Abstract
The inflammatory response to Pseudomonas aeruginosa is not properly regulated in the lungs of cystic fibrosis patients (CF). In the lung epithelium of individuals with wild-type (WT) CF Transmembrane Conductance Regulator (CFTR), lipid rafts containing CFTR are rapidly formed in response to P. aeruginosa infection and this response is closely linked to resistance to infection and disease. We found these rafts also contained high levels of caveolin -1 (Cav-1) and thus examined the sensitivity of Cav-1 knock-out (KO) mice to P. aeruginosa challenge in both acute and chronic P. aeruginosa infection models. We found that Cav-1 KO mice had increased sensitivity to P. aeruginosa infection, as represented by an increased mortality rate, elevated bacterial burdens recovered from lungs and spleens and elevated inflammatory responses. These findings correlated with the decreased ability of Cav-1 deficient neutrophils to phagocytose P. aeruginosa. In addition, P. aeruginosa colonized Cav-1 KO mice much better compared to the WT controls in a model of chronic infection, indicting an important contribution of Cav-1 to innate host immunity to P. aeruginosa infection in the setting of both acute pneumonia and chronic infection typical of CF.
Keywords: caveolin, P. aeruginosa, acute pneumonia
Introduction
While Pseudomonas aeruginosa is a frequent cause of acute nosocomial pneumonia in patients undergoing mechanical ventilation, burn victims, patients with corneal trauma, patients with healing surgical wounds (1) and cystic fibrosis (CF) (2, 3), healthy individuals are generally highly resistant to serious infection. There are clearly multiple, redundant innate immune mechanisms contributing to this resistance. However, it is also clear a key factor is the cystic fibrosis transmembrane conductance regulator (CFTR), inasmuch as >80% of CF patients eventually develop chronic lung infections with P. aeruginosa (3, 4). As perhaps the only human genetic disease where infectious consequences, including most of the morbidity and ultimate life-shortening mortality, are so overwhelmingly associated with a single pathogen, the molecular and cellular consequences from having defective CFTR molecules provides a focused setting for the detailed study of mammalian innate immunity to this important pathogen.
A major rapid, early wild-type (WT) host response to P. aeruginosa infection is the CFTR-dependent uptake of this pathogen by nasal and bronchial epithelial cells via a lipid raft-dependent mechanism (5, 6). Lipid rafts are specialized areas of the plasma membrane that are enriched in cholesterol and sphingolipids (5). It is thought that bacterial infection by P. aeruginosa stimulates formation of lipid rafts containing CFTR that permits the bacterium to invade the epithelial cell, initiate protective host inflammatory responses followed by apoptosis and shedding of the superficial layer of epithelial cells with internalized bacteria (5, 6). Lack of functional CFTR prevents this response, allowing P. aeruginosa to establish an early colonization in the thickened mucus of CF patients.
Lipid rafts components are usually isolated by density-gradient separation, partitioning into Triton-X100 insoluble fractions. These fractions also retain the caveolin-1 (Cav-1) and caveolin-2 (Cav-2) proteins. Caveolin-1 is the major component of caveolae, morphologically identifiable plasma membrane invaginations that are distinct from clathrin coated pits. Caveolae appear as non-coated pits in the plasma membrane or as 50 to 100-nm flask or Ω–shaped invaginations (7). Caveolae are involved in pinocytosis, endocytosis, and cell signaling and are very abundant in lung cells, where they are found in endothelial cells, type I pneumocytes, and bronchial epithelial cells (8). Disruption of the cav1 gene leads to loss of caveolae (9) and exogenous expression of cav1 induces caveolae formation in cells lacking both caveolae and Cav-1 (10, 11). Unlike Cav-1, Cav-2 is insufficient to induce caveolae biogenesis (12). Caveolin-1 oligomerizes into high-molecular structures of about 400 kDa. In contrast, Cav-2 is incapable of forming high–molecular-weight oligomers, a property which may explain why Cav-2 by itself can not stimulate formation of caveolae (12). However, Cav-2 forms heterocomplexes with Cav-1.
Studies using caveolin deficient mice have begun to unravel the complex role of caveolins as regulators of various biologic processes. In general, caveolin-deficient mice are viable, but each caveolin knockout seems to present with different phenotypes. For example caveolin-1-deficient mice display elevated nitric oxide synthase activity in vascular endothelial cells and enhanced susceptibility to carcinogen-induced tumor development (9, 13). Mice deficient in both caveolin-1 and caveolin-2 display severe pulmonary abnormalities attributed to endothelial hyperproliferation (9). In contrast, caveolin-3 deficient mice develop severe cardiac hypertrophy (14). However, information regarding the role of caveolins during bacterial infection is limited.
Recent research demonstrated Cav-2 facilitates P. aeruginosa entry into rat primary bronchial cells and the mouse lung epithelial (MLE) cell line 12 (15). Knock-down of either Cav-1, which also eliminates Cav-2 synthesis (12, 16), or Cav-2 alone, significantly decreased the uptake of P. aeruginosa by these cell lines by roughly 50% (17). We have shown that during bacterial entry into cells, P. aeruginosa co-localizes with Cav-1 and CFTR in variety of human cell lines(18) in association with the CFTR-receptor for P. aeruginosa (19, 20). Caveolin-1 was also identified as a prominent protein found in lipid rafts of cells with WT CFTR after only 15 min of infection with P. aeruginosa (20). Here we examined the role of Cav-1 in both an acute and chronic P. aeruginosa infection model. We found that Cav-1 knock–out (Cav-1 KO) mice have increased sensitivity to P. aeruginosa infection, as represented by an increased mortality rate, elevated bacterial burdens recovered from lungs and spleens and elevated inflammatory responses. These findings correlated with the decreased ability of neutrophils to phagocytose P. aeruginosa. In addition, P. aeruginosa colonized Cav-1 KO mice much better compared to the WT controls in a model of chronic infection, indicting an important contribution of Cav-1 to innate host immunity to P. aeruginosa infection in the setting of CF.
Materials and methods
Mice
Breeding pairs of cav1 KO and control mice (B6129SF2/J) were obtained from Jackson Laboratory. Mice were housed and bred in the Channing Laboratory Animal Facilities. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Harvard Medical Area Office for Research Subject Protection.
Acute P. aeruginosa pneumonia model
Gender matched 6-9 weeks old cav1 KO and control mice were sedated with ketamine hydrochloride (65 mg/kg) and xylazine (13 mg/kg), then infected intranasally (IN) with doses of P. aeruginosa from 2 × 107 cfu to 2 × 108 cfu, using strains PAO1 or PAK as described (20). Mice were sacrificed at 6 h or 16 h post-infection by intravenous injection of phenobarbital. Lungs were inflated by instillation of PBS, removed, weighed, homogenized in 10% FBS in DMEM, and aliquots were plated on P. aeruginosa selective cetrimide plates to enumerate bacterial levels.
Flow cytometry analysis of cells in bronchoalveolar lavage
Following 20 h of infection of cav1 KO and control animals with P. aeruginosa strain PAO1, mice were euthanized with pentobarbital then bronchiolar lavage (BAL) fluid obtained by infusion, via an intratracheal needle, of 1 ml of PBS that was then recovered via the same needle. This process was repeated two more times. Cells were recovered from the BAL by centrifugation, the concentration determined in a hemocytometer, then 1 ×106 cells stained with antibody to the PMN markers CD11b (M1/70) and Gr-1 (RB6.8C5 clone) using phycoerythrin-conjugated or FITC-conjugated antibodies, respectively (BD Pharmingen) as previously described (21). Stained cells were analyzed by flow cytometry using FACS Calibur (BD Biosciences, San Jose, CA) with CellQuest software (BD Biosciences, San Jose, CA). Neutrophils were identified based on a combination of cell surface marker characteristics, size and granularity as described (22, 23).
Inflammatory cytokine profiling
Levels of mouse cytokines in BAL were simultaneously measured using a Meso Scale Discovery (MSD) multiplex 7-spot electrochemiluminescence (ECL) assay read by an ultra low noise charge-coupled device (CCD) Imager 2400 (Meso Scale Discovery, Gaithersburg, MD, USA). The cytokines included interleukin (IL)-1β, IL-6, IL-12p70, IL-10, IFNγ and the alpha chemokine neutrophil attractant and activator CXCL1/GRO (also known as KC). The MSD ECL platform has been previously validated against cytokine standards recommended by WHO and U.K. National Institute for Biological Standards and Control (NIBSC) and by comparison to traditional ELISA (24).
Phagocytosis assay
One ml of mouse blood was collected into heparinized tubes using 20 or 22 G needles to obtain blood from the heart of euthanized animals The blood from 3-4 mice was pooled for these experiments. The blood was centrifuged at 200 x g at 23°C for 10 min to collect the buffy coat layer. The cellular suspension was overlaid onto Histopaque 1077 (Sigma) and Histopaque 1119 (Sigma) gradients and centrifuged for 30 min at 700 x g. The neutrophil fraction was collected at the interface of Histopaque 1077 and Histopaque 1119 layers. 1 × 106 purified PMN were resuspended in RPMI, 10% FBS, and mixed with P. aeruginosa strain PO1 at the ratio of 100 PMN: 1 bacteria. Aliquots of PMN/bacteria were incubated at 37°C for 180 min. Extracellular bacteria was quantified by plating an aliquot of the mixture on P. aeruginosa selective cetrimide plates. The phagocytosed bacteria was quantified by lysing the cells with TSB/0.5% Triton and plating on cetrimide plates.
Chronic model of P. aeruginosa infection
Establishment of chronic infection of mice, first developed for transgenic CF mice, was carried out as described (25). I this model, the recovery of P. aeruginosa on throat swabs and development of a significant antibody response are taken as documentation that long term infection of the respiratory tract had been established. Mice (N=10 per group) were treated for 5 days with oral levofloxacin in the drinking water to clear out enteric flora that colonize the murine oropharynx and interfere with establishment of P. aeruginosa infection (25). Oropharyngeal infection was induced by placing the bacteria in the drinking water (107 cfu/ml) for 5-7 days. The infected water was replaced with acidified water (pH 4.5) to prevent bacterial growth in the water and cross-infection within a cage (25). Oropharyngeal throat swabs were taken after the infection period to verify that all mice were exposed to P. aeruginosa. evaluated by ELISA as described (25). At the age of 9 months animals were sacrificed, lungs removed for histopathology as described for chronically infected transgenic CF mice (25).
Serum samples were collected from the chronically colonized animals. Serum dilutions were analyzed by ELISA for the presence of antibodies to P. aeruginosa. Briefly, P. aeruginosa strain N6 (an LPS-smooth, non-mucoid early clinical isolate from a CF patient) was grown overnight on TSA plates. A bacterial suspension was prepared by inoculating 10 ml of PBS with P. aeruginosa to an OD450 nm = 2.0. This bacterial stock was treated with NaN3 to kill the bacterial cells then used at 1/1000 dilution in 0.05M carbonate/bicarbonate buffer pH9.6 to coat Immunolon HBX microtiter plates (Fisher Scientific, Pittsburg, PA,USA) overnight at 4°C. Subsequently plates were washed with PBS/0.05%Tween 20, blocked with 5% BSA/PBS for 2h at 37°C, serial two fold dilutions of sera starting at 1:200 added and plates incubated for 90 min at 37°C, plates washed with PBS/0.05%Tween 20, incubated with P. aeruginosa adsorbed-anti-mouse IgG-alkaline phosphatase (AP) conjugate (A4656, Sigma, Saint Louis, MI, USA) for 1h at 37°C, washed with PBS/0.05% Tween 20 and developed with p-nitrophenyl phosphate substrate (pNPP) (N2765, Sigma, Saint Louis, MI, USA). Plates were read at 405 nm within 30 min of substrate addition. To adsorb the anti-mouse IgG AP conjugate with P. aeruginosa, P. aeruginosa strain N6 was grown overnight at 37°C on a TSA plate. A bacterial suspension was prepared by washing the bacteria off the TSA plate into 50 ml of 5%BSA/PBS then 10 μl of the anti-mouse IgG-AP conjugate added. After 30 min at 4°C, the bacteria were removed by centrifugation, the supernatant filtered and used in the ELISA.
Histopathology analysis
Lung tissues were fixed in 1% formaldehyde then embedded in paraffin using a routine histologic procedure. Four μm sections were cut and stained by hematoxylin-eosin at the Harvard Medical Area Rodent Histopathology Core and examined for differences in morphology after infection by the Histopathology Core Facility personnel.
Statistical analysis
Determinations of the significance of the differences in outcomes between cav1 KO and wild type control animals after P. aeruginosa infection were calculated by Kaplan–Meier survival curve comparisons and the P values derived from a Log Rank test. We also derived a hazard ratio and 95% confidence intervals (C.I.) associated with lack of Cav-1 (Prism 4, GraphPad). The bacterial loads in lungs and spleens were compared between cav1 KO and WT mice by a Mann-Whitney U test, as were antibody titers (Prism 4, GraphPad). As previously described (25) the marginal probability of oropharyngeal colonization of the mice by P. aeruginosa was estimated on the basis of irregularly timed repeated measures by solving generalized estimating equations (26) using the R software for statistical computing (www.r-project.org). The throat culture results obtained over time were used to calculate an overall probability of colonization of a given strain of mouse with a given strain of P. aeruginosa. Two-sided significance tests of the differences in the probability of colonization between mouse strains were obtained by using the ratio of the estimated difference to its robust standard error (26), which follows a standard normal distribution. Cytokine levels were analyzed for significance by two-tailed T tests (Prism 4, Graph Pad).
Results
Caveolin regulates the susceptibility to P. aeruginosa induced acute pneumonia
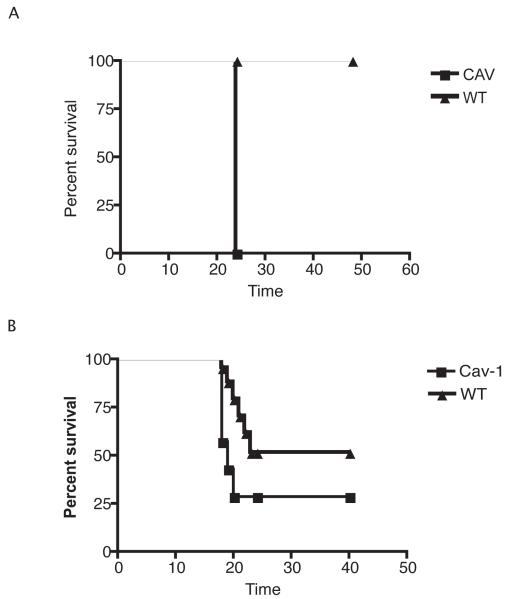
To determine if Cav-1 affects susceptibility to P. aeruginosa lung infection, groups of cav1 KO and genetically-related WT mice were challenged with various doses of two strains of P. aeruginosa: PAO1 or PAK. When mice were challenged with 1 × 108 or 4 × 108 cfu/animal of strain PAO1, cav1 KO mice displayed increased lethality (Log Rank Test, p=0.02) (Fig.1). Although the differences in the overall survival were small (about 20%), the calculated hazard ratio (3.041) indicated an increased hazard for early death of about 3-fold with a 95% confidence interval (CI) from 1.26 to 16. When cav1 KO and WT control mice were challenged with a different P. aeruginosa strain – PAK (2 × 108 cfu/mouse)-- increased mortality among cav1 KO mice was also seen. Seven out of seven animals succumbed to infection within 24 h, whereas all the wild type animals survived the challenge (P=0.0006, Fisher’s exact test for overall survival; odds ratio=0.004, 95% CI 0.00008-0.26). These findings indicate that Cav-1 is needed for full resistance to P. aeruginosa infection.
Figure 1. Caveolin-1 attenuates the survival from P. aeruginosa induced acute pneumonia.
A. Cav-1 KO (Cav-1) mice and WT control animals were infected with 1x108 cfu/mouse of P. aeruginosa strain PAO1. Survival is represented by Kaplan-Meier Survival Curves (P=0.02, Log Rank Test). B. cav1 KO (Cav-1) and WT control mice were infected with 2 ×108 cfu/mouse of P. aeruginosa strain PAK. Survival is represented by Kaplan-Meier Survival Curves (P=0.0003, Log Rank Test).
Caveolin-1 deficiency is associated with elevated bacterial burdens in the lung
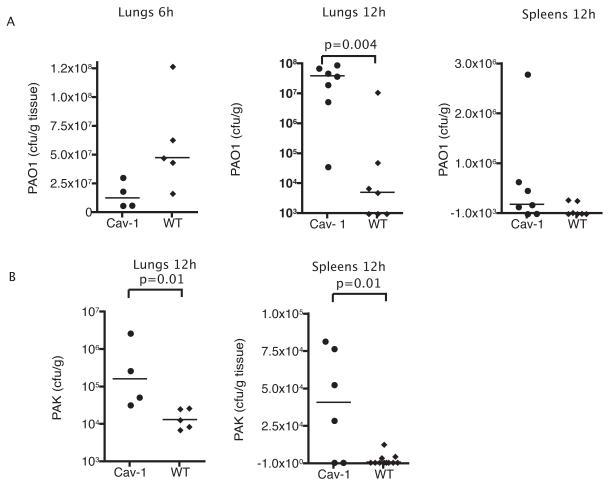
The cause of mortality from acute P. aeruginosa lung infection in mice is strongly associated with systemic bacterial spread to major organs, of which the spleen is the most sensitive indicator. To determine the bacterial burden in lungs and spleens of cav1 KO and WT mice, animals were challenged intranasally with 1 × 108 cfu/mouse of P. aeruginosa strain PAO-1 and sacrificed at different time points after the infection. Interestingly, 6 h after infection, cav1 KO mice showed a non-significant tendency for decreased levels of bacteria, when compared to the WT mice (Fig. 2A), However, after 12 h of infection, cav1-KO mice showed three fold higher levels of strain PAO1 in the lungs when compared to the WT control mice (Fig. 2) (P=0.004, Mann-Whitney U test). Elevated numbers of PAO1 bacteria were also found in the spleens of cav1 KO animals when compared to WT infected animals (Fig. 2A, P=0.05, Mann Whitney U test). A similar outcome in the lung was obtained when mice were challenged with PAK strain (Fig. 2B).
Figure 2. Caveolin-1 deficiency results in elevated bacterial burdens.
A. Bacterial counts in the lungs or spleens of cav1 KO (Cav-1) and WT mice. Groups of mice (n=7) were infected IN with 1 × 108 cfu/mouse of P. aeruginosa strain PAO1. Animals were sacrificed at 6 h and 12 h after the infection, lungs were extracted, homogenized and the bacterial burden was established by plating different dilutions of the homogenate on cetrimide plates. The data are representative of two individual experiments. B. Groups of mice (n=7) were infected with 1 × 107 cfu/mouse of P. aeruginosa strain PAK IN. The data are representative of two individual experiments.
Caveolin deficiency modifies P. aeruginosa-induced inflammation
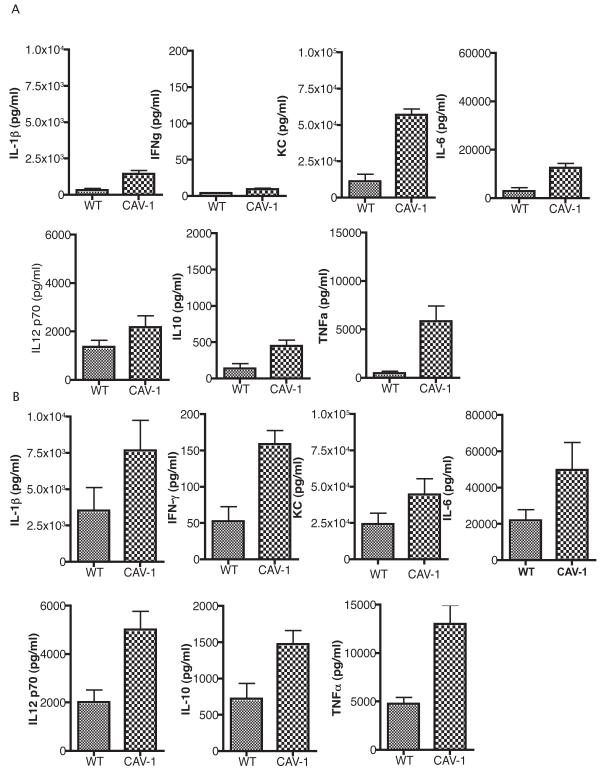
Since caveolae harbor a variety of signaling components and the Cav-1 protein has been implicated as an important regulator of inflammatory responses, we analyzed the cytokine profile induced in the lung of cav1 KO and WT mice 6-12 h following P. aeruginosa infection (Fig. 3) (27). The cytokine measurements demonstrated that Cav-1 deficient mice had elevated levels of IL-1β, TNF-α, IFN-γ, KC, IL-6, IL-10 and IL-12p70 when compared to WT control mice (P<0.05). These differences were observed as early as 6 h after infection and remained at later time points e.g. 12 h after infection (Fig. 3A and 3B). KC levels were dramatically increased in cav1 KO mice at 6 h after challenge with P. aeruginosa strain PAO1 when compared to WT mice. This tendency was preserved at the later time points where CAV-1 deficient mice maintained two to three fold elevated KC and IL-6 levels when compared to WT mice. Twelve hours post challenge with strain PAO1, IL-1β levels in the CAV-1 deficient mice were raised almost five-fold over that in the BAL from the WT mice demonstrating that the inflammatory response induced by P. aeruginosa that is characterized by production of IL-1β, TNF–α, KC and IL-6 is enhanced in the absence of Cav-1 protein.
Figure 3. Caveolin-1 modifies the inflammatory responses to P. aeruginosa lung infection.
cav1 KO (Cav-1) (n=7) and WT controls (n=7) were infected with 1 × 108 cfu/mouse of P. aeruginosa strain PAO1. Bronchoalveolar lavage fluid was harvested at 6 h (A) and 12 h (B) after infection and the cytokines levels were quantified. Bars represent means and SD from duplicate measurements from a representative experiment.
Analysis of phagocytosis of P. aeruginosa strain PAO1 by cav1-deficient and sufficient PMNs
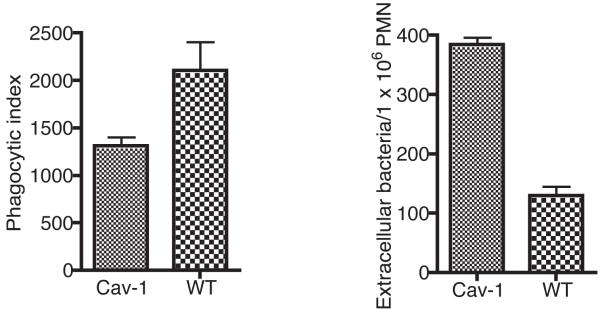
To determine if Cav-1 deficient PMN ingest live P. aeruginosa as efficiently as the PMNs obtained from WT mice, we performed phagocytic studies. Consistent with a previously described defect in cav1-deficient macrophages to phagocytose bacteria (28), we found that cav1 deficient PMNs also phagocytosed significantly less P. aeruginosa bacteria than did the WT control PMNs (Fig. 4).
Figure 4. Caveolin-1 deficiency inhibits the ability of neutrophils to phagocytose P. aeruginosa.
PMN derived from cav1 KO and WT mice were exposed to P. aeruginosa for 180 min to allow for the phagocytosis to occur. PMNs were lysed and aliquots were plated to determine the number of ingested bacteria (A). The phagocytic index represents the number of ingested bacteria per 1 × 106 PMNs. The number of extracellular bacteria was determined by plating and aliquot from the phagocytic reaction on P. aeruginosa selective media without lysing the PMNs. Bars represent means and error bars the SD from a representative experiment. P values determined by a Student T test.
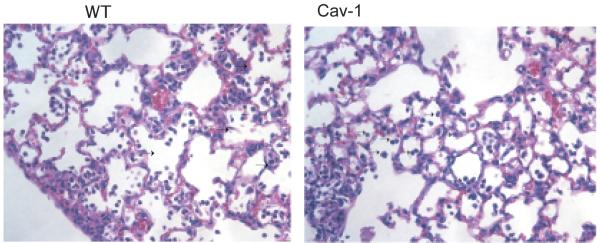
Histopathologic analysis of lungs in cav1 KO mice
To determine how the infection with P. aeruginosa affected lung pathology, tissue sections were stained with hematoxylin-eosin. The micrographs showed no dramatic differences in the morphology of the infected groups in either cav1 KO or WT mice. Both groups of infected mice showed signs of acute inflammation, with elevated neutrophil infiltrates (Fig. 5). Thus, the effect of the loss of Cav-1 on susceptibility to P. aeruginosa infection was not manifest as a major change in the histopathology of the lung during acute infection, indicating that the effects on phagocytosis and control of inflammatory responses were the primary driving force in the different outcomes from infection.
Figure 5. Caveolin-1 deficient mice present with comparable morphology after acute infection with P. aeruginosa strain PAO1 to that of WT control mice.
Groups of 7 cav1 KO and WT mice were infected with 1 × 108 cfu of P. aeruginosa strain PAO1and sacrificed at 12 h after infection. Lungs were extracted and embedded in paraffin. Sections were analyzed by H&E staining. Representative images are shown out of two individual experiments.
Caveolin deficiency results in increased chronic lung colonization with P. aeruginosa
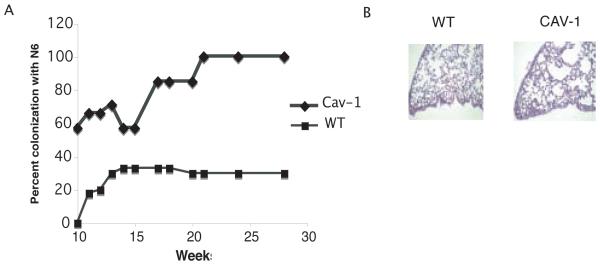
Since Cav-1 deficiency resulted in increased mortality from acute P. aeruginosa pneumonia, it was of interest to establish if this defect would allow for chronic lung infection to develop following infection via the drinking water with a clinical isolate of P. aeruginosa, strain N6, obtained from a CF patient early in the course of colonization. Cultures of throat swabs obtained right after levofloxacin treatment confirmed that all mice were initially free of detectable P. aeruginosa. After exposure to P. aeruginosa in the drinking water for 5 days, followed by replacement of the contaminated water with acidified water that prevents bacterial growth in this medium, all of the animals had positive throat culture swabs for P. aeruginosa, indicating both groups had initially acquired the pathogen via the water. After 7 weeks, to allow for establishment of lung infection, all mice were treated for two weeks with meropenem (1 mg/L) in their drinking water to kill bacteria residing in the upper oropharynx but not those in the lower respiratory tract. After antibiotic treatment about 60% of the Cav-1 KO mice had positive throat cultures for P. aeruginosa within 1 week, while only 20% of the WT mice had cultures positive for P. aeruginosa. The colonization with P. aeruginosa was then followed weekly for an additional 20 weeks (Fig. 6). The percent of P. aeruginosa colonized cav1 KO mice reached 100%, while only 30% percent of the WT had positive throat cultures for P. aeruginosa (P=0.0017; generalized estimating equation).
Figure 6. Caveolin-1 deficient mice are readily colonized with P. aeruginosa strain N6.
A. cav1 KO (Cav-1) and WT mice (N=10) were exposed to P. aeruginosa strain N6 in the sterile drinking water for one week, subsequently placed on acid water and monitored for oropharyngeal colonization. Seven weeks later the mice were treated with the antibiotic meropenem in the drinking water for two weeks and subsequently reinstated on acid water. Animals were monitored for the presence of P. aeruginosa in the throat by swab cultures after the antibiotic treatment was withdrawn. The percent of oropharyngeal colonization of cav1 KO and WT mice at each week is plotted. Differences in the probability of colonization of cav1 KO mice versus WT mice are significant at a level of P<0.001 using generalized estimating equations (26). B. Lung sections harvested from chronically colonized cav1 KO or WT mice and stained with H&E. Images shown are representative examples of infected lungs from Cav-1 deficient and WT animals.
To determine if chronic colonization with P. aeruginosa resulted in changes in lung morphology, lung tissue was obtained from chronically colonized WT and cav1 KO mice, embedded in paraffin, sectioned and stained with hematoxylin and eosin. No gross changes in morphology were observed (Fig. 6), consistent with prior results with CFTR-deficient mice chronically infected with non-mucoid P. aeruginosa (25) who also showed only modest changes in the lungs after 6-9 months of chronic infection.
An additional response to lung infection not seen in mice with only oropharyngeal colonization but not lung infection is the development of antibody responses to P. aeruginosa cells. Mice were tested for IgG antibody to killed P. aeruginosa N6 cells. cav1 KO mice had higher titers of IgG antibodies to P. aeruginosa cells than did the WT controls (Table 1, Mann-Whitney test, p=0.01). These differences were manifested as early as four weeks after the withdrawal of meropenem from the drinking water and were maintained throughout the study. The WT mice with positive throat cultures did not have higher serum IgG antibody titers than the WT with negative throat cultures, indicating that in these mice the colonization was confined to the upper oropharynx and had not reached tissue sites such as the lung, where antibody responses are induced. Overall, all 10 cav1 KO mice developed chronic P. aeruginosa lung infection, as evidenced by positive throat cultures and antibody responses, whereas none of the WT controls had both of these measured of chronic infection (P<0.0001, Fisher’s exact test).
Table 1. Antibody titers in sera of caveolin-1 knock out mice or wild type animals in a chronic colonization model using P. aeruginosa strain N6.
| Weeks post infection | Antibody Titers Cav-1 KO |
Antibody Titers WT |
|---|---|---|
| Pre-immune sera | 275±103 | 490±900 |
| 4 | 1828±1343 | 866±1200 |
| 8 | 5257±9837 * | 780±1280 |
| 20 | 5257±9837 * | 780±1280 |
Antibody titers represent the average values of the individual mouse antibody titers±standard deviations. To determine the individual titers, serial dilutions of mouse sera were analyzed by ELISA. The assigned titer value is indicative of the last dilution in which the antibody was detected (22). The titer was considered to be the value of the last dilution, that had an OD 405 reading two fold higher than the background. The star depicts that the titers of cav1 deficient mice at weeks 8 and 20 are significantly different from the corresponding titers of the wild type mice by Mann-Whitney test at a P value of <0.01.
Discussion
Prior results (15, 17, 29) have associated caveolins with lung epithelial cell responses to P. aeruginosa, but the actual importance of these responses in regard to susceptibility and resistance to infection was not investigated. While numerous studies have indicated caveolins are important components of innate immune responses to pathogen virulence factors like LPS (9, 10, 30), there are only a limited number of studies that aimed to clarify the significance of Cav-1 in modulating innate immune responses against live pathogens.
In this study we tested the hypothesis that the P. aeruginosa-dependent recruitment of Cav-1 to lipid rafts of airway epithelial cells (29) represented a significant host factor involved in controlling infection with this pathogen. cav1 KO mice were significantly more likely to have a lethal outcome from P. aeruginosa lung infection, and this phenotype was associated with higher production of inflammatory cytokines, elevated bacterial burdens and a decreased ability of neutrophils to phagocytose P. aeruginosa.
Lisanti and co-workers (31) showed that Cav-1 KO mice displayed a significant decrease in survival when challenged with Salmonella enterica serovar Typhimurium. The increased sensitivity correlated with elevated bacterial burdens in the spleen and increased production of inflammatory cytokines, chemokines, and nitric oxide suggesting that cav1 KO mice were unable to control systemic infection with Salmonella (31). However, it was surprising that the authors did not find differences in bacterial ingestion between cav1 KO and WT macrophages, indicating that the elevated mortality in cav1 KO mice could be due to their inability to control the inflammatory responses. This phenotype is comparable to our findings, wherein during an acute pneumonia that develops into a systemic bacterial infection, Cav-1 modifies inflammation. We found that when cav1 KO and WT control mice were infected with P. aeruginosa, the Cav-1 deficient mice had elevated inflammatory cytokines in their BAL including IL-1β, TNF-α, IL-6, IFN-γ, KC. These results show that in a setting of an acute systemic infection, Cav-1 plays a key role in regulating inflammatory responses to P. aeruginosa infection so they are not over-abundant.
In addition to the dramatic differences in cytokines found in cav1 KO mice infected with P. aeruginosa compared with WT controls, we also found significant differences in ability of neutrophils to ingest bacteria. These data are consistent with a previously published report that describes phagocytic defects in Cav-1 deficient macrophages that are unable to maximally ingest E. coli K-12 bioparticles (32). Subsequent studies demonstrated that Leishmania chagasi or Francesella tularensis use caveolin-dependent phagocytosis to gain access to macrophages (28, 33). These observations signify that a common phagocytic mechanism that is shared by PMNs and macrophages is the use of caveolin-rich platforms to mediate phagocytosis and/or facilitate vesicle fusion. In the setting of live bacterial infection, Cav-1 deficiency may contribute to inefficient bacterial clearance and potentially in decreases in survival by compromising efficient phagocytosis.
After finding a role for Cav-1 in resisting acute P. aeruginosa lung infection we also investigated whether this factor also increased the susceptibility of mice to chronic colonization with this organism. The Cav-1 KO mice became chronically colonized over a 9 month period and made robust serum IgG antibody responses to P. aeruginosa cells, whereas WT mice showed no clear evidence of chronic infection after a 2-week antibiotic treatment used to reduce upper-respiratory tract colonization limited to the oropharynx. Similar colonization experiments previously performed with CFTR deficient and IL-1 receptor deficient mice also showed that chronic colonization with P. aeruginosa can persist in a fashion comparable to that observed in CF patients early in the course of chronic infection (34, 35). Because of the constraints imposed by the life expectancy of a mouse and the time frame involved in studying chronic infection, it is difficult to continue experiments in this model beyond 9 months. Of note, some chronically infected CF mice yield mucoid variants of P. aeruginosa after 6-9 months of infection (25, 36), whereas no such variants were recovered from either the cav1 KO mice studied here or the IL-1 receptor deficient mice previously reported (35).
Our results differ from a recently published paper reporting that Cav-1 deficiency decreases lethality from P. aeruginosa acute pneumonia (37). The differences are likely due to the use of different strains of P. aeruginosa and the methods of infection. Notably, Zaas et al. (37) used a poorly characterized isolate of P. aeruginosa in their infection studies given as a single challenge dose wherein about 50% of the cav1 KO mice survived versus none of the WT mice. Whether their strain expresses a full set of P. aeruginosa virulence factors is not known, as it is for the well-studied strains PAK and PAO1 used here. They also used trans-tracheal instillation of P. aeruginosa which likely delivers the challenge inoculum to different locations within the lung compared with our inhalation method of delivery. In analyzing the role of caveolin-1 in acute pneumonia using strains PAK and PAO1 we observed comparable phenotypes. Both strains are invasive, non-cytotoxic and induce similar inflammatory responses in vitro. In contrast, when we infected mice with the Exotoxin U-positive, cytotoxic P. aeruginosa strain PA14 we did not find differences in the survival or bacterial levels recovered from the lungs of the infected Cav-1 KO or WT mice (data not shown). These findings likely reflect well-known differences in the virulence of cytotoxic and invasive strains of P. aeruginosa (38-41) wherein cytotoxic strains generally can be used at lower challenge doses in experimental murine lung infection models, which likely impacts the host’s innate immune response to infection. These differences in outcomes achieved by use of different P. aeruginosa strains emphasize the fact that variant strains of this organism likely promote diverse innate immune responses and drawing conclusions about which ones are relevant to resistance and susceptibility to infection needs to be placed in the context of the bacterial strains and their pathogenic properties and potentials. In spite of the differences in outcomes from acute pneumonia in cav1 KO mice found by us and by Zaas et al. (37) data from both groups demonstrate that caveolin modifies inflammation and that this host factor likely plays a role in the general resistance of mammals to P. aeruginosa infection.
In conclusion, we have established that Cav-1 is an important component of the innate host immune response to the majority of non-cytotoxic strains of P. aeruginosa by promoting bacterial clearance during acute pneumonia and chronic colonization. Lack of Cav-1 was found to reduce PMN recruitment and increase inflammatory cytokine production during acute pneumonia, two host factors known to affect host resistance to infection. From prior studies identifying Cav-1 as one of 150 proteins recruited to lipid rafts of bronchial epithelial cells 15 min after infection with P. aeruginosa (29) we have in this study validated that Cav-1 makes a major contribution to host innate immunity to P. aeruginosa. Obviously with such a large number of proteins found to rapidly respond to P. aeruginosa infection, innate immune resistance to this pathogen is quite complex and dependent on a potentially large number of interacting factors. Nonetheless, the results here validate that Cav-1 has a demonstrable effect in resistance to both acute and chronic P. aeruginosa infection.
Glossary
Abbreviations
- (Cav)
caveolin
- (CFTR)
cystic fibrosis transmembrane regulator
- (WT)
wild type mice
Footnotes
Conflicts of interest: None
References
- 1.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 2.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 3.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Grassme H, Becker KA, Zhang Y, Gulbins E. Ceramide in bacterial infections and cystic fibrosis. Biol Chem. 2008 doi: 10.1515/BC.2008.162. [DOI] [PubMed] [Google Scholar]
- 6.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J. Immunol. 2004;172:418–425. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- 7.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrhardt C, Kneuer C, Laue M, Schaefer UF, Kim KJ, Lehr CM. 16HBE14o- human bronchial epithelial cell layers express P-glycoprotein, lung resistance-related protein, and caveolin-1. Pharm Res. 2003;20:545–551. doi: 10.1023/a:1023230328687. [DOI] [PubMed] [Google Scholar]
- 9.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 10.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 11.Chung K, Elwood P, Heuser J. Caveolin is necessary and sufficient for caveolae formation. Mol Biol Cell. 1996;7:276a. [Google Scholar]
- 12.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci U S A. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003;162:2029–2039. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi T, Oka N, Koga A, Miyazaki H, Ohmura H, Imaizumi T. Behavior of caveolae and caveolin-3 during the development of myocyte hypertrophy. J Cardiovasc Pharmacol. 2005;45:204–210. doi: 10.1097/01.fjc.0000152029.53997.57. [DOI] [PubMed] [Google Scholar]
- 15.Abraham SN, Duncan MJ, Li G, Zaas D. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J. Investig. Med. 2005;53:318–321. doi: 10.2310/6650.2005.53609. [DOI] [PubMed] [Google Scholar]
- 16.Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. Expression of Caveolin-1 Is Required for the Transport of Caveolin-2 to the Plasma Membrane. RETENTION OF CAVEOLIN-2 AT THE LEVEL OF THE GOLGI COMPLEX. J. Biol. Chem. 1999;274:25718–25725. doi: 10.1074/jbc.274.36.25718. [DOI] [PubMed] [Google Scholar]
- 17.Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas Invasion of Type I Pneumocytes Is Dependent on the Expression and Phosphorylation of Caveolin-2. J. Biol. Chem. 2005;280:4864–4872. doi: 10.1074/jbc.M411702200. [DOI] [PubMed] [Google Scholar]
- 18.Bajmoczi M, Gadjeva M, Alper SL, Pier GB, Golan DE. Cystic fibrosis transmembrane conductance regulator and caveolin-1 regulate epithelial cell internalization of Pseudomonas aeruginosa. Am J Physiol Cell Physiol. 2009;297:C263–277. doi: 10.1152/ajpcell.00527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci U S A. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerceker AA, Golan DE, Pier GB. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc. Natl. Acad. Sci. U S A. 2002;99:6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J Immunol. 2003;171:5363–5371. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 22.Gadjeva M, Verschoor A, Brockman MA, Jezak H, Shen LM, Knipe DM, Carroll MC. Macrophage-derived complement component C4 can restore humoral immunity in C4-deficient mice. J Immunol. 2002;169:5489–5495. doi: 10.4049/jimmunol.169.10.5489. [DOI] [PubMed] [Google Scholar]
- 23.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J. Immunol. 2003;171:5363–5371. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 24.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk JM, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE., Jr. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008;80:4741–4751. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman FT, Mueschenborn S, Meluleni G, Ray C, Carey VJ, Vargas SO, Cannon CL, Ausubel FM, Pier GB. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc. Natl. Acad. Sci. U S A. 2003;100:1949–1954. doi: 10.1073/pnas.0437901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, Choi AM. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez NE, Gaur U, Wilson ME. Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cell Microbiol. 2006;8:1106–1120. doi: 10.1111/j.1462-5822.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 29.Kowalski MP, Dubouix-Bourandy A, Bajmoczi M, Golan DE, Zaidi T, Coutinho-Sledge YS, Gygi MP, Gygi SP, Wiemer EA, Pier GB. Host resistance to lung infection mediated by major vault protein in epithelial cells. Science. 2007;317:130–132. doi: 10.1126/science.1142311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 31.Medina FA, de Almeida CJ, Dew E, Li J, Bonuccelli G, Williams TM, Cohen AW, Pestell RG, Frank PG, Tanowitz HB, Lisanti MP. Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006;74:6665–6674. doi: 10.1128/IAI.00949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Scherl A, Medina F, Frank PG, Kitsis RN, Tanowitz HB, Sotgia F, Lisanti MP. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle. 2005;4:1599–1607. doi: 10.4161/cc.4.11.2117. [DOI] [PubMed] [Google Scholar]
- 33.Tamilselvam B, Daefler S. Francisella targets cholesterol-rich host cell membrane domains for entry into macrophages. J Immunol. 2008;180:8262–8271. doi: 10.4049/jimmunol.180.12.8262. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, Pier GB. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol. 2001;166:7410–7418. doi: 10.4049/jimmunol.166.12.7410. [DOI] [PubMed] [Google Scholar]
- 35.Reiniger N, Lee MM, Coleman FT, Ray C, Golan DE, Pier GB. Resistance to Pseudomonas aeruginosa chronic lung infection requires cystic fibrosis transmembrane conductance regulator-modulated interleukin-1 (IL-1) release and signaling through the IL-1 receptor. Infect Immun. 2007;75:1598–1608. doi: 10.1128/IAI.01980-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu D, Eisinger VM, Head NE, Pier GB, Yu HD. ClpXP proteases positively regulate alginate over-expression and mucoid conversion in Pseudomonas aeruginosa. Microbiol. 2008;154:2119–2130. doi: 10.1099/mic.0.2008/017368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaas DW, Swan ZD, Brown BJ, Li G, Randell SH, Degan S, Sunday ME, Wright JR, Abraham SN. Counteracting signaling activities in lipid rafts associated with the invasion of lung epithelial cells by Pseudomonas aeruginosa. J. Biol. Chem. 2009 doi: 10.1074/jbc.M808629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 2000;68:3998–4004. doi: 10.1128/iai.68.7.3998-4004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Schulert GS, Feltman H, Rabin SD, Martin CG, Battle SE, Rello J, Hauser AR. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J. Infect. Dis. 2003;188:1695–1706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- 41.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]