Abstract
Purpose
To determine the response, toxicity, and survival for children with progressive or recurrent medulloblastoma and germinoma using a single myeloablative course of chemotherapy supported by autologous hematopoietic stem cells.
Patients and Methods
Subjects were in second remission or had minimal residual disease at the time of study entry. The conditioning regimen consisted of cyclophosphamide 6,000 mg/m2 plus melphalan 180 mg/m2.
Results
Twenty nine evaluable pediatric patients were accrued. The most frequent major toxicities were myelosuppression, infections, and stomatitis, but no toxic deaths were recorded. Best responses were: CR=6, CCR=13, PR=6, SD=2, and PD=2. There were 6 medulloblastoma and 3 germinoma survivors with a median follow-up of 7.5 years (range= 2.8–10). Two germinoma survivors received radiotherapy after autografting for presumptive progressive disease.
Conclusion
Myeloablative chemotherapy consisting of cyclophosphamide and melphalan was tolerable in the relapsed brain tumor setting with 19/29 cases achieving CR or CCR status and 9/29 becoming long term survivors.
Keywords: medulloblastoma, germinoma, chemotherapy, autograft
INTRODUCTION
Central nervous system tumors represent the most common solid tumor group in childhood. Long term survival rates of greater than 70% have been reported for medulloblastoma and germinoma using multiple different frontline therapeutic regimens.1–4 However, patients who develop disease progression or sustain relapse have a very poor outcome, irrespective of treatment employed.5–9 With the background of general chemosensitivity for these two tumor types, it was postulated that increased dose intensity supported by autologous hematopoietic stem cells may be effective salvage therapy.
A plan was developed by the Pediatric Oncology Group (POG) to investigate high dose cyclophosphamide plus melphalan chemotherapy with autograft support. The phase I portion of this strategy (POG 9079) defined maximum tolerated doses of cyclophosphamide and melphalan for phase II analysis.10 This paper reports results of the follow-up study (initially designated POG 9430), a phase II trial for children with medulloblastoma or germinoma who achieved second remission or minimal residual disease status.
PATIENTS and METHODS
Patient Eligibility
Subjects were to be more than 2 years and less than 26 years of age. Acceptable diagnoses included medulloblastoma and central nervous system germ cell tumors that progressed or relapsed after frontline therapy. Pathologic review of the primary tumor was mandated. Repeat biopsy prior to study entry was encouraged, but not required. At the time of autografting, patients needed to be in second complete remission or have minimal residual disease with tumor bulk less than or equal to 1.5 cm in maximal dimension. Informed consent was obtained according to institutional guidelines before treatment was initiated.
Bone marrow free of tumor plus absolute neutrophil count greater than 1,000/microliter and platelet count greater than 100,000/microliter were stipulated. Serum creatinine less than 1.2 mg/dl, bilirubin less than 1.5 mg/dl, and SGPT less than 80 IU were required. Normal cardiac function, documented ideally by gated nuclear cardiac scan, was necessary. Patients were to be free of active infections with minimum Karnofsky score of 80% and estimated life expectancy of at least 8 weeks.
Prior Therapy
No more than one primary treatment regimen and one prior salvage therapy at relapse were allowed. Pre-transplant surgery or radiotherapy boost was acceptable, but no therapy within four weeks, except corticosteroids. Prior cyclophosphamide or ifosfamide chemotherapy did not preclude eligibility.
Collection of Hematopoietic Stem Cells
Bone marrow and/or peripheral blood hematopoietic stem cells were allowed for rescue after high dose chemotherapy. The quantity of bone marrow was targeted to be greater than 2 × 108 nucleated cells/kg. For peripheral blood, it was recommended that at least 4 × 108 nucleated cells/kg and 2 × 106 CD34 positive cells/kg be collected.
Protocol Chemotherapy
Cyclophosphamide 1,500 mg/m2 was infused over 1 hour daily for four days, day -8 thru day -5. Mesna 360 mg/m2 was given over 1 hour concomitantly with each cyclophosphamide and an additional dose as a 3 hour infusion immediately afterwards. Mesna 360 mg/m2 was repeated as a bolus at hour 4, hour 7, and hour 10 after cyclophosphamide initiation. Melphalan 60 mg/m2 over 15 minutes was administered daily for three days, day -4 thru day -2, inclusive. The total doses of cyclophosphamide and melphalan were 6,000 mg/m2 and 180 mg/m2, respectively (Table I).
TABLE I.
CHEMOTHERAPY OUTLINE
| Day -8 | Cyclophosphamide 1,500 mg/m2 |
| Day -7 | Cyclophosphamide 1,500 mg/m2 |
| Day -6 | Cyclophosphamide 1,500 mg/m2 |
| Day -5 | Cyclophosphamide 1,500 mg/m2 |
| Day -4 | Melphalan 60 mg/m2 |
| Day -3 | Melphalan 60 mg/m2 |
| Day -2 | Melphalan 60 mg/m2 |
| Day -1 | Rest |
| Day 0 | Hematopoietic stem cell infusion |
Supportive Care
Post transplant supportive care was suggested according to Pediatric Oncology Group and Children’s Oncology Group guidelines, but was delivered ultimately according to institutional standards. Of note, it was recommended that platelets should be transfused for count less than 40,000/mcl during the first 28 days after hematopoietic stem cell infusion and that myeloid synthesis should be augmented with sargramostim (granulocyte-macrophage colony stimulating factor, GM-CSF). The latter was based on preceding phase I study experience from POG 9079.10 Sargramostim 500 mcg/m2 was prescribed as a daily 2-hour intravenous infusion until the absolute neutrophil count exceeded 2,000 cells/mcl on three consecutive days.
Evaluation of Response and Toxicity
Computerized tomography or magnetic resonance imaging of central nervous system tumors was scheduled on days +28, +56, then every 3 months for 2 years. Responses were defined as follows: complete response (CR)- resolution of all tumors, partial response (PR)- at least 50% decrease in tumor dimensions as measured by the sum of the products of the maximal perpendicular diameters of all measurable disease, progressive disease (PD)- greater than 25% increase in tumor dimensions or appearance of new lesions, stable disease (SD)- patients with tumor status not encompassed by CR, PR or PD criteria. Toxicity was defined according to National Cancer Institute’s Common Toxicity Criteria. The cutoff date for this report was January 2007.
Statistical Analysis
The primary endpoints for statistical analysis included response following hematopoietic stem cell infusion, progression free survival (PFS), as defined as the time from study entry to disease progression/relapse and overall survival (OS), defined as time from study entry to death from any cause. Nonparametric estimates of PFS and OS probabilities were obtained using the product limit (Kaplan-Meier) estimate with standard errors computed using the Greenwood formula. Fisher’s Exact test was used to determine if there is an association between amount of residual disease at the time of autografting and long term outcome.
RESULTS
Patient Characteristics and Accrual
Protocol 9430 was active from September 1994 to September 2003 with an accrual goal of 30 subjects. There were 31 patient enrollments, 29 who fulfilled all eligibility criteria in the final analysis. One child was deemed ineligible due to an incomplete pre-study evaluation as well as exceeding prior treatment limits. Another subject had more than minimal residual disease at time of study enrollment. Of the 29 eligible participants, 17 were male and 12 were female with median age of 9.8 years (4.3–17.1). There were 22 cases of medulloblastoma and 7 with germinoma.
Toxicity Data
The most common major toxicities were myelosuppression, stomatitis, and infections. Median day to absolute neutrophil count of 500 was 12 days (9–26) with all, but one child achieving this level by day 18. No toxic deaths were recorded. Table II enumerates grade 3–4 toxicities that occurred in three or more patients.
TABLE II.
GRADE 3–4 TOXICITIES IN 29 PATIENTS
| Toxicity | Count | Percent |
|---|---|---|
| Neutropenia | 29 | 100% |
| Thrombocytopenia | 29 | 100% |
| Anemia | 29 | 100% |
| Infection NOS | 15 | 52% |
| Stomatitis | 12 | 41% |
| Bacterial sepsis | 7 | 24% |
| Diarrhea | 7 | 24% |
| Nausea/vomiting | 7 | 24% |
| Pulmonary (clinical) | 5 | 17% |
| Viral infection | 5 | 17% |
| Fungal infection | 4 | 14% |
Response and Survival Data
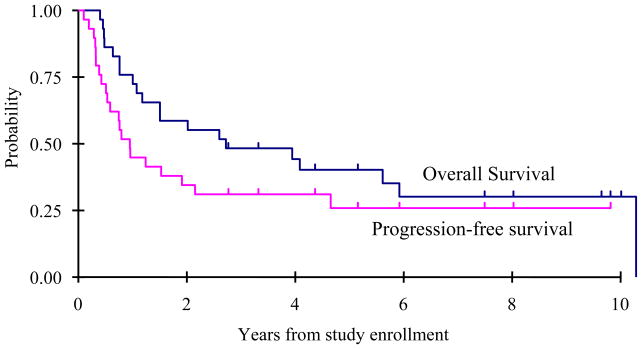
The best responses following hematopoietic stem cell infusion were as follows: complete response= 6, continuous complete response= 13, partial response= 6, stable disease= 2, and progressive disease= 2 (Table III). One medulloblastoma patient classified initially as a partial responder survives 8 years later without further therapy implying that residual radiographic abnormalities were not active tumor. This child was retrospectively re-classified as a CR. Two subjects with germinoma received craniospinal irradiation at 4 and 6 months after autografting for presumptive disease progression and survive 9 years later. The 2-year overall survival was 59 +/− 9% with 2-year progression free survival of 34 +/− 9% (Figure 1).
TABLE III.
BEST RESPONSE TO PROTOCOL THERAPY
| Response Category | Number |
|---|---|
| Complete Response | 6 |
| Continuous Complete Response | 13 |
| Partial Response | 6 |
| Stable Disease | 2 |
| Progressive Disease | 2 |
| Total Cases | 29 |
Figure 1.
Overall survival and progression free survival.
Prior to autografting, the 9 survivors had relapsed after a median of 1 year 5 months (range= 4 months- 2 years 5 months), then were followed for a median of 7 years 6 months (range= 2 years 10 months- 10 years) after relapse. Treatment and response details for the 9 long term survivors are summarized in Table IV. Five of the 9 survivors (4 medulloblastoma and 1 germinoma) had metastatic disease at time of relapse. Based on disease status at the time of autografting, 2 of 16 patients with measurable residual disease became long term survivors versus 7 of 13 who were in complete remission (p=0.02).
TABLE IV.
TREATMENT AND RESPONSE DATA FOR 9 SURVIVORS
| Initial Therapy | Relapse Sites | Pre-Autograft Therapy | Autograft Response | Follow-up |
|---|---|---|---|---|
| Germinoma Cases | ||||
| X + C | primary | C | CCR | 10 yrs 0 mos* |
| X + C | metastatic | C | CCR | 9 yrs 4 mos* |
| X + C | primary | C | CCR | 7 yrs 6 mos |
| Medulloblastoma Cases | ||||
| C | metastatic (M4) | C | CR | 9 yrs 8 mos |
| X** | metastatic (M2) | C | CCR | 8 yrs 0 mos |
| X | primary | S | CCR | 5 yrs 1 mo |
| C | metastatic (M3) | X + C | CCR | 4 yrs 4 mos |
| X + C | primary | S | CCR | 3 yrs 3 mos |
| X + C | metastatic (M3) | C | CR | 2 yrs 10 mos |
Note: The first 2 germinoma cases received post-autograft radiotherapy (see text); Treatment abbreviations; C= chemotherapy, X= radiotherapy, S= surgery;
Initially considered to be a PR but was later reclassified as a CR.
DISCUSSION
The prognosis for children with relapsed or progressive central nervous system (CNS) malignancies remains unfavorable. During the time period concurrent with this study, Pediatric Oncology Group (POG) and Children’s Oncology Group (COG) phase II evaluations of new agents demonstrated negligible to modest activity across the major central nervous system tumor histologies. Trials of idarubicin, taxol, topotecan, temozolomide, and irinotecan recorded few responses with nearly all patients developing further tumor progression.11–15
For selected children who are able to attain second remission or minimal residual disease status (especially those who demonstrate chemosensitivity), the strategy of high dose chemotherapy supported by autologous hematopoietic stem cells has been implemented. The scientific rationale and results of numerous relatively small series have been the subject of multiple reviews and recently established international conference series.16–18 Long term survival has been reported using a spectrum of high dose chemotherapy regimens. Due to modest patient numbers, it remains difficult to draw definitive conclusions between the varied approaches.
High dose cyclophosphamide plus melphalan chemotherapy was evaluated in a multi-center study based at Duke University Medical Center and by the Pediatric Oncology Group.10,19 Investigators from Duke escalated melphalan (75–180 mg/m2) with a fixed dose of cyclophosphamide (6,000 m/m2).19 Fifteen children and young adults with recurrent medulloblastoma were treated with this drug combination, including nine at the top dose of melphalan (180mg/m2). Four patients with localized recurrence became long term disease free survivors with follow-up of 27+, 42+, 47+ and 49+ months. Two additional subjects with mixed germ cell tumors were both alive and disease free 22+ and 30+ months after treatment.
The initial POG phase I protocol (9079) utilized the opposite drug escalation strategy from the Duke trial.10 Cyclophosphamide was escalated from 3,000 mg/m2 to 6,000 mg/m2 with a fixed melphalan dose of 180 mg/m2. Both studies concluded that cyclophosphamide 6,000 mg/m2 and melphalan 180 mg/m2 were the appropriate doses to move into subsequent phase II trials. In POG 9079, four of eight medulloblastoma cases responded (1 complete response and 3 partial responses) while both children with CNS germinoma achieved a complete response.
In the POG-COG protocol reported here (9430), 29 eligible subjects were accrued between 1994 and 2003. This pilot trial remained open for an extended period, in part, related to transition of the Pediatric Oncology Group into the amalgamated Children’s Oncology Group setting. The long study duration has resulted in mature follow-up data for the 9 survivors, i.e. median of 7.5 years (range= 2.8–10). Four medulloblastoma survivors had metastatic disease at relapse which contrasts to the Duke series in which all 4 long term survivors had local relapse only.19 Children with the most favorable survival rate were those in complete remission at time of autograft performance (7 of 13 patients surviving).
A small minority of pediatric patients with recurrent or progressive malignant central nervous system tumors will attain second remission or minimal residual disease status in order to benefit potentially from high dose chemotherapy supported by hematopoietic stem cells. This concept has been substantiated by two recent comprehensive population analyses performed in England and Germany.8,9 In those studies, only 5–15% of children with relapsed or progressive medulloblastoma became disease free survivors. The denominator for the 6 medulloblastoma survivors applicable to the current protocol is not known, but data from this trial plus POG-COG phase II new agent results cited above suggest consistency with the European reports.
In summary, high dose chemotherapy, including cyclophosphamide and melphalan, with autologous hematopoietic stem cell support may salvage a few selected children with relapsed or progressive medulloblastoma and germinoma. This drug combination was well tolerated, but efficacy was not clearly better than other high dose chemotherapy programs. Larger trials will be necessary to assess relative efficacy of the various preparative regimens and strategies. Recently, COG has begun to explore feasibility of performing collaborative protocols between COG and non-COG institutions beyond North America. In addition, a new international conference series devoted to high dose chemotherapy and autografting for pediatric central nervous system tumors was initiated in late 2006 and may promote future progress.18
Acknowledgments
This study was supported by Children’s Oncology Group grant numbers CA98543.A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm
Footnotes
A preliminary report was presented at the 39th Annual Congress of the International Society of Paediatric Oncology in Mumbai, India on November 1, 2007.
References
- 1.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 3.Bouffet E, Baranzelli MC, Patte C, et al. Combined treatment modality for intracranial germinomas: result of a multicentre SFOP experience. Br J Cancer. 1999;79:1199–1204. doi: 10.1038/sj.bjc.6690192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretschmar C, Kleinberg L, Greenbert M, et al. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;48:285–291. doi: 10.1002/pbc.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbertson RJ, Gajjar A. Molecular biology of medulloblastoma: will it ever make a difference to clinical management? J Neuro-Oncol. 2005;75:273–278. doi: 10.1007/s11060-005-6750-z. [DOI] [PubMed] [Google Scholar]
- 6.Siegel MJ, Finlay JL, Zacharoulis S. State of the art chemotherapeutic management of pediatric brain tumors. Expert Rev Neurother. 2006;6:765–779. doi: 10.1586/14737175.6.5.765. [DOI] [PubMed] [Google Scholar]
- 7.Fouladi M, Blaney SM, Poussaint TY, et al. Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: A Pediatric Brain Tumor Consortium study. Cancer. 2006;107:2291–2297. doi: 10.1002/cncr.22241. [DOI] [PubMed] [Google Scholar]
- 8.Bode U, Simon A, Hasan C, et al. The role of high dose chemotherapy in the treatment of therapy-resistant CNS PNETs: HIT-REZ results. Haematol Rep. 2006;2:2. (abstr) [Google Scholar]
- 9.Pizer B, Stevens S, Robinson K, et al. A UKCCSG study of the treatment of relapsed CNS primitive neuroectodermal tumours using high dose chemotherapy. Haematol Rep. 2006;2:12. (abstr) [Google Scholar]
- 10.Mahoney DH, Strother D, Camitta B, et al. High-dose melphalan and cyclophosphamide with autologous bone marrow rescue for recurrent/progressive malignant brain tumors in children: A pilot Pediatric Oncology Group study. J Clin Oncol. 1996;14:382–388. doi: 10.1200/JCO.1996.14.2.382. [DOI] [PubMed] [Google Scholar]
- 11.Kadota RP, Stewart CF, Horn M, et al. Topotecan for the treatment of recurrent or progressive central nervous system tumors- A Pediatric Oncology Group phase II study. J Neuro-Oncol. 1999;43:43–47. doi: 10.1023/a:1006294102611. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz C, Strauss LC, Kepner JL, et al. Paclitaxel for the treatment of progressive or recurrent childhood brain tumors: A Pediatric Oncology Group phase II study. J Pediatr Hematol/Oncol. 2001;23:277–281. doi: 10.1097/00043426-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Dreyer ZE, Kadota RP, Stewart C, et al. A phase II study of idarubicin in pediatric brain tumors: A Pediatric Oncology Group study. Neuro-Oncol. 2003;5:261–267. doi: 10.1215/S115285170200056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: A report from the Children’s Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 15.Bomgaars L, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: A Children’s Oncology Group study. J Clin Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 16.Dunkel IJ, Finlay JL. Hematopoietic cell transplantation for brain tumors. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. Malden, MA: Blackwell; 2004. pp. 1345–1353. [Google Scholar]
- 17.Gururangan S, Gardner S, Finlay J. Autologous hematopoietic stem cell transplantation after high-dose chemotherapy for primary malignant tumors of the central nervous system. In: Atkinson K, Champlin R, Ritz J, et al., editors. Clinical Bone Marrow and Blood Stem Cell Transplantation. Cambridge, UK: Cambridge University Press; 2004. pp. 716–731. [Google Scholar]
- 18.Finlay J, Fossati-Bellani F, Massimino M. A consensus and state-of-the-art workshop: Marrow ablative chemotherapy with hematopoietic stem cell rescue for malignant brain tumors of childhood and adolescence. Haematol Rep. 2006;2:1–16. doi: 10.1002/pbc.22374. [DOI] [PubMed] [Google Scholar]
- 19.Graham ML, Herndon JE, Casey JR, et al. High-dose chemotherapy with autologous stem-cell rescue in patients with recurrent and high-risk pediatric brain tumors. J Clin Oncol. 1997;15:1814–1823. doi: 10.1200/JCO.1997.15.5.1814. [DOI] [PubMed] [Google Scholar]