Abstract
We have previously showed that platinum drugs up-regulate SSAT and SMO and down-regulate ODC and SAMDC in the polyamine pathway. Several studies including our own established that platinum drugs combined with polyamine analog DENSPM produces synergistic increase in SSAT activity with polyamine depletion. Since polyamine pathway is an important therapeutic target, we investigated whether agents containing both platinum and polyamines have similar effects on the polyamine pathway. Two complexes i) Pt-spermine with two cisplatin molecules linked to a spermine in the center and ii) Pd-spermine with similar structure i, but Pd (II) substituted for Pt (II) were analyzed with respect to their effect on the expression of genes in polyamine pathway, SSAT and SMO protein expression, SSAT activity and polyamine pools. Pt-, Pd-spermine complexes induced significant down-regulation of SMO, arginase 2 and NRF-2, with no change in SSAT, while cisplatin as a single agent or in combination with DENSPM induced significant up-regulation of SSAT and SMO. The SSAT activity was not induced by either Pt- or Pd-spermine in A2780 cells; SMO protein levels were significantly elevated compared to the no-drug control and to a similar extent as cisplatin/DENSPM. The Pd-spm treatment induced a fall in putrescine levels to 33%, spermidine to 62% and spermine to 72% while Pt-spm did not induce such a decline. Comparative cytotoxicity studies in A2780 cells indicated the potency to be cisplatin> Pd-Spm>Pt-Spm. Although both complexes exhibit a lower potency, the degree of resistance itself is much lower for Pt-spermine and Pd-spermine in that order (2.5 and 7.5, respectively) compared to cisplatin (~12) as tested in cisplatin resistant A2780/CP cells. These studies suggest that Pd (II)-polyamine complexes may constitute a promising group of inorganic compounds for further studies in the development of novel chemotherapy/adjuvant chemotherapy strategies.
Keywords: spermine complexes, polyamine, cisplatin, ovarian carcinoma
Introduction
Cisplatin is the prototype platinum drug that plays a central role in cancer chemotherapy. The mode of action of this platinum drug and mechanisms for resistance are well documented over the years. The principal mechanism of action is believed to be the damage to DNA by forming adducts with it, that not only prevents DNA from replication and transcriptional activities, but the DNA damage also induces down-stream signaling leading to apoptotic cell death (1). Mechanisms of resistance to the drug include impaired drug accumulation, inactivation of the drug by thiols, enhanced DNA repair and altered down-stream signaling (2-5). Our Affymetrix studies have shown that as single agents, platinum drugs impact on the polyamine pathway, up-regulating the key catabolic pathway gene spermine, spermidine N1-acetyl transferase (SSAT) and down-regulating the biosynthetic pathway genes ornithine decarboxylase (ODC) and S-adenosylmethionine decaboxylase (SAMDC) (6). Our studies have shown that while platinum drugs oxaliplatin and cisplatin are potent inducers of SSAT gene expression, the mRNA is not translated proportionally to SSAT activity, but when combined with the polyamine analog N1N11-diethylnorspermine (DENSPM) a synergistic increase in SSAT activity occurs, resulting in significant polyamine pool depletion (7,8). Polyamines are required for cell proliferation (9) and the levels of polyamines are higher in tumor tissue compared with the non-tumor counterparts (10,11). Polyamine pool depletion has been used as cancer therapeutic strategy (12). DENSPM up-regulates SSAT and down-regulates the biosynthetic pathway enzymes ODC and SAMDC causing depletion of polyamine pools and this analog has been tested in Phase I and Phase II clinical trials (13-16) and had no demonstrable single agent activity. Our studies and others have shown that when platinum drugs are combined with DENSPM, the synergistic SSAT activity and polyamine pool depletion that occurs in tumor cells is significantly greater than that by either of the single agents (7,17,18).
The biogenic polyamine spermine is able to form chelates with several metal ions providing flexible linkers and conferring hydrophobic character to the molecule, which is important for drug uptake and enables a distinct interaction with DNA when compared to cisplatin. The quest for new anticancer agents is not limited to the investigation of new ligands, but also accounts for the substitution of platinum by other metal centers such as palladium. In fact, despite the initial belief that Pd (II) compounds were inactive as antineoplastic agents, many have been synthesized and shown to be not only more active than cisplatin (19-21), but also more effective than their Pt (II) counterparts (22-24). In this regard, multinuclear Pt (II)- and Pd (II)-spermine complexes comprising cisplatin-like moieties linked by variable length alkanediammine chains were synthesized and constitute a promising class of anticancer agents, the most successful case being the trinuclear compound BBR3464 (25). These spermine complexes have been shown to possess antiproliferative properties towards human cancer cells lines (22,23,26), the Pd (II)-spermine complex having a damaging interaction with both DNA and the cytoskeleton (Fiuza et al, unpublished data).
Since our studies have shown that platinum drugs in combination with polyamine analog DENSPM impact the polyamine pathway, we hypothesized that the Pt-spermine and Pd-spermine complexes may similarly affect the expression of polyamine pathway genes and SSAT activity. As single agents these compounds consist of either two cisplatin moieties, or Pd II substituted for Pt II in cisplatin molecule, each conjugated to the polyamine spermine in the center (Fig. 1). The study presented here characterizes the effect of Pt-spermine and Pd-spermine on the expression of genes in the polyamine pathway and the genes involved in platinum drug action that were identified using oxaliplatin/DENSPM and cisplatin/DENSPM combinations in A2780 ovarian carcinoma cell line (27,28). Further, the ability of these new agents to affect the proteins levels of SSAT and SMO, and SSAT activity in these cells is tested relative to cisplatin/DENSPM. In addition, comparative cytotoxicity studies of these complexes with cisplatin were performed in parental A2780 cells and in a cisplatin resistant variant A2780/CP.
Figure 1.
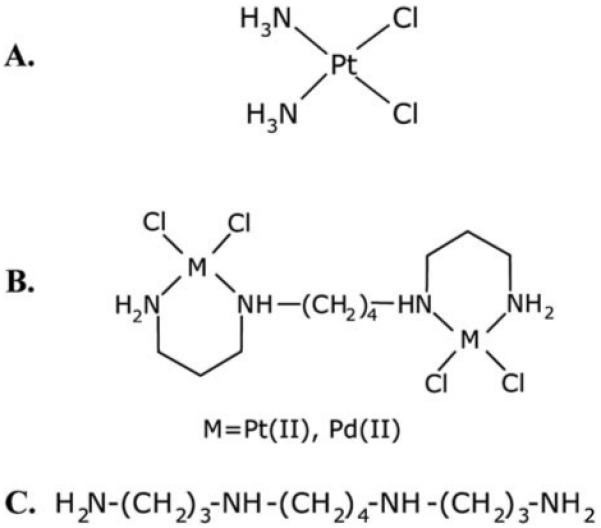
Structures of cisplatin (A), Pt, Pd-spermine complexes (B) and spermine (C).
Materials and methods
Cell lines and materials
The A2780 human ovarian carcinoma cell line and the cisplatin resistant A2780/CP cell line were a gift from Dr R. Ozols (Fox Chase Cancer Center, Philadelphia, PA). Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO). DENSPM was generously provided by Dr R. Merriman from Pfizer Pharmaceuticals (Ann Arbor, MI). The Pt-spermine and Pd-spermine complexes have been synthesized as described before (26). [14C]-acetyl-Coenzyme A (no. NEC-313) was purchased from Perkin Elmer Life Sciences, Inc. (Waltham, MA). Protein assay reagents were from Bio-Rad laboratory (Hercules, CA).
Drug treatment conditions for polyamine pathway effects
Our previous studies have shown that high synergistic SSAT induction (both mRNA and activity) is achieved after a simultaneous treatment of cells by platinum drugs (10 μM) and DENSPM (10 μM) for 20 h, cells were washed thoroughly with PBS and incubated in drug-free medium for 24 h (7,8,17). The post drug treatment incubation of 24 h in drug-free medium was based on our previous studies (6) which indicated that both cisplatin and oxaliplatin induced mRNA levels that increase with time after drug exposure with a maximum at 16-24 h. Similar concentrations of 10 μM were chosen for comparative studies for Pt-spermine and Pd-spermine cells with 20 h exposures and 24 h incubation in drug free medium. At this point, cells were collected and assayed for SSAT gene expression, enzyme activity and polyamine pools.
Gene expression using Taqman® low density array
We have custom designed a 32 gene array using Taqman Low Density Array (TLDA) technology (Applied Biosystems Inc. Foster City, CA). TLDA was carried out using the 384-well micro-fluidic card, with 30 target genes and 2 endogenous standards. For the 32 gene format the assays were run in triplicate with 4 sample capacity per card. The TLDA cards with pre-deposited gene expression assays were purchased from AB (Foster City, CA). The card includes all the polyamine target genes, genes relevant to platinum drug action, along with some of the important cell cycle, apoptosis and antioxidant pathway genes with ß-actin and GAPDH as the endogenous standard (Table I).
Table I.
Genes on Taqman low density array
| Gene symbol | Gene name | Ref. NM no. |
|---|---|---|
| Polyamines | ||
| ODC1 | Ornithine decarboxylase 1 | NM_002539 |
| AMD1 | Adenosylmethionine decarboxylase 1 | NM_001634 |
| SRM | Spermidine synthase | NM_003132 |
| SMS | Spermine synthase | NM_004595 |
| SAT | Spermidine/spermine N1-acetyltransferase 1 | NM_002970 |
| SMOX | Spermine oxidase | NM_175839 |
| PAOX | Polyamine oxidase (exo-N4-amino) | NM_152911 |
| ARG2 | Arginase, type II | NM_001172 |
| NFE2L2 | Nuclear factor, erythroid derived 2, like 2 | NM_006164 |
| Platinum | ||
| SLC22A1 | Solute carrier family 22 (organic cation transporter), member 1 | NM_003057 |
| SLC22A2 | Solute carrier family 22 (organic cation transporter), member 2 | NM_153191 |
| ERCC1 | Excision repair cross-complementing rodent repair deficiency, complementation group 1 (includes overlapping antisense sequence) (Homo sapiens) |
NM_001983 |
| XPA | Xeroderma pigmentosum, complementation group A | NM_000380 |
| GCLC | Glutamate-cysteine ligase (γ-glutamylcysteine synthetase) | NM_001498 |
| GGT1 | γ-glutamyltransferase 1 | NM_013430 |
| SLC22A1 | Solute carrier family 22 (organic cation transporter), member 1 | NM_003057 |
| SLC22A2 | Solute carrier family 22 (organic cation transporter), member 2 | NM_153191 |
| ERCC1 | Excision repair cross-complementing rodent repair deficiency, complementation group 1 (includes overlapping antisense sequence) (Homo sapiens) |
NM_001983 |
| Antioxidant | ||
| GPX1 | Glutathione peroxidase 1 | NM_000581 |
| SOD1 | Superoxide dismutase 1 | NM_000454 |
| GSR | Glutathione reductase | NM_000637 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
NM_000963 |
| OGG1 | 8-oxoguanine DNA glycosylase | NM_016819 |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (p105) | NM_003998 |
| PPARG | Peroxisome proliferative activated receptor, γ | NM_138712 |
| GPX1 | Glutathione peroxidase 1 | NM_000581 |
| Cell cycle | ||
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467 |
| MDM2 | Transformed 3T3 cell double minute 2, p53 binding protein (mouse) | NM_002392 |
| CCND1 | Cyclin D1 | NM_053056 |
| PCNA | Proliferating cell nuclear antigen | NM_002592 |
| Apoptosis | ||
| BCL2 | B-cell CLL/lymphoma 2 | NM_000633 |
| BAX | BCL2-associated X protein | NM_138761 |
| FAS | Fas (TNF receptor superfamily, member 6) | NM_000043 |
| BIRC5 | Baculoviral IAP repeat-containing 5 (survivin) | NM_001168 |
| Control | ||
| ACTB | actin, ß | NM_001101 |
TLDA was performed using ABI 7900 HT Fast Real-Time RT-PCR system with SDS 2.2 software. The concept behind it is the same as for the original Taqman Real-Time RT-PCR assays using gene specific primers and probes and quantification using comparative CT method with the endogenous standards run simultaneously on these low density arrays. The results presented here are using ß-actin as the endogenous standard as has been customary for platinum, polyamine combination studies. The RQ shown in the gene expression plots is the relative quantification measure that calculates the relative quantities of expression of each of the target genes in different samples relative to a corresponding calibrator. In our experiments the calibrator is the control where cells were not treated with any drug. TLDA uses approximately 50 μl of cDNA sample plus 50 μl of Universal Master Mix (AB, Foster City, CA) in each of the eight ports (which equates to 1 μl of the cDNA sample, mixed with 1 μl of Universal Master Mix per well). Centrifugal capillary action then pulls the cDNA from the loading ports, into the 48 reaction chambers containing the genes of interest. The total RNA extraction and cDNA generation were as described previously using RNeasy columns (Qiagen, Valencia, CA) and Superscript II respectively (7).
Western blots for SSAT and SMO
The cells were lysed in RIPA buffer and equal amounts of total protein from different cell lines were loaded (for SSAT 80 μg and for SMO 60 μg) onto 12 and 10% SDS-PAGE gels, respectively, followed by transfer to polyvinylindene difluoride membrane and immunoblotted with specific antibodies. SSAT was detected as described previously (29,30) and SMO protein was detected using a SMO-specific antibody developed by our laboratories (Vujcic et al, unpublished). Untreated cells were used as a control. NIH3T3 cells treated with DENSPM and A2780 treated with oxaliplatin and DENSPM were used as positive control for SSAT. HCT-116 cells treated with DENSPM, and A2780 treated with oxaliplatin and DENSPM were used as positive controls for SMO.
SSAT activity
SSAT activity assay was performed as described previously (31). In brief, the reaction mixture contained [14C]acetylCoA (60 mCi/mmol, NEN Radiochemicals, Waltham, MA) spermidine and cell extract in Tris-HCl buffer, pH 7.5. The [14C]acetylated spermidine product generated by the enzyme reaction is captured on discs followed by counting of radioactivity. Protein in the cell lysate was determined by the Bradford assay (32). The activity reported is pmol/min/mg protein.
Polyamine pools
Intracellular polyamine pools and acetylated polyamine pools were extracted with 0.6 N perchloric acid, dansylated and analyzed using reverse phase HPLC with fluorescence detection as previously described (33). Protein was determined by Bradford assay (32). Polyamine pools were expressed as pmol/mg protein.
Cytotoxicity assays
Cells were plated in a 96-well plate (1×103 cells/well) on day 0 followed by exposure to cisplatin, Pt-spermine or Pd-spermine on day 1. Cells were exposed to drug concentrations ranging from 0.1 to 100 μM for 72 h, at which point cells were fixed and subjected to the sulforhodamine-B micro-culture colorimetric assay (SRB) (34). Percent survival were determined as [OD570 (treated cells)/OD570 (untreated cells)]*100.
Results
Effect of Pt-spermine and Pd-spermine on gene expression
The effect of the Pt- and Pd (II) spermine agents on our custom panel of 30 genes relevant to platinum and polyamine drug action (TLDA) in comparison to cisplatin/DENSPM was studied in A2780 ovarian carcinoma cells. A representative experiment for the gene expression data obtained from the Pt (II) spermine and Pd (II) spermine complexes is shown in Fig. 2. Data from cisplatin, DENSPM or cisplatin/DENSPM treatment of A2780 cells is shown in Fig. 3. Key for the genes on the TLDA card is included in Table I. The gene expression plots in Figs. 1 and 2 show the log RQ for each of the genes. RQ is the gene expression normalized to ß-actin for each of the genes in each sample and presented relative to the no-drug control. The log plots of RQ show the down-regulation of genes much better than the linear plots and present a balanced view of the up or down-regulated genes which makes it easier to compare the gene expression differences between treatments. The gene expression plots show the data in alphabetical order (as dictated by the software) and not as genes in select groups shown in Table I. Note that the order of plots for each gene and for each of the treatments in Figs. 2 and 3 are also in alphabetical order; thus, no-drug controls are shown in the 3rd position in Fig. 3. The error bars here represent the confidence interval (CI) based on a t-test and not standard deviations. The CI for each of the genes in no-drug controls represents the variability of control replicates for those specific genes and makes it easier to select the CI non-overlapping genes between drug treated and controls as the significantly up- or down-regulated genes after the drug treatments. Of those genes that showed significant changes, the extent of up- or down-regulation (RQ values) after each of the drug treatments relative to the no-drug controls is summarized in Table II.
Figure 2.
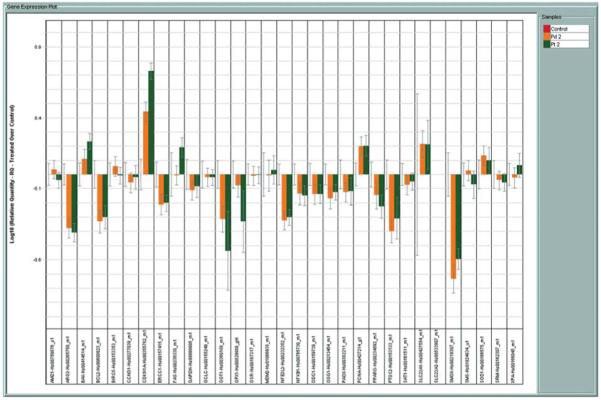
Expression of genes represented on TLDA in A2780 human ovarian carcinoma cells treated with Pt-spermine and Pd-spermine complexes. Bars are log10 RQ. RQ is the quantity of expression of a given gene relative to the drug untreated control. Bars going upward represent up-regulation and those going downwards down-regulation. Error bars are the confidence intervals compared to the no-drug controls in the first position of each cluster.
Figure 3.
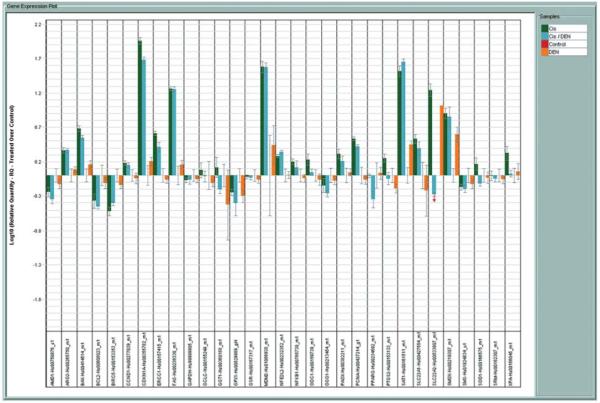
Expression of genes represented on TLDA in A2780 ovarian carcinoma cells treated with cisplatin, DENSPM or cisplatin/DENSPM. Bars are log10 RQ. RQ is the quantity of expression of a given gene relative to the drug untreated control. Bars going upward represent up-regulation and those going downwards down-regulation. Error bars are the confidence intervals compared to the no-drug controls in the third position of each cluster.
Table II.
Comparison of changes in gene expression (increase or decrease relative to untreated control)a in A2780 cells following treatment with Pt (II) spermine, Pd (II) spermine and Cispt, DENSPM or Cispt/DENSPM under the same treatment conditions
| Gene | CisPt | DENSPM | CisPt/DEN | Pt (II)-spermine | Pd (II)-spermine |
|---|---|---|---|---|---|
| SSAT | 33 | 3 | 49 | NC | NC |
| SMO | 6 | 3 | 5.9 | 0.18 | 0.13 |
| SAMDC | 0.6 | NC | 0.5 | NC | NC |
| ARG2 | 2.3 | NC | 2.4 | 0.33 | 0.38 |
| NRF2 | 1.75 | NC | 2.1 | 0.42 | 0.46 |
| FAS | 16 | NC | 17 | 1.7 | NC |
| BAX | 4.5 | NC | 3.5 | 1.7 | 1.5 |
| BCL2 | 0.43 | NC | 0.37 | 0.5 | 0.5 |
| BIRC5 (Survivin) | 0.3 | NC | 0.4 | NC | NC |
| P21 | 93 | NC | 51 | 5 | 2.7 |
| MDM2 | 26 | NC | 29.7 | NC | NC |
| PCNA | 3.3 | NC | 2.8 | 1.5 | 1.6 |
| ERCC1 | 4 | NC | 2.6 | 0.6 | 0.6 |
| GGT1 | NC | NC | NC | 0.23 | 0.25 |
| OCT,1 | 3 | NC | 2.3 | NC | NC |
| OGG1 | NC | NC | 0.6 | 0.65 | 0.62 |
| PPARG | NC | NC | 0.5 | 0.6 | NC |
| PTGS2 (Cox2) | 1.8 | 0.7 | NC | 0.45 | 0.4 |
Values are RQ (relative expression, treated/control); rounded off. NC, no change. RQ value >1 denotes up-regulation and the value itself describes the fold increase in expression; RQ value <1 denotes down-regulation and the value itself is expression as a fraction of control.
As seen from log RQ plots (Fig. 2) the Pt and Pd complexes have a very similar profile of up- or down-regulation of the genes represented on this card, suggesting that it may be the polyamine portion of the molecule conferring the changes in gene expression rather than the metal. The Pt-spermine and Pd-spermine complexes induced more down-regulation of genes than up-regulation (Fig. 2), as opposed to cisplatin and cisplatin/DENSPM combinations that induced more up-regulation of genes (Fig. 3). From the plots and the data summarized in Table II, it can be seen that the most significant differences (directional differences in expression) are in genes related to the polyamine pathway. Pt-, Pd-spermine complexes induced significant down-regulation of SMO, arginase 2 and NRF-2, while no significant change was noted for SSAT. Under the same treatment conditions, cisplatin as single agent or in combination with DENSPM induced significant up-regulation of both SSAT and SMO and down-regulated SAMDC. For the most part, Pt- and Pd-spermine complexes showed similar effects on apoptosis and cell cycle genes as cisplatin and cisplatin/DENSPM (↑FAS, ↑BAX, ↓BCL-2, ↑p21, ↑PCNA), but to a significantly lower degree. While cisplatin or cisplatin/DENSPM up-regulated ERCC1, the Pt-, Pd-spermine complexes down-regulated the expression of this gene. BIRC5 (survivin) was down-regulated by cisplatin and cisplatin/DENSPM combination while the Pt- and Pd-spermine complexes did not have an effect on this gene. Pt-, Pd-spermine complexes down-regulated the PTGS2 (Cox-2) gene.
SSAT and SMO protein or activity
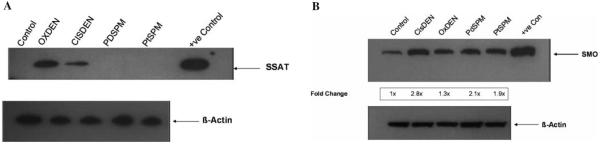
Consistent with the gene expression data, the SSAT protein (Fig. 4A) or activity (Table III) was not induced by either Pt- or Pd-spermine in A2780 cells. Induction of SSAT protein is evident under the same conditions for cisplatin/DENSPM combination (Fig. 4A). Although SMO gene expression showed a significant down-regulation after both Pt- and Pd-spermine, interestingly SMO protein levels were significantly elevated compared to the no-drug control and to a similar extent as the cisplatin/DENSPM treated cells (Fig. 4B).
Figure 4.
Western blot analysis of SSAT (A) and SMO (B). A2780 cells were treated with Pd-spermine, Pt-spermine or cisplatin/DENSPM, all at 10 μM concentration each for 20 h followed by 24 h incubation in drug free medium. The cells were harvested thereafter and 60-80 μg of whole cell extracts were used for Western blot and probed with human SSAT antibody (A) or SMO antibody (B). Untreated cells were used as control for both. NIH3T3 cells treated with DENSPM were used as a positive control for SSAT (A) and HCT-116 cells treated with oxaliplatin/DENSPM were used as a positive control for SMO (B). A2780 cells treated with oxaliplain/DENSPM were also used as a positive control for SSAT and SMO. Note that fold changes could not be presented in 4A, as controls had no measurable SSAT protein.
Table III.
Spermidine/Spermine N1 acetyltransferase (SSAT)a activity after treatment with Pt-spermine or Pd-spermine
| Treatment | SSAT activity (pmols/min/mg) |
|---|---|
| Control | 15.5±3.3 |
| Pd-Spm | 11.5±4.6 |
| Pt-Spm | 12.1±0.8 |
A2780 cells were treated with Pd-spermine or Pt-Spermine (10 μM) for 20 h followed by 24 h in drug free medium. The cells were harvested thereafter and assayed for SSAT. Untreated cells were used as controls. Data are an average of 3 measurements.
Polyamine pools
Changes in polyamine pools in A2780 cells after Pt-spermine or Pd-spermine are shown in Table IV. The Pd-spm treatment decreased putrescine levels to 33%, spermidine to 62% and spermine to 72% relative to the no-drug controls, while Pt-spm did not induce such a decline.
Table IV.
Polyamine pools after treatment with Pt-spermine or Pd-spermine
| Treatment | Putrescine (pmols/mg protein) |
Spermidine (pmols/mg protein) |
Spermine (pmols/mg protein) |
|---|---|---|---|
| Control | 2,151±537 | 28,269±1,187 | 20,347±703 |
| Pd-spermine | 714±401 | 17,606±1,359 | 14,673±1,344 |
| Pt-spermine | 2,479±627 | 33,379±6,142 | 25,838±4,948 |
A2780 cells were treated with Pd-spermine or Pt-Spermine (10 μM ) for 20 h followed by 24 h in drug free medium. The cells were harvested thereafter and assayed for polyamine pools. Untreated cells were used as controls. Data are an average of 3 measurements.
Cytotoxicity profiles of the Pt- and Pd-spermine complexes in relation to cisplain in A2780 cells
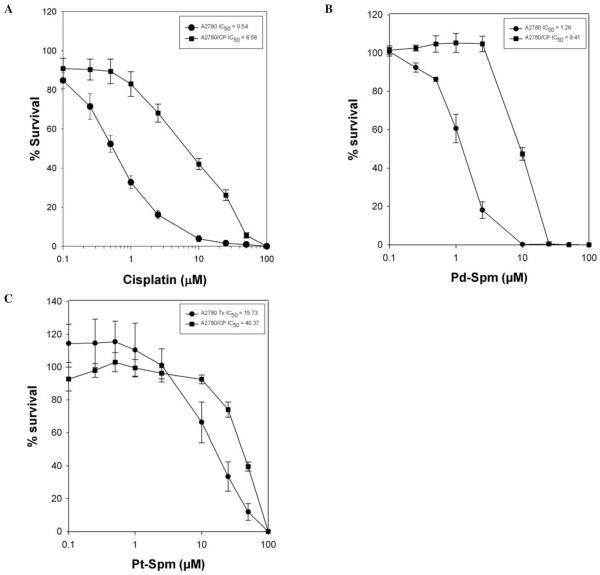
Comparative cytotoxicity profiles of cisplatin, Pt-Spm and Pd-Spm in A2780 cells shown in Fig. 5 indicate the potency to be cisplatin>Pd-Spm>Pt-Spm with IC50 values of 0.51, 1.25 and 15.72 μM, respectively.
Figure 5.
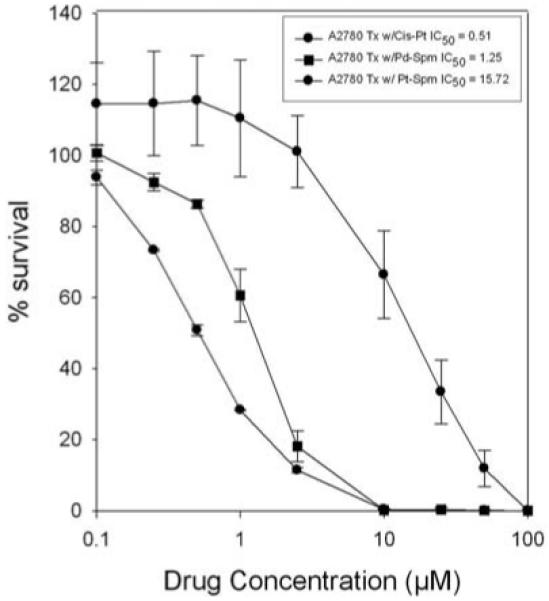
Relative cytotoxic potency of cisplatin, Pt-spermine and Pd-spermine in A2780 cells. Cells were plated in a 96 well plate (1×103 cells/well) on day ‘0’, followed by treatment with cisplatin or Pd-Spermine or Pt-spermine on day 1 at concentrations ranging from 0.1 to 100 μM for 72 h. Cells were then fixed and subjected to sulforhodamine-B-microculture colorimetric assay (SRB). Percent survival was determined as (OD570 treated cells/OD570 untreated cells) x100. Data presented are an average of 2 separate experiments, each experiment consisting of 5 replicates of each agent tested.
Cross-resistance patterns of the complexes with cisplatin in a cisplatin resistant ovarian carcinoma cell line A2780/CP
The A2780/CP cell line is ~12-fold resistant to cisplatin (Fig. 6). A comparison of the Pt-spermine and Pd-spermine cytotoxicity in A2780/CP cells and parental A2780 cells indicate that their potency is lower than cisplatin, however, the Pt-spermine exhibits only a 2.5-fold cross resistance and the Pd-spermine exhibiting ~7.5-fold cross resistance. Thus, cisplatin resistant cells appear to be sensitive to Pt-spermine.
Figure 6.
Cross resistance patterns for Pt (II) spermine and Pd (II) spermine in A2780/CP cisplatin resistant cells. Comparison of cytotoxicity in A2780 vs. A2780/CP cells for (A) cisplatin, (B) Pd-spermine and (C) Pt-spermine complexes. Data presented are an average of 2-3 separate experiments, each experiment consisting of 5 replicates of each agent tested.
Discussion
A significant body of studies accumulated in the last several years indicate that platinum drugs have an effect on the polyamine pathway (6,7,17,18,35,36). Our own Affymetrix experiments with oxaliplatin and cisplatin indicate that both of these drugs up-regulated the polyamine catabolic pathway enzymes SSAT and SMO and down-regulated the biosynthetic pathway enzymes SAMDC and ODC (6). A study from our laboratory and others have shown that while platinum drugs are potent inducers of SSAT gene expression, the gene expression does not translate into activity (7,17,18). However, when oxaliplatin is combined with a polyamine analog such as DENSPM a synergistic increase in SSAT activity occurs (7,17,18) with concurrent polyamine pool depletion (7,17). We hypothesized that Pt-, Pd-spermine complexes may possibly have an impact on polyamine pathway and polyamine pools since they represent a composite of two cisplatin molecules (or Pd-diammine dichloride molecules) that are linked to a spermine in the center.
Using TLDA we were able to compare the effect of these complexes with that of cisplatin and cisplatin/DENSPM combination on the expression of genes not only in the polyamine pathway, but also those in other pathways relevant to platinum drug action. As indicated from the data presented, cisplatin up-regulated the expression of FAS, BAX, P21, MDM-2 and PCNA and down-regulated the expression of the anti-apoptotic gene BCL-2 as known from its mode of action (5). Combining DENSPM with cisplatin, did not alter cisplatin effects on these genes. The Pt- and Pd-spermine complexes showed qualitatively similar changes as cisplatin (or cisplatin/DENSPM) in the expression of the above mentioned cell cycle and apoptosis genes although with a much lower magnitude, indicating that these complexes may kill the cells with similar mechanisms as cisplatin.
The most significant difference between cisplatin (or cisplatin/DENSPM) and the Pt-, Pd-spermines were found in their potential to affect on the polyamine pathway. When cisplatin up-regulated the expression of SSAT and SMO genes, the Pt- or Pd-spermine treated A2780 cells exhibited down-regulation of SMO and no-change in the expression of SSAT compared to the no-drug controls. Unlike cisplatin, the Pt-, Pd-spermine complexes down-regulated the expression of ARG2 and NRF2. Although SMO gene expression is down-regulated, it is intriguing that SMO protein levels are comparatively elevated by Pt-, Pd-spermine complexes similar to cisplatin/DENSPM or oxaliplatin/DENSPM treated cells. It may possibly be that these complexes stimulate translation, and stabilize the SMO protein similar to that known for polyamine interactions with SSAT (37). However, this remains to be determined.
It is quite interesting that while both complexes induced an increase in SMO protein and had no effect on SSAT protein or activity, only the Pd-spm treatment induced a fall in putrescine levels to 33%, spermidine to 62% and spermine to 72% relative to the no-drug controls. It is unclear exactly what these changes are due to, since at the gene expression level neither of these compounds had an effect on the biosynthetic pathway genes ODC and SAMDC. It may also be related to intracellular concentration and/or metabolism attained for these complexes and further studies are necessary to unravel some of these differences.
We also tested the cytotoxicity profiles of these compounds in comparison to cisplatin in the A2780 ovarian carcinoma cells, the cells used for the gene expression and polyamine pathway alterations discussed above. These studies indicated the potency for these 3 agents to be cisplatin > Pd-Spm > Pt-Spm with IC50 values of 0.52, 1.32 and 12.7 μM, respectively. Thus some of the differences seen between Pt-spermine and Pd-spermine may be related to the potency of these compounds, related to differences in uptake, further metabolism, or effective concentrations for reaching the target. We also tested the two drugs for their cytotoxicity in a cisplatin resistant cell line (A2780/CP) relative to the parental cell line, to understand their cross resistance patterns to cisplatin. As evident from the data, although both drugs exhibit a lower potency relative to cisplatin, the degree of resistance itself is much lower for Pt-spermine and Pd-spermine in that order (2.5 and 7.5, respectively) compared to cisplatin (~12). While Pt-spermine may be more effective in cisplatin resistant cells in vitro, because of the very low potency, the concentrations required to kill the cells may not be achievable in an in vivo setting. However, the overall data with respect to polyamine pool depletion as well as the potency and cross-resistance patterns suggest that the Pd-spermine complex may prove to be a useful agent for further investigations in vitro and in vivo.
Our previously obtained results point to a different mechanism of action of Pd-spermine as compared to cDDP, leading to a synergetic interaction when these two agents are co-administered. It was also verified that Pd-spermine is responsible for a damaging interaction with DNA, although the exact nature of this interplay remains to be understood (experiments performed by the authors to check for interstrand crosslinks with DNA were not conclusive, Fiuza et al, unpublished data). In light of these results, Pd (II)-polyamine complexes constitute a promising group of inorganic compounds for the development of novel chemotherapy/adjuvant chemotherapy strategies.
Acknowledgements
This study was supported by RO1CA109619 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute and the National Institutes of Health. We acknowledge the support of NCI Comprehensive Cancer Center grant CA10656 for the PK/PD Core Facility usage.
Abbreviations
- DENSPM
N1N11-diethylnorspermine
- Pt
platinum
- Put
putrescine
- Spd
spermidine
- Spm
spermine
- SSAT
spermidine/spermine N1-acetyltransferase (also known as SSAT-1)
- SMO
spermine oxidase
- ODC
ornithine decarboxylase
- SAMDC
S-adenosylmethionine decarboxylase
- SRB-B
sulforhodamine blue
- TLDA
Taqman low-density array
References
- 1.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Andrews PA. Mechanisms of acquired resistance to cisplatin. Cancer Treat Res. 1994;73:217–248. doi: 10.1007/978-1-4615-2632-2_11. [DOI] [PubMed] [Google Scholar]
- 3.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosland M, Lum B, Schimmelpfennig J, Baker J, Doukas M. Insights into mechanisms of cisplatin resistance and potential for its clinical reversal. Pharmacotherapy. 1996;16:16–39. [PubMed] [Google Scholar]
- 5.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 6.Varma R, Hector S, Greco WR, Clark K, Hawthorn L, Porter C, Pendyala L. Platinum drug effects on the expression of genes in the polyamine pathway: time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells. Cancer Chemother Pharmacol. 2007;59:711–723. doi: 10.1007/s00280-006-0325-3. [DOI] [PubMed] [Google Scholar]
- 7.Hector S, Porter CW, Kramer DL, Clark K, Prey J, Kisiel N, Diegelman P, Chen Y, Pendyala L. Polyamine catabolism in platinum drug action: Interactions between oxaliplatin and the polyamine analogue N1N11-diethylnorspermine at the level of spermidine/spermine N1-acetyltransferase. Mol Cancer Ther. 2004;3:813–822. [PubMed] [Google Scholar]
- 8.Tummala R, Porter CW, Diegelman P, Vujcic S, Clark K, Prey J, Kisiel N, Kramer D, Pendyala L. Platinum drug effects on polyamine enzymes in A2780 human ovarian carcinoma cells. Proc Am Assoc Cancer Res. 2007;48 Abs 365. [Google Scholar]
- 9.Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter C, Herrera-Omelas L, Pera P, Petrelli NF, Mittleman A. Polyamine biosynthetic activity in normal and neoplastic human colorectal tissues. Cancer. 1987;60:1275–1281. doi: 10.1002/1097-0142(19870915)60:6<1275::aid-cncr2820600619>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Leveque J, Foucher F, Bansard JY, Havouis R, Grall JY, Moulinoux JP. Polyamine profiles in tumor, normal tissue of the homologous breast, blood, and urine of breast cancer sufferers. Breast Cancer Res Treat. 2000;60:99–105. doi: 10.1023/a:1006319818530. [DOI] [PubMed] [Google Scholar]
- 12.Thomas T, Balabhadrapathruni S, Gallo MA, Thomas TJ. Development of polyamine analogs as cancer therapeutic agents. Oncol Res. 2002;13:123–135. [PubMed] [Google Scholar]
- 13.Creaven PJ, Perez R, Pendyala L, Meropol NJ, Loewen G, Levine E, Berghorn E, Raghavan D. Unusual central nervous system toxicity in a Phase I study of N1N11 diethylnorspermine in patients with advanced malignancy. Invest New Drugs. 1997;15:227–234. doi: 10.1023/a:1005827231849. [DOI] [PubMed] [Google Scholar]
- 14.Streiff RR, Bender JF. Phase 1 study of N1N11-diethylnorspermine (DENSPM) administered TID for 6 days in patients with advanced malignancies. Invest New Drugs. 2001;19:29–39. doi: 10.1023/a:1006448516938. [DOI] [PubMed] [Google Scholar]
- 15.Hahm HA, Ettinger DS, Bowling K, Hoker B, Chen TL, Zabelina Y, Casero RAJ. Phase I study of N1N11-diethylnorspermine in patients with non-small cell lung cancer. Clin Cancer Res. 2002;8:684–690. [PubMed] [Google Scholar]
- 16.Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, Casero RAJ, Davidson NE. A Phase II study of the polyamine analog N1N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2001;9:5922–5928. [PubMed] [Google Scholar]
- 17.Hector S, Tummala R, Kisiel ND, Diegelman P, Vujcic S, Clark K, Fakih M, Kramer DL, Porter CW, Pendyala L. Polyamine catabolism in colorectal cancer cells following treatment with oxaliplatin, 5-fluorouracil and N1N11-diethylnorspermine. Cancer Chemother Pharmacol. 2008;62:517–527. doi: 10.1007/s00280-007-0633-2. [DOI] [PubMed] [Google Scholar]
- 18.Allen WL, McLean EG, Boyer J, McCulla A, Wilson PM, Coyle V, Longley DB, Casero RA, Jr, Johnston PG. The role of spermidine/spermine N1-acetyltransferase in determining response to chemotherapeutic agents in colorectal cancer cells. Mol Cancer Ther. 2007;6:128–137. doi: 10.1158/1535-7163.MCT-06-0303. [DOI] [PubMed] [Google Scholar]
- 19.Ray S, Mohan R, Singh JK, Samantaray MK, Shaikh MM, Panda D, Ghosh P. Anticancer and antimicrobial metallo-pharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J Am Chem Soc. 2007;129:15042–15053. doi: 10.1021/ja075889z. [DOI] [PubMed] [Google Scholar]
- 20.Kuduk-Jaworska J, Puszko A, Kubiak M, Pelczynska M. Synthesis, structural, physico-chemical and biological properties of new palladium (II) complexes with 2,6-dimethyl-4-nitro-pyridine. J Inorg Biochem. 2004;98:1447–1456. doi: 10.1016/j.jinorgbio.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Mansuri-Torshizi H, Ghadimy S, Akbarzadeh N. Synthesis, characterization, DNA binding and cytotoxic studies of platinum (II) and palladium (II) complexes of the 2,2′-bipyridine and an anion of 1,1-cyclobutanedicarboxylic acid. Chem Pharm Bull. 2001;49:1517–1520. doi: 10.1248/cpb.49.1517. [DOI] [PubMed] [Google Scholar]
- 22.Fiuza SM, Amado PJ, Oliveira VA, Sardao VA. Batista de Carvalho LAE and Marques MPM: Pt (II) vs Pd (II) polyamine complexes as new anticancer drugs: a structure-activity study. Lett Drug Des Discov. 2006;3:149–151. [Google Scholar]
- 23.Teixeira LJ, Seabra M, Reis E, da Cruz MT, de Lima MC, Pereira E, Miranda MA, Marques MP. Cytotoxic activity of metal complexes of biogenic polyamines: polynuclear platinum (II) chelates. J Med Chem. 2004;47:2917–2925. doi: 10.1021/jm0311238. [DOI] [PubMed] [Google Scholar]
- 24.Butour JL, Wimmer S, Wimmer F, Castan P. Palladium(II) compounds with potential antitumour properties and their platinum analogues: a comparative study of the reaction of some orotic acid derivatives with DNA in vitro. Chem Biol Interact. 1997;104:165–178. doi: 10.1016/s0009-2797(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 25.Jodrell DI, Evans TR, Steward W, Cameron D, Prendiville J, Aschele C, Noberasco C, Lind M, Carmichael J, Dobbs N, Camboni G, Gatti B, De BF. Phase II studies of BBR3464, a novel tri-nuclear platinum complex, in patients with gastric or gastro-oesophageal adenocarcinoma. Eur J Cancer. 2004;40:1872–1877. doi: 10.1016/j.ejca.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Soares AS, Fiuza SM, Concalves MJ, de Caravalho LAEB, Marques MPM, Urbano AM. Effect of metal center ib tge antitumor activity of the analogous dinuclear spermine chelates (PdCl2)2(spermine) and (PtCl2)2(spermine) Lett Drug Des Discov. 2007;4:460–463. [Google Scholar]
- 27.Varma R, Hector S, Greco WR, Clark K, Hawthorn L, Porter C, Pendyala L. Platinum drug effects on the expression of genes in the polyamine pathway: time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells. Cancer Chemother Pharmacol. 2007;59:711–723. doi: 10.1007/s00280-006-0325-3. [DOI] [PubMed] [Google Scholar]
- 28.Brun YF, Varma R, Hector SM, Pendyala L, Tummala R, Greco WR. Simultaneous modeling of concentration-effect and time-course patterns in gene expression data from microarrays. Cancer Genomics Proteomics. 2008;5:43–53. [PubMed] [Google Scholar]
- 29.Fogel-Petrovic M, Vujcic S, Brown PJ, Haddox MK, Porter CW. Effects of polyamines, polyamine analogs, and inhibitors of protein synthesis on spermidine-spermine N1-acetyltransferase gene expression. Biochemistry. 1996;35:14436–14444. doi: 10.1021/bi9612273. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Kramer DL, Jell J, Vujcic S, Porter CW. Small interfering RNA suppression of polyamine analog-induced spermidine/spermine N1-acetyltransferase. Mol Pharmacol. 2003;64:1153–1159. doi: 10.1124/mol.64.5.1153. [DOI] [PubMed] [Google Scholar]
- 31.Porter CW, Ganis B, Libby PR, Bergeron RJ. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51:3715–3720. [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;52:5115–5118. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Vujcic S, Halmekyto M, Diegelman P, Gan G, Kramer DL, Janne J, Porter CW. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J Biol Chem. 2000;275:38319–38328. doi: 10.1074/jbc.M003270200. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein LV, Shoemaker RH, Paull KD, et al. Comparison of in vitro anticancer drug screening data generated with a tetra-zolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell P, Longley DB, Latif T, Boyer J, Allen W, Lynch M, McDermott U, Harkin D, Allegra CJ, Johnston PG. Identification of 5-fluorouracil-inducible target genes using cDNA microarray profiling. Cancer Res. 2003;63:4602–4606. [PubMed] [Google Scholar]
- 36.Boyer J, Allen WL, McLean EG, Wilson PM, McCulla A, Moore S, Longley DB, Caldas C, Johnston PG. Pharmacogenomic identification of novel determinants of response to chemotherapy in colon cancer. Cancer Res. 2006;66:2765–2777. doi: 10.1158/0008-5472.CAN-05-2693. [DOI] [PubMed] [Google Scholar]
- 37.Casero RAJ, Pegg AE. Spermidine/spermine N1-acetyltransferase-the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]