Abstract
It has been reported that allergies are associated with depression and possibly suicide in women. Aggression is an important behavioral component that predisposes depressed individuals to suicidal acts. In the present study we examined the relationship between allergies and aggression to determine a potential contribution of allergies in factors of risk for suicidal behavior. Because stress plays a critical role in the manifestation of clinical symptoms of allergies and also in suicidal behavior, we also studied the role of acute stress. Female inbred Brown Norway rats known for their susceptibility to respiratory allergies were sensitized and challenged with a mixture of tree pollen and evaluated in the resident-intruder test for detection of aggressive behaviors. They were also subjected to acute stress by sessions of inescapable forced swimming and re-evaluated in the resident intruder test. Animals made allergic to tree pollen and subjected to acute stress displayed increased aggressive-like behavior as compared with control-saline treated animals or to their own aggressive scores previous to the stress session. These results suggest that allergies and stress increases aggressive-like behavior, indicating that these conditions may be important factors promoting altered emotional reactivity with the potential to influence suicidal behavior.
Keywords: neuroimmune, intranasal, Brown Norway, inbred, female, resident-intruder
Introduction
Suicide is a very complex behavior that is associated with different factors including among others, socioeconomic, demographic and genetic (1-3). One of the most important risk factors for attempted and completed suicide is suffering from a mental disorder, and major depression is the most common psychopathology that has been consistently associated with suicidal behavior (1,4-6). Other mental disorders include bipolar disorder, borderline personality disorder, substance abuse and schizophrenia (1,5,6). Certain personality traits are important factors that contribute to increase the risk for suicidal behavior in depressed individuals including impulsivity neuroticism and aggression (6-10). Studies about the contribution of these traits in increasing the risk for suicide in individuals suffering from major depression concluded that aggression, but not impulsivity or neuroticism, is the trait with clinical relevance that better predicts increased suicide risk in people with depression (11). Thus, an aggressive state in depressed individuals significantly increases risk for suicidal behavior; it has been said that to be able to kill oneself, one should be “ready to die and ready to kill”. Moreover, based on the fact that only a relative small percentage of psychiatric patients attempt suicide, it has been hypothesized that a secondary stressor in people at high risk is likely to act as a precipitant for suicidal behavior (1). The interaction of a stressor such as an acute psychosocial crisis in psychiatric patients with a predisposition for suicidal behavior (the stress-diathesis model) explains and also predicts the occurrence of suicidal behavior (1,12).
Epidemiological studies show that depression and suicide manifest in the population with marked oscillations on their incidence. An increased rate of depression as measured by hospitalization has been documented during early spring followed by an increase incidence in suicide rates during late spring (13). It is important to note that the spring peak in suicide is more prominent for violent suicides than for non violent suicides (14). The reasons for these seasonal patterns in disease fluctuation are highly debated. Several additional epidemiological studies reported that in women, suffering from an atopic condition increases the risk for depression (15,16) and seasonality of completed suicide (17) and that seasonal aeroallergen exposure may be related with suicide completion (18). Based on these associations, we have previously hypothesized that seasonal allergies may be a factor contributing to increased incidence in suicide during spring, particularly in women (18,19). However, the behavioral traits that may be influenced by allergies leading to depression and suicide are unknown. Moreover, the interactions of these factors during a stressful situation have not been explored.
To determine if seasonal allergies influence behavioral traits that may contribute to suicidal behavior in females, we evaluated in the resident intruder test female rats made allergic to tree pollen. The resident intruder test is used to detect aggression based on elements of rat behavior expressed during social encounters (20,21). To explore the contribution of an acute stressful event in this paradigm, we introduced in this model inescapable forced swim sessions known to induce learned helplessness in rodents (22,23).
Methods
Allergy to Pollen
Inbred Female Brown Norway (BN/NHhsd) rats (n = 18) were obtained from Harlan-Sprague Dawley (Indianapolis, IN). This strain was selected based on their susceptibility to develop respiratory allergic reactions after repeated exposure with an antigen (24,25). They were sensitized by repeated intranasal instillations with a mixture of equal parts of tree pollen (oak, cedar, maple and birch) (Greer Laboratories, Lenoir, NC) dissolved in saline (250 mg/ml pollen mixture) for 5 consecutive days. The animals were slightly anesthetized with isofluorane in induction chambers and at the moment of awakening were administered with 100 μl per nostril of saline solution or pollen mixture. The animals received an intranasal dose at day 15 after the last administration to boost the immune reaction. The rats were challenged 7 days later after the boosting administration for 5 consecutive days with the same solutions. Control animals were treated with saline under the same schedule. Allergic animals developed mild allergic symptoms evidence by watery runny nose.
Stress Exposure
Groups of rats (n = 9) consisted of control saline and pollen treated animals evaluated on a first session of resident intruder test on day five of the challenge with tree pollen. Five days later control and pollen treated rats were subjected to acute stress exposure by forced swimming in a vertical glass cylinder (diameter 22.5 cm and height 60 cm) containing 35 cm of water maintained at 25?C for 15 min. Twenty-four hours later the animals were re-tested in the resident intruder test. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore.
Resident Intruder Test
Resident and intruder rats were separated 21 days before testing. Rats were housed in Plexiglas cages with standard food pellets and water available ad libitum in a room at a constant temperature of 23° C. All animals were maintained on a 12:12 L:D cycle (lights on at 07:00 hr) and each rat remained in its original cage throughout the experimental procedures. Resident intruder tests were performed between 10:00 AM - 12:00 PM each day. The resident rat remained in its original home cage placed in the testing area for ten minutes prior to intruder entry to ensure the resident was fully acclimated to its surroundings. An unfamiliar intruder was taken from a separate cage and placed in the resident cage to begin the test. Tests were videotaped from a horizontal angle with a Sony Mini DV camcorder in sessions of ten minutes. Aggressive behavior was measured by two observers both during the test, and re-scored on video after the test. Behavior that was considered aggressive was measured solely from the resident directed towards the intruder and was scored based on the number of aggressive actions that occurred. After the ten-minute test, the intruder was removed from the cage and returned to its original cage. Data was analyzed with a one way ANOVA followed by Neuman-Keuls as post hoc comparisons. Statistical significance was set at alpha 0.05.
Results
Identification of Agonistic Behaviors in Female Brown Norway Rats
Based on the descriptions of DeBold and Miczeck, 1984 (26) and Blanchard 1982 (27) and Mitchell 2005 (20,21) the following agonistic behaviors were identified in all groups and conditions tested: a) Lunging toward the intruder b) placing paws on intruder's back c) pursuing or following the intruder d) cornering (backing intruder into a corner of the cage). The following agonistic behaviors were not identified in any group or condition tested: a) bite, b) boxing c) nipping.
Additional behavioral categories identified included exploration (locomotion and rearing) and investigation (nose to nose sniff, genitalia sniff) and maintenance (grooming, digging). The relative percentage of these behaviors during the 10 minutes test is shown in table 1.
Table 1.
Mean percentage values ± standard error mean of different behavioral categories identified during 10 minutes of an encounter with an unfamiliar intruder conspecific in control saline rats or rats sensitized and challenged with tree pollen. The relative time spent on each behavior was grouped according to the motivational state as follow: locomotion and rearing (Exploration); nose to nose sniff and genitalia sniff (Investigation); lunging toward the intruder, placing paws on intruders back, pursuing or following the intruder and backing intruder into a corner of the cage (Aggression); grooming and digging (Maintenance); (adapted from Mitchell, 2005 (20))
| Before Stress | After Stress | |||
|---|---|---|---|---|
| Control | Pollen | Control | Pollen | |
| Exploration | 42 ± 3 | 39 ± 4 | 34 ± 3.6 | 28 ± 4.5 |
| Investigation | 47 ± 4.4 | 49 ± 3.7 | 51 ± 6.5 | 57 ± 8.4 |
| Aggression | 4.2 ± 6.5 | 3.9 ± 2.5 | 5.1 ± 1.8 | 9.6 ± 2.2 |
| Maintenance | 6.8 ± 2.7 | 8.4 ± 1.1 | 8.2 ± 2.3 | 5 ± 1.3 |
Stress-Induced Aggressive-Like Behavior in Rats Allergic to Tree Pollen
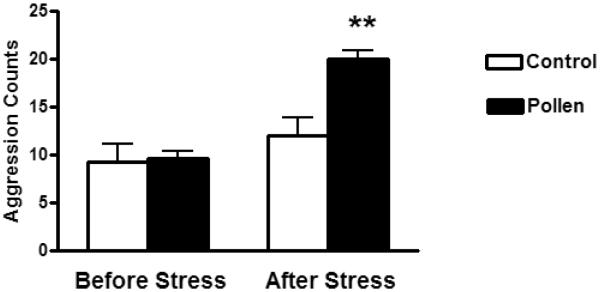
Based on the consideration that all of the agonistic behaviors identified correspond to the same category of low intensity aggressive behaviors, the frequency of each individual agonistic behavior for each animal was summarized giving a total aggression count corresponding to the 10 minutes of test. The analysis of variance ANOVA for total aggression counts showed a significant difference between groups [F (1, 20) = 12.66; p < 0.0001]. Post hoc analysis showed that animals made allergic to tree pollen and subjected to the stress session had increased aggression scores compared with the rest of the groups (Figure 1). No differences were found for pollen treated animals with respect to control if they were not subjected to the stressor.
Figure 1.
Total number of aggressive behaviors (lunging toward the intruder, placing paws on intruders back, pursuing or following the intruder and backing intruder into a corner of the cage) identified during 10 minutes of an encounter with an unfamiliar intruder conspecific in control saline rats or rats sensitized and challenged with tree pollen. Before Stress: animals tested before acute stress session. After Stress: animals tested after an acute stressor consisting of 15 minutes of inescapable forced swimming.
** p < 0.0001.
Discussion
The present study showed that allergies increase low intensity aggressive-like behaviors only when they interacted with an acute stressor. Since acute stressors are unpredictable in nature, the present results lead us to speculate that seasonal allergies are a necessary, yet not sufficient factor that may contribute to increase the risk for suicidal behavior during spring via increased aggressive behavior. Moreover, the present study analyzes only one aspect of the full spectrum of behavioral risk factors for suicide, the contribution of allergic processes in depression, anxiety, impulsivity, and other suicide risk factors including alterations in sleep patterns have not been investigated.
Studies on patterns of aggressive behaviors displayed by female out-bred Norway rats indicates that high intensity aggressive behaviors in female rats are rare and that they may represent defensive behaviors towards the intruder rather than offensive actions (26-28). However, these behaviors which include striking bites towards the face have been documented and they were not observed in the present study. As mentioned earlier, all the aggressive postures observed are considered to be of low intensity aggression (26-28). This has been documented previously for the resident-intruder test in male inbred Brown Norway rats (29). Whether or not these features represent aggressive traits in humans with any relevance for suicidal behavior is only speculation. In any case, the present data shows that acute stress during an allergic process clearly influences agonistic behaviors as evidenced by the doubling of these encounters that represent altered social interactions. Altered social interactions are recognized as characteristic of pre-suicidal states in humans, and the inability to cope with a stressful situation is a hallmark of many mental disorders (23).
Several mechanisms may explain how allergies influence aggressive-like behaviors. One of them is through the effect that cytokines released during an allergic process may have on rage and aggression. It has been reported that certain cytokines such as interleukin-1β (IL-1β) administered in several brain regions potentiates rage and aggression in cats (30). Moreover, the authors showed that interleukin-2 may suppress or enhance defensive rage in cats depending on the region of the brain in which it was administered (31). Another study demonstrated that peripheral administration of IL-1β inhibits inter male agonistic behavior in a dose dependent manner (32). Conversely, it has been shown that mice deficient for interleukin-6 display high degrees of aggressive behavior (33). In humans, hostility, physical and verbal aggression has been positively correlated with tumor necrosis factor-alpha expression in stimulated monocytes (34). Although studies on the behavioral effects of the major cytokines involved in allergic inflammation have received little attention, it is possible that these cytokines including interleukin-4, 5 and 13 among others may modulate aggressive behavior through interactions with cytokines such as IL-6, IL-1β and TNF-α.
Despite the fact that immune challenge and cytokine administration have been shown capable of modifying components of aggressive behaviors, in the present study the manifestation of aggressive-like behaviors was not seen in allergic animals but only after they have been exposed to an acute stressor. This is an important consideration because it may point to an important mechanism of neuro-immune interaction that have been consistently reported in the literature. It is known that the relationship between stress and allergic conditions is reciprocal where stress worsens atopic disorders (35,36) and atopic disorders influence emotional reactivity (37,38). The core physiology of this reciprocal connection involves the hypothalamic-pituitary-adrenal (HPA)-axis that can both modulate immune function through the effects of glucocorticoids, and its activity can be modulated by immune function through the effects of cytokines (39-41). In addition, activation of mast cells by corticotrophin-releasing factor (CRF), that is also a potent activator of the HPA-axis, has been proposed as a contributing mechanism (36). Therefore, nasal allergies induced by tree pollen may lead to a similar situation to that we have recently reported in which inflammation in the nasal cavities produces a sub-syndromal state of sickness increasing cytokine expression and priming the HPA-axis to release large amounts of glucocorticoids under stress such as forced swimming (42) and potentially influencing later behavioral responses.
Conclusions
In conclusion, altered agonistic behaviors were observed in female rats made allergic to tree pollen and subjected to acute stress. This relationship may represent an important factor of altered emotional reactivity and its behavioral expression in individuals that suffer from both atopic and mood disorders. Further studies are necessary to fully characterize and determine the mechanisms of this association.
References
- 1.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4(10):819–28. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 2.Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to socioeconomic, demographic, psychiatric, and familial factors: a national register-based study of all suicides in Denmark, 1981-1997. Am J Psychiatry. 2003;160(4):765–72. doi: 10.1176/appi.ajp.160.4.765. [DOI] [PubMed] [Google Scholar]
- 3.Agerbo E, Mortensen PB, Eriksson T, Qin P, Westergaard-Nielsen N. Risk of suicide in relation to income level in people admitted to hospital with mental illness: nested case-control study. BMJ. 2001;322(7282):334–5. doi: 10.1136/bmj.322.7282.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann JJ, Currier D. A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Arch Suicide Res. 2007;11(1):3–16. doi: 10.1080/13811110600993124. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med. 2003;33(3):395–405. doi: 10.1017/s0033291702006943. [DOI] [PubMed] [Google Scholar]
- 6.Arsenault-Lapierre G, Kim C, Turecki G. Psychiatric diagnoses in 3275 suicides: a meta-analysis. BMC Psychiatry. 2004;4:37. doi: 10.1186/1471-244X-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGirr A, Renaud J, Bureau A, Seguin M, Lesage A, Turecki G. Impulsive-aggressive behaviours and completed suicide across the life cycle: a predisposition for younger age of suicide. Psychol Med. 2007;1:11. doi: 10.1017/S0033291707001419. [DOI] [PubMed] [Google Scholar]
- 8.Brezo J, Paris J, Turecki G. Personality traits as correlates of suicidal ideation, suicide attempts, and suicide completions: a systematic review. Acta Psychiatr Scand. 2006;113(3):180–206. doi: 10.1111/j.1600-0447.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 9.Dumais A, Lesage AD, Alda M, Rouleau G, Dumont M, Chawky N, Roy M, Mann JJ, Benkelfat C, Turecki G. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. Am J Psychiatry. 2005;162(11):2116–24. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- 10.McGirr A, Renaud J, Seguin M, Alda M, Benkelfat C, Lesage A, Turecki G. An examination of DSM-IV depressive symptoms and risk for suicide completion in major depressive disorder: a psychological autopsy study. J Affect Disord. 2007;97(1-3):203–9. doi: 10.1016/j.jad.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Keilp JG, Gorlyn M, Oquendo MA, Brodsky B, Ellis SP, Stanley B, Mann JJ. Aggressiveness, not impulsiveness or hostility, distinguishes suicide attempters with major depression. Psychol Med. 2006;36(12):1779–88. doi: 10.1017/S0033291706008725. [DOI] [PubMed] [Google Scholar]
- 12.Grunebaum MF, Ramsay SR, Galfalvy HC, Ellis SP, Burke AK, Sher L, Printz DJ, Kahn DA, Mann JJ, Oquendo MA. Correlates of suicide attempt history in bipolar disorder: a stress-diathesis perspective. Bipolar Disord. 2006;8(5 Pt 2):551–7. doi: 10.1111/j.1399-5618.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin FK, Jamison KR, Ghaemi SN. Manic-depressive illness: bipolar and recurrent depression. 2nd ed edition Oxford Univ Press; New York, NY: 2007. [Google Scholar]
- 14.Maes M, Cosyns P, Meltzer HY, De Meyer F, Peeters D. Seasonality in violent suicide but not in nonviolent suicide or homicide. Am J Psychiatry. 1993;150(9):1380–5. doi: 10.1176/ajp.150.9.1380. [DOI] [PubMed] [Google Scholar]
- 15.Timonen M, Jokelainen J, Hakko H, Silvennoinen-Kassinen S, Meyer-Rochow VB, Herva A, Rasanen P. Atopy and depression: results from the Northern Finland 1966 Birth Cohort Study. Mol Psychiatry. 2003;8(8):738–44. doi: 10.1038/sj.mp.4001274. [DOI] [PubMed] [Google Scholar]
- 16.Timonen M, Jokelainen J, Herva A, Zitting P, Meyer-Rochow VB, Rasanen P. Presence of atopy in first-degree relatives as a predictor of a female proband's depression: results from the Northern Finland 1966 Birth Cohort. J Allergy Clin Immunol. 2003;111(6):1249–54. doi: 10.1067/mai.2003.1546. [DOI] [PubMed] [Google Scholar]
- 17.Timonen M, Viilo K, Hakko H, Sarkioja T, Meyer-Rochow VB, Vaisanen E, Rasanen P. Is seasonality of suicides stronger in victims with hospital-treated atopic disorders? Psychiatry Res. 2004;126(2):167–75. doi: 10.1016/j.psychres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Postolache TT, Stiller JW, Herrell R, Goldstein MA, Shreeram SS, Zebrak R, Thrower CM, Volkov J, No MJ, Volkov I, Rohan KJ, Redditt J, Parmar M, Mohyuddin F, Olsen C, Moca M, Tonelli LH, Merikangas K, Komarow HD. Tree pollen peaks are associated with increased nonviolent suicide in women. Mol Psychiatry. 2005;10(3):232–5. doi: 10.1038/sj.mp.4001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman A, Tonelli LH, Roberts D, Stiller JW, Jackson MA, Soriano JJ, Yousufi S, Rohan KJ, Komarow H, Postolache TT. Mood-worsening with high-pollen-counts and seasonality: a preliminary report. J Affect Disord. 2007;101(1-3):269–74. doi: 10.1016/j.jad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell PJ. Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol. 2005;526(1-3):147–62. doi: 10.1016/j.ejphar.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell PJ, Redfern PH. Animal models of depressive illness: the importance of chronic drug treatment. Curr Pharm Des. 2005;11(2):171–203. doi: 10.2174/1381612053382250. [DOI] [PubMed] [Google Scholar]
- 22.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47(4):379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 23.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9):775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 24.Steerenberg PA, Dormans JA, van Doorn CC, Middendorp S, Vos JG, van Loveren H. A pollen model in the rat for testing adjuvant activity of air pollution components. Inhal Toxicol. 1999;11(12):1109–22. doi: 10.1080/089583799196619. [DOI] [PubMed] [Google Scholar]
- 25.Motta A, Peltre G, Dormans JA, Withagen CE, Lacroix G, Bois F, Steerenberg PA. Phleum pratense pollen starch granules induce humoral and cell-mediated immune responses in a rat model of allergy. Clin Exp Allergy. 2004;34(2):310–4. doi: 10.1111/j.1365-2222.2004.01872.x. [DOI] [PubMed] [Google Scholar]
- 26.DeBold JF, Miczek KA. Aggression persists after ovariectomy in female rats. Horm Behav. 1984;18(2):177–90. doi: 10.1016/0018-506x(84)90041-2. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Ann Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- 28.DeBold JF, Miczek KA. Sexual dimorphism in the hormonal control of aggressive behavior of rats. Pharmacol Biochem Behav. 1981;14(Suppl 1):89–93. doi: 10.1016/s0091-3057(81)80015-9. [DOI] [PubMed] [Google Scholar]
- 29.Berton O, Ramos A, Chaouloff F, Mormde P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behav Genet. 1997;27(2):155–66. doi: 10.1023/a:1025641509809. [DOI] [PubMed] [Google Scholar]
- 30.Zalcman SS, Siegel A. The neurobiology of aggression and rage: role of cytokines. Brain Behav Immun. 2006;20(6):507–14. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Bhatt S, Zalcman S, Hassanain M, Siegel A. Cytokine modulation of defensive rage behavior in the cat: role of GABAA and interleukin-2 receptors in the medial hypothalamus. Neuroscience. 2005;133(1):17–28. doi: 10.1016/j.neuroscience.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 32.Cirulli F, Pistillo L, de Acetis L, Alleva E, Aloe L. Increased number of mast cells in the central nervous system of adult male mice following chronic subordination stress. Brain Behav Immun. 1998;12(2):123–33. doi: 10.1006/brbi.1998.0505. [DOI] [PubMed] [Google Scholar]
- 33.Alleva E, Cirulli F, Bianchi M, Bondiolotti GP, Chiarotti F, De Acetis L, Panerai AE. Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur J Neurosci. 1998;10(12):3664–72. doi: 10.1046/j.1460-9568.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 34.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29(9):1119–28. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Vig RS, Forsythe P, Vliagoftis H. The role of stress in asthma: insight from studies on the effect of acute and chronic stressors in models of airway inflammation. Ann N Y Acad Sci. 2006;1088:65–77. doi: 10.1196/annals.1366.023. [DOI] [PubMed] [Google Scholar]
- 36.Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann N Y Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 37.Hashizume H, Takigawa M. Anxiety in allergy and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2006;6(5):335–9. doi: 10.1097/01.all.0000244793.03239.40. [DOI] [PubMed] [Google Scholar]
- 38.Buske-Kirschbaum A, Hellhammer DH. Endocrine and immune responses to stress in chronic inflammatory skin disorders. Ann N Y Acad Sci. 2003;992:231–40. doi: 10.1111/j.1749-6632.2003.tb03153.x. [DOI] [PubMed] [Google Scholar]
- 39.Mastorakos G, Ilias I. Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci. 2006;1088:373–81. doi: 10.1196/annals.1366.021. [DOI] [PubMed] [Google Scholar]
- 40.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29(4-5):891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6(4):318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonelli LH, Holmes A, Postolache TT. Intranasal Immune Challenge Induces Sex-Dependent Depressive-Like Behavior and Cytokine Expression in the Brain. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]