institutes of Health (HL57307, HL63972, and HL076540; to S.C.R.).
Abbreviations in this paper
- ATP
adenosine triphosphate
- HGF
hepatocyte growth factor
- IL
interleukin
- LSEC
liver sinusoidal endothelial cells
- VEGF
vascular endothelial growth factor
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2008.07.025.
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 3.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 4.LeCouter J, Moritz DR, Li B, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 5.Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 6.Kaczmarek E, Koziak K, Sevigny J, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 7.Enjyoji K, Sevigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 8.Goepfert C, Sundberg C, Sevigny J, et al. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 9.Thevananther S, Sun H, Li D, et al. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39:393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 10.Cressman VL, Lazarowski E, Homolya L, et al. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(−) transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- 11.Homolya L, Watt WC, Lazarowski ER, et al. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y(2) receptor (−/−) mice. J Biol Chem. 1999;274:26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- 12.Inderbitzin D, Studer P, Sidler D, et al. Regenerative capacity of individual liver lobes in the microsurgical mouse model. Microsurgery. 2006;26:465–469. doi: 10.1002/micr.20271. [DOI] [PubMed] [Google Scholar]
- 13.Beldi G, Wu Y, Banz Y, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seye CI, Yu N, Gonzalez FA, et al. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279:35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- 15.Jackson SW, Hoshi T, Wu Y, et al. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol. 2007;171:1395–1404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goepfert C, Imai M, Brouard S, et al. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumoto N, Kumamoto T, Robson SC, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 19.von Albertini M, Palmetshofer A, Kaczmarek E, et al. Extracellular ATP and ADP activate transcription factor NF-κB and induce endothelial cell apoptosis. Biochem Biophys Res Commun. 1998;248:822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- 20.Ross MA, Sander CM, Kleeb TB, et al. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135–1148. doi: 10.1053/jhep.2001.29624. [DOI] [PubMed] [Google Scholar]
- 21.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Kanno S, Oda N, et al. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000;902:201–207. doi: 10.1111/j.1749-6632.2000.tb06314.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Liao Z, Camden J, et al. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279:8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.