Abstract
In the behavioral literature, self-echoic behavior has been hypothesized to play an important role in, for example, emergent conditional discriminations (e.g., Lowenkron, 1991), emergent verbal operants (Horne & Lowe, 1996), and problem solving (Skinner, 1957). Although early behavioral intervention programs for children with autism emphasize the establishment of accurate echoic repertoires, the type of stimulus control that defines a self-echoic response is typically not addressed. We report the development of a self-echoic assessment procedure that was administered to children with and without autism spectrum disorders. Preliminary results indicated that a discrepancy between echoic and self-echoic repertoires was more likely to be present among participants with autism than among typically developing participants. Future research should evaluate the extent to which interventions to establish self-echoic responding might produce other collateral benefits.
Keywords: echoic, self-echoic, verbal behavior, language assessment
Skinner (1957) defined the echoic as verbal vocal behavior under the control of, and with common sound units to, an immediate auditory stimulus. The size of the echoic may vary considerably from small partial units (e.g., single phoneme) to large units (e.g., sentence) and may include nonspeech properties such as intonation. Through direct “educational reinforcement” (p. 56), the verbal community establishes verbal repertoires, with acceptable articulation, necessary for a child to be an effective speaker within the community. For example, under appropriate motivating operations (Laraway, Snycerski, Michael, & Poling, 2003), the transfer of verbal functions (e.g., from echoic to tact or mand) expands the child's verbal repertoire when a member of the community simultaneously presents a vocal model in the presence of a novel or desired object and encourages an echoic response. Contingent on the child's satisfactory response, the echoic is strengthened by demand removal and possibly praise or receipt of a desired object. Once established by the verbal community, the echoic is maintained by “indirect reinforcement” (Skinner, p. 57), where the repetition of a statement may allow a speaker time to compose a reply or where a repeated statement may control the verbal behavior of another to provide a more complete explanation of a point of discussion.
Given that one's own vocal behavior is heard by a speaker as listener, Skinner (1957) described the self-echoic as behavior under the control an immediate auditory stimulus produced by oneself and reinforced automatically if it “strengthens stimulation to control one's own verbal behavior” (p. 64). As with the echoic, such control can be effective whether the response occurs overtly or covertly, although perhaps its effects are more subtle when controlling stimuli are less salient. For example, mnemonic devices are auditory stimuli produced by a speaker to strengthen a partial self-echoic related to another verbal operant. When encountering an acquaintance whose name one does not recall readily, one might have learned to recite the alphabet covertly (e.g., “a,” “b”), because stimulation from the vocal response has strengthened tact control over saying a person's name (e.g., “b,” “buh,” “Bob,” “Bill!”). Self-echoic behavior also may be a useful bridging response to increase the probability of responding effectively in the future (e.g., self-echoing a telephone number until a pen is found). In addition, self-echoic behavior may be useful in editing one's verbal behavior. That is, one may self-echo part of a statement to observe the emotional effects of such verbal behavior on oneself. If the effect is unpleasant, one might change the wording until a more desired outcome is produced.
Although the echoic and self-echoic appear to be similar, there are several differences in their controlling contingencies. First, the auditory stimulus that evokes an echoic does not originate from the person emitting the response, whereas, by definition, the antecedent stimulus for the self-echoic and its ensuing response are produced by the same person. Another difference is that reinforcement for echoic behavior is often provided in a social context (e.g., removal of threat), whereas effective consequences for the self-echoic occur as a result of increased stimulation of one's own verbal behavior that may result in performing effectively, either verbally or nonverbally (e.g., recalling a name). Finally, the verbal community establishes motivating operations (e.g., threat of disapproval) for the echoic, whereas the self-echoic arises from events in which conditions to perform effectively are currently unavailable (e.g., forgetting a friend's name). The strength of a self-echoic repertoire may be influenced by a person's learning history to attend to and listen to his own verbal behavior and to manipulate this behavior when presented with relevant motivating events.
In the recent behavioral literature, self-echoic behavior has been hypothesized to play an important role in the emergence of novel stimulus control and novel responses. For example, the self-echoic is a crucial component of Lowenkron's (1991, 1998) joint control account of relational responding. Lowenkron defined joint control as “a discrete event, a change in stimulus control that occurs when a response topography evoked by one stimulus … and preserved by rehearsal, is emitted under the additional (and thus joint) control of a second stimulus” (1998, p. 332); rehearsal consists of self-echoic or otherwise self-duplic (Michael, 1982) behavior. Joint control thus occurs when one is emitting a particular response topography as a self-echoic and encounters a stimulus that has previously acquired control over that same topography. The original source of the self-echoed topography may be, for example, an echoic, a tact, an intraverbal, or a textual response to a prior stimulus that need not share any physical features with the subsequently encountered stimulus. As an example, a student taking a multiple-choice test might encounter the question “What is the capital of France?,” which evokes the previously acquired intraverbal response “Paris.” If the student then self-echoes “Paris” while scanning the response options, joint control occurs when the student encounters the visual stimulus “Paris,” which evokes as a textual response the same topography (“Paris”) that is already occurring as a self-echoic. Assuming an appropriate history of reinforcement for informing listeners of the occurrence of joint control, the student may then go on to select the response option “Paris.” Of note, the selection response may occur even if the student has no prior history that involves matching the written names of countries to the written names of capitals, having previously acquired only textual responses to the written stimuli and topography-based intraverbal responses that involve countries and capitals. Although this is a simple example, Lowenkron (1998) explains how a joint control analysis may be applied to a wide range of novel performances, including derived symmetry and transitivity in stimulus equivalence experiments and other forms of derived relational responding. In addition, the joint control account has been applied to more elementary phenomena, such as the acquisition and recombinative generalization of conditional discriminations (Lowenkron, 2006). A weak self-echoic repertoire, according to this account, might thus be hypothesized to impede the acquisition of a basic listener repertoire as well as a variety of more complex verbal or cognitive skills.
Horne and Lowe (1996) hypothesized the role that self-echoic behavior plays in the development of naming, a higher order operant proposed to consist of bidirectional speaker and listener relations related to particular stimulus classes. Once a child has acquired this higher order relation, reinforcement of new listener relations (e.g., pointing to a picture of a tyrannosaurus after hearing the animal's name) can result in the emergence of novel, corresponding speaker relations (e.g., the tact “tyrannosaurus”) and vice versa. However, a prerequisite for the fusion of speaker and listener behavior into naming is the occurrence of child–caregiver interactions in which the child emits echoic responses to the caregiver's tacts of items while simultaneously responding as a listener by orienting to those same items, followed by continued self-echoic responses. Horne and Lowe applied the naming account not only to emergent speaker and listener relations but also to other types of emergent performances hypothesized to be functionally related to naming, such as derived symmetry, transitivity, and functional equivalence. As a result, a weak self-echoic repertoire would be expected to impair not only the acquisition of naming but also the acquisition of various novel performances.
The joint control account and the naming hypothesis each have generated a body of supporting evidence (e.g., Horne, Lowe, & Randle, 2004; Lowe, Horne, Harris, & Randle, 2002; Lowenkron, 1988, 2006; Tu, 2006). Additional research is needed on the role of the self-echoic in some of the complex performances that it has been hypothesized to affect (Horne & Lowe, 1996; Lowenkron, 1988). However, to the extent that self-echoic behavior is in fact functionally related to these phenomena, one might expect individuals with deficient self-echoic repertoires to exhibit developmental delays. A self-echoic repertoire could be deficient in at least two ways. First, self-echoic responses might fail to occur because a person's vocalizations are not under the control of his or her own immediately prior vocalizations. It is even conceivable that a person might have an intact echoic repertoire under the stimulus control of vocalizations that originate with another person, but his or her own vocalizations might fail to evoke similar responses. Second, it is possible that self-echoic vocalizations might occur under appropriate stimulus control in certain contexts, for example, when instructed (e.g., “What did you say?”), but might fail to occur spontaneously in other contexts in which such responses might be useful. In other words, self-echoic responding might not occur under all relevant stimulus conditions. In either case, individuals with such deficits might be hypothesized to benefit from intervention on self-echoic responding.
Of potential relevance to applied implications of self-echoic behavior are studies that have assessed verbal rehearsal during memory tasks in children with developmental disabilities (e.g., Bebko & Ricciuti, 2000; Jarrold, Baddeley, & Hewes, 2000; Joseph, Steele, Meyer, & Tager-Flusberg, 2005; Rosenquist, Conners, & Roskos-Ewoldsen, 2003). These studies have produced mixed findings with respect to whether or not their respective target groups actually showed impaired rehearsal compared to typically developing peers, with some suggesting that it may depend on the intellectual functioning of the target group (Bebko & Ricciuti) or on the specific task that is used (Bebko & Ricciuti; Joseph et al.). However, studies that have included intervention on verbal rehearsal have found that it improved performance on memory tasks. Loomes, Rasmussen, Pei, Manji, and Andrew (2008) measured the performance of children with fetal alcohol syndrome in a digit span task in which participants were instructed to repeat numerals following a 10-s delay. Instructions to use whispering as a strategy to remember the digits during the delay resulted in improved performance compared to a control group that did not receive instructions. Conners, Rosenquist, Arnett, Moore, and Hume (2008) evaluated the effects of parent-implemented rehearsal training that consisted of modeling and prompting. The participants were children with Down syndrome, and results indicated that the procedure improved performance on a digit span task as well as on a phonological similarity task. Although such results might be consistent with the notion of benefits of self-echoic repertoires, the procedures employed in these studies did not permit a stimulus control analysis of failures of self-echoic responses to occur. That is, it is unclear whether those participants who failed to rehearse had defective echoic repertoires or defective self-echoic repertoires, or if there was defective contextual control over self-echoic repertoires. Thus, it is also unclear which type of stimulus control might have been addressed in training.
The purpose of the current study was to assess the extent to which self-echoic repertoires of children diagnosed with autism might lag behind their echoic repertoires, and to compare their echoic and self-echoic repertoires to those of typically developing children of similar age. A procedure was developed to assess self-echoic behavior by comparing the accuracy of children's echoic responses to an experimenter's vocalizations to the accuracy of instructed self-echoic responses. The assessment procedure was administered to 8 children with autism and to 14 children who had no known language or developmental delays.
METHOD
Participants and Setting
Participants were 6 boys and 2 girls, 3 to 8 years old, with a diagnosis of autism. Each child had been enrolled for at least 1 year in a classroom that provided language-focused behavioral instruction that included both structured and naturalistic teaching sessions. Exceptions were the 3-year-old participants (n = 3) who had been enrolled in a behavioral (applied behavior analysis) classroom at the age of 3 years. A speech and language pathologist evaluated the language repertoires of each child within 2 months prior to the study. All demonstrated deficits in listener, tact, and intraverbal repertoires, with performance falling below either Level 2 (developmentally equivalent to 30 months of age) or Level 3 (developmentally equivalent to 48 months of age) on the Verbal Behavior Milestones Assessment and Placement Program (Sundberg, 2008).
In addition, 14 nondevelopmentally delayed children participated (8 boys and 6 girls). They ranged in age from 2 to 7 years and, according to parent report, had no diagnoses of developmental delays.
Each participant attended a single 20- to 30-min session in a quiet area in his or her school or day-care center. Session items consisted of a video camera, small table and chairs, recording data sheets, token cards, tokens, and a container of novel toys.
Preexperimental Assessments
Echoic placement pretest
All participants were given an echoic placement pretest immediately prior to the experimental session to determine the maximum number of digits each was able to repeat. Children were asked to repeat numerals at increasing levels of digits from one digit (Level 1) to nine digits (Level 9). Each level consisted of three sets of numerals that were randomly generated and did not appear more than once per set. To standardize presentations, no sets contained the two-syllable numeral 7; all numerals consisted of only one syllable. To illustrate the arrangement, Level 2 numerals were 9-5 (Set 1), 10-4 (Set 2), and 1-6 (Set 3). The experimenter said numerals at a steady rate without emphasis such as separation into groups (e.g., 2-4-6-3 instead of 2-4, 6-3), changes in intonation, or repetitions. Participants passed each level after correctly repeating any two of the three sets. The echoic pretest was terminated at the level at which participants missed two of the three sets. The last level each participant passed was used for the experiment. Any participant who was unable to echo any two of the three sets at Level 1 (one digit) was excused from the study.
Self-echoic lead pretest
To identify an appropriate instructional lead (e.g., “What?”) for the self-echoic (SE) probes, the experimenter presented three to five pictures of common objects (e.g., cat) that previously had been shown to reliably evoke tact responses and asked the participant “What's this?” Following the child's tact response, the picture was removed and the experimenter asked the child to repeat by saying one of several leads to the child (e.g., “What?” “Huh?” “What did you say?”). All leads that resulted in an SE response were selected for use in the SE component of the assessment (see Procedure, below). Any participant who failed to emit tact or SE responses on this pretest was excused from the study.
Response Definition and Data Collection
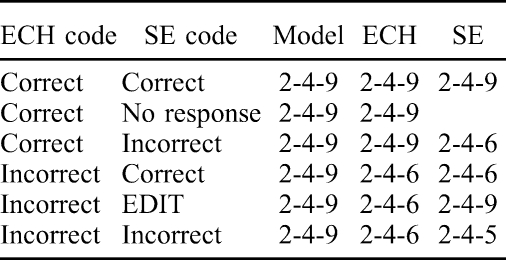
Responses to 10 echoic models (numerals) and to a self-echoic instructional lead (e.g., “What?” “Huh?” “What did you say?”) were recorded during the session and from session videotapes as they occurred in a single experimental session, with randomly interspersed distracter tasks. Echoic responses (ECH) were scored as correct, incorrect, or no response. An ECH response was scored correct when it matched with point-to-point correspondence the antecedent auditory stimulus (e.g., 8-2-5) and occurred immediately (within 2 s) of that model. Echoic responses that did not match the model or did not occur within 2 s of the model were scored as incorrect. Approximated responses that differed from the model in articulation (e.g., “thikth” for “six”) and prosody (e.g., pitch, loudness) were scored as correct. SE responses were scored as correct, incorrect, no response, or edited (EDIT). An SE response was scored correct when it matched with point-to-point correspondence the participant's own preceding correct or incorrect ECH response and occurred immediately (within 2 s) after the antecedent lead (e.g., “What?”). Thus, an SE response following an incorrect ECH was scored as a correct SE (albeit an incorrect ECH) if it matched the incorrect ECH. An SE response was scored incorrect if it did not match with point-to-point correspondence the participant's ECH, even if the response included elements of a correct response (e.g., “three eight, no, I mean, three five”). No response was scored when a participant failed to respond to the SE lead within 5 s. An SE response that matched the experimenter's echoic model, but not the participant's incorrect ECH response, was scored as EDIT. Table 1 depicts scoring codes to possible ECH and SE responses.
Table 1.
Scoring Codes for Sample Echoic (ECH) and Self-Echoic (SE) Responses to Model
Interobserver Agreement
Two independent observers recorded target responses, either during the session or from session videotapes. Interobserver agreement was calculated on a trial-by-trial basis for ECH, SE, and EDIT. An agreement for each trial was scored when both observers recorded the same ECH and SE response (correct, incorrect, no response) or an EDIT response. A disagreement was scored when observers recorded the occurrence of different ECH, SE, and EDIT responses for the same trial. Interobserver agreement was calculated by dividing the number of agreements by the sum of agreements plus disagreements and multiplying by 100%. Agreement was calculated for 75% of sessions for participants with autism and for 43% of sessions for typically developing participants. Average agreement for participants with autism was 95% for ECH responses (range, 90% to 100%) and 97% for SE responses (range, 80% to 100%). Average agreement for typically developing participants was 98% for ECH responses and 97% for SE responses (range, 90% to 100%).
Procedure
The purpose of this descriptive study was to determine differences in the occurrence of ECH and SE responses. Each 20-trial session consisted of 10 ECH/SE trials randomly interspersed with 10 distracter trials of receptive (point to) and visual performance (match) activities. Immediately prior to Trial 1, participants completed placement pretests to determine appropriate models (number of digits) for ECH trials and effective instructional leads to evoke a response on SE trials.
ECH/SE trials
The experimenter said randomly determined numerals at a rate of approximately 3 per second and asked the participant to repeat the series (e.g., 5-3-2) by saying “Ready? Say —.” A 2-s delay followed the participant's response, followed by an instructional lead selected during the placement pretest for a self-echoic (e.g., “What?”). Participants who scored less than 50% correct on the ECH component were retested at the next lower level. For example, if a participant scored below 50% responding to echoic stimuli of four syllables, the testing procedure was repeated with echoic stimuli consisting of three syllables.
Distracter trials
Distracter trials leading to token presentations were interspersed with ECH/SE trials to encourage continued responding and to interrupt repeated ECH/SE trials. Distracter trials occurred on Trials 2, 5, 6, 8, 10, 13, 14, 16, 17, and 20. The experimenter presented a receptive task (e.g., point to shoe) or a visual perceptual task (e.g., match the cat); neither task required vocal behavior from the participant. Tasks were selected from mastered skills provided by the participant's teacher. A least-to-most prompt hierarchy was used to prompt the participant to respond on tasks that were not completed independently. After task completion, the experimenter delivered praise, the token board, and a token.
Token boards
Token boards and tokens (clothespins or pennies) were used to maintain motivation during the pretest and test sessions. The placement pretest token board had two smiley faces on which tokens could be placed. Prior to the pretest, participants were given 1 to 2 min of access to a toy box. After completing each section of the placement pretest (ECH, SE lead), they received a token to put on the token board with a reminder that tokens could be exchanged for toy-box access at the end of the session. Following the placement pretest, participants exchanged tokens for access to the toy box for 2 to 3 min. During sessions, no tokens were given after echoic or self-echoic trials. Tokens were given after completion of each distracter task. At the end of the 20-trial session, each participant again exchanged the filled token boards for access to the toy box.
Procedural Integrity
A trained observer viewed session videotapes for all participants with autism and randomly selected 43% of session videotapes for typically developing participants to assess procedural integrity on all trials (target and distracter). ECH and SE trials were scored as either completely correct or incorrect. Correct target trials were defined as (a) presentation of the programmed ECH model (e.g., 3-5-1), (b) a 2-s pause after an ECH response, then (c) a request to repeat the ECH response (e.g., “What?”). Correct distracter trials were defined as (a) presentation of the distracter task immediately following the previous trial (ECH/SE or distracter), (b) visual prompts if responding on the distracter task did not readily occur, and (c) presentation of praise and the token card and token immediately following the prompted or unprompted response. A procedural integrity score was calculated for each participant by dividing the number of correctly presented trials by the sum of correct and incorrect trials and multiplying by 100%. For participants with autism, procedural integrity was 95% (range, 85% to 100%). Average procedural integrity for typically developing participants was 95% (range, 85% to 100%).
RESULTS
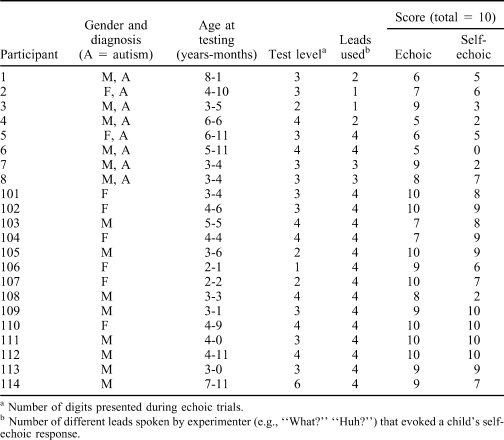
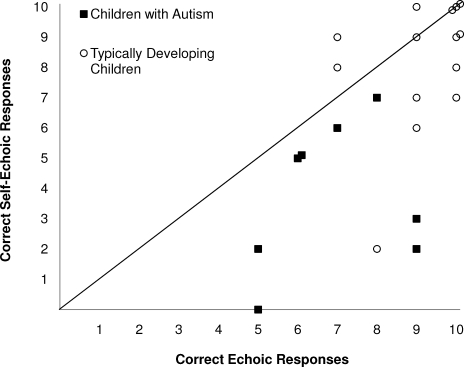
Table 2 shows the level (i.e., number of digits presented) at which each participant was tested, along with the number of correct ECH and SE responses. On average, typically developing participants responded more accurately than participants with autism on both ECH (Ms = 9.14 and 6.88) and SE (Ms = 8.14 and 3.75) trial components. Figure 1 contains a graphic representation of ECH and SE test scores. Data points that fall on the diagonal line represent an equal number of correct responses on ECH and SE trial components. Data points below the line represent fewer correct SE than ECH trials, and data points above the line represent more correct SE than ECH responses (i.e., participants who sometimes correctly self-echoed an incorrect ECH response). All participants with autism had fewer correct SE than ECH responses, whereas 7 of 14 typically developing participants had either an equal number of correct ECH and SE responses or a greater number of correct SE responses. A difference score was calculated for each participant by subtracting their SE score from their ECH score. The mean difference score was 3.12 (SD = 2.53) for participants with autism and 1.00 (SD = 2.08) for typically developing participants. The difference between the groups was statistically significant, t(20) = 2.14, p = .045.
Table 2.
Participant Characteristics and Test Results Summary
Figure 1.
Correct echoic and instructed self-echoic responses in typically developing children (open circles) and children with a diagnosis of autism (filled squares) across a single 10-trial assessment.
DISCUSSION
This study presented a procedure for assessing self-echoic behavior and the extent of its discrepancy with echoic behavior on a content-identical task (repeating a set of digits) that immediately preceded it. Performances of children with autism were compared to those of typically developing peers and were found to lag behind the peer group in accuracy of both ECH and SE responses overall. Furthermore, there was a significantly greater mean discrepancy between ECH and SE performance among participants with autism than among most typically developing participants. However, there was not a perfect correlation between difference scores and diagnosis. Half of the participants with autism had a difference score of only 1, and one of the typically developing participants (108) had a difference score of 6. It is possible that this participant had unidentified language delays that contributed to the large discrepancy. However, it is also plausible that, for this participant, the discrepancy was related to his relatively strong echoic repertoire. Participant 108 was tested at Level 4, whereas all other 3-year-old participants (with the exception of Participant 105), as well as some 4-year-olds, were tested at Level 3 (Table 2).
The level at which participants were tested was selected on the basis of a placement pretest to increase the probability that they would make correct echoic responses during the assessment, enabling assessment of self-echoic repertoires independent of echoic repertoires. In addition, participants whose performance on the echoic component was less than 50% accurate were retested at a lower level. As a result, the difference between the two groups' performance on the echoic component was unexpected. It is unclear why the placement pretest failed to predict consistently a level at which participants would make mostly correct echoic responses. Interestingly, participants with autism and typically developing participants performed similarly on the echoic portion of the placement pretest. As seen in Table 2, all participants with autism were tested at Level 2, 3, or 4, and 13 of 14 typically developing participants were tested at Levels 1 through 4; 1 participant (the oldest) was tested at Level 6. Thus, the difference in echoic repertoires emerged only when participants were exposed to the combined echoic and self-echoic trials. Fatigue when presented with a larger number of trials is one possible explanation for this finding. Another possible explanation is that echoic responses were punished by the presentation of the self-echoic lead, which occurred during the self-echoic assessment but not during the placement pretest. Anecdotally, some participants with autism emitted responses such as “I don't know” or “I already told you that” when presented with the SE lead, whereas such responses were rarely observed among the typically developing participants. Interestingly, typically developing children responded to all four SE leads during the pretest, whereas only 2 of 8 children with autism responded to all four SE leads (Table 2), suggesting different learning histories with respect to SE responses and the antecedent stimuli controlling them (e.g., “Huh?” “Say it again”). It is possible that the study would have been strengthened if a higher cutoff score had been used for retesting at a lower level, to ensure more equal performance on the echoic component. Nevertheless, the larger discrepancy between echoic and self-echoic responses among participants with autism than among typically developing participants suggests that the weaker self-echoic repertoires of these participants were not due to exclusively weaker echoic repertoires.
Although the reasons why children with autism might have weaker self-echoic repertoires than typically developing children of the same age are unknown, it is likely that this finding is related to other differences between the verbal or nonverbal repertoires of the two groups. It is possible, for example, that the participants with autism lacked prerequisite skills that are required for self-echoic responding; for example, attending to one's own vocal behavior or even one's behavior in general. Also, the greater number of SE leads available for typically developing children compared to those available for children with autism may suggest a different history for typically developing children that requires them to attend to their own vocal behavior and repeat what they just said. Although speculative, it is possible that parents and teachers are less likely to ask children with autism to repeat their own responses, presenting fewer opportunities to respond as listeners to their own vocal behavior and thereby to acquire stronger self-echoic repertoires. Thus, stimuli produced by the children's own vocalizations may not acquire stimulus control over subsequent vocal matching responses. Alternatively, it is possible that children with autism are more likely to have a history of being asked to “do something again” only when they have previously made an incorrect response to an instruction. Indeed, it is plausible that some of the participants with autism responded incorrectly on self-echoic trials due to a history of reinforcement for responding differently than the first time. In that case, their responses may indeed have been under the stimulus control over their own prior vocalizations, but the auditory product of their vocalizations exerted control over nonmatching responses instead of matching responses. However, to the extent that such stimulus control might interfere with the occurrence of self-echoic responses in situations in which they might be beneficial, it nevertheless suggests a potential reason to intervene.
Early behavioral intervention programs for children diagnosed with autism spectrum disorders typically emphasize the early establishment of accurate echoic repertoires (Greer & Ross, 2008; Leaf & McEachin, 1999; Lovaas, 2003; Maurice, Green, & Luce, 1996; Sundberg & Partington, 1998). However, no protocols have been described in the early intervention literature for establishing or increasing the accuracy of self-echoic responding. Because the present results suggest that at least some children's self-echoic responding may significantly lag behind that of most typically developing peers, it is possible that attention to establishing self-echoic skills is needed. Of course, such a recommendation is warranted only if it is in fact the case that self-echoic repertoires facilitate acquisition or performance of other skills among children with autism. Some evidence exists that self-echoic training may help overcome speaker–listener independence in this population (Tu, 2006). Future research should further investigate the extent to which self-echoic training may facilitate the acquisition of various functional skills, as implied by Lowenkron's (1988) joint control account or Horne and Lowe's (1996) naming hypothesis. Subsequent research could then focus on the identification of optimal intervention strategies to develop the self-echoic. Although it may be premature to speculate on specific intervention techniques, examples of self-echoic training procedures may be found in both the joint control literature (Tu, 2006) and in the verbal rehearsal literature (e.g., Conners et al., 2008).
It is important to note that the present study addressed only instructed self-echoic responses. However, even if instructed self-echoic repertoires were improved, self-echoic responding might not occur without instruction when needed (e.g., problem solving, remembering). As a result, it also might be important to examine the stimulus conditions necessary for uninstructed self-echoic responding; for example, motivating operations that might evoke self-echoic responding and behavior facilitated by such responding.
It is not clear whether the present findings are limited to vocal self-echoic responses or whether they might also be seen when self-duplic behavior is emitted nonvocally (e.g., sign language). Future research should address this issue. It is also unknown whether the same results would be obtained with the testing of self-echoic responses to a verbal operant other than an echoic response. Thus, future research might examine prompted self-echoic responses following instructions to tact a series of stimuli or following intraverbal responses. Finally, analysis of self-echoic performances in other language-impaired populations (e.g., poststroke, Alzheimer's) may be useful in the identification of important treatment components for these individuals.
A potential limitation of the present study is that the 2-s delay may not have been sufficient to ensure that the response on the self-echoic trial was truly self-echoic and not simply under the control of the original auditory stimulus presented by the experimenter. The performance of typically developing Participants 103, 104, and 109 suggests that their responses on self-echoic trials were indeed controlled by their previous vocalizations, because they sometimes made self-echoic responses that matched an incorrect echoic response, rather than the original auditory stimulus. However, such responses were not observed among the participants with autism. It is possible that these children simply continued to respond to the auditory stimulus provided by the experimenter, as opposed to responding to their own vocalizations. Future research might attempt to address this issue by varying the delay of the self-echoic prompt to increase the time between the prompt and the auditory echoic model of the experimenter, thus making the child's echoic auditory stimulus more recent. In addition, research on self-echoic responses to operants other than the echoic would help to separate potential sources of stimulus control.
A second possible limitation is that verbal repertoires of typically developing participants were not assessed. We assumed that these children had better verbal skills than the participants with autism; however, this was not objectively verified. Anecdotally, however, all of the typically developing participants readily tacted, manded, and engaged in conversation with the experimenter.
The present findings should be regarded as preliminary, in that the study had several limitations and provided only a documentation of differences between echoic and self-echoic performance, but did not present evidence that self-echoic repertoires affect any other functional skills. Nevertheless, given the theoretical significance of self-echoic repertoires in accounting for complex human behavior (e.g., problem solving, rehearsal, remembering; see Donahoe & Palmer, 1994), the difference in echoic and self-echoic performance is potentially an important area for researchers to consider.
Acknowledgments
This research was supported in part by a grant from the Texas Christian University Research and Creative Activities Fund and Junior Faculty Summer Research Program. Portions of the data were collected for Jordon D. McCart's honors thesis at Texas Christian University, under the supervision of Anna Ingeborg Petursdottir. We thank Charlotte L. Carp, Jessica Clothier, Laura Donner, and Maleah Goss for assistance with participant recruitment.
REFERENCES
- Bebko J.M, Ricciuti C. Executive functioning and memory strategy use in children with autism: The influence of task constraints on spontaneous rehearsal. Autism. 2000;4:299–320. [Google Scholar]
- Conners F.A, Rosenquist C.J, Arnett L.T, Moore M.S, Hume L.E. Improving memory span in children with Down syndrome. Journal of Intellectual Disability Research. 2008;52:244–255. doi: 10.1111/j.1365-2788.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- Donahoe J.W, Palmer D.C. Learning and complex behavior. Needham Heights, MA: Allyn & Bacon; 1994. [Google Scholar]
- Greer R.D, Ross D.E. Verbal behavior analysis: Inducing and expanding new verbal capabilities in children with language delays. Boston: Pearson; 2008. [Google Scholar]
- Horne P.J, Lowe C.F. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65:185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P.J, Lowe C.F, Randle V.R.L. Naming and categorization in young children: II. Listener behavior training. Journal of the Experimental Analysis of Behavior. 2004;81:267–288. doi: 10.1901/jeab.2004.81-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C, Baddeley A.D, Hewes A.K. Verbal short-term memory deficits in Down syndrome: A consequence of problems in rehearsal. Journal of Child Psychology and Psychiatry. 2000;41:223–244. [PubMed] [Google Scholar]
- Joseph R.M, Steele S.D, Meyer E, Tager-Flusberg H. Self-ordered pointing in children with autism: Failure to use verbal mediation in the service of working memory. Neuropsychologia. 2005;43:1400–1411. doi: 10.1016/j.neuropsychologia.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Michael J, Poling A. Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis. 2003;36:407–414. doi: 10.1901/jaba.2003.36-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf R, McEachin J, editors. A work in progress: Behavioral management strategies and a curriculum for intensive behavioral treatment of autism. New York: DRL Books; 1999. [Google Scholar]
- Loomes C, Rasmussen C, Pei J, Manji S, Andrew G. The effect of rehearsal training on working memory span of children with fetal alcohol spectrum disorder. Research in Developmental Disabilities. 2008;29:113–124. doi: 10.1016/j.ridd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Lovaas O.I. Teaching individuals with developmental delays: Basic intervention techniques. Austin, TX: Pro-Ed; 2003. [Google Scholar]
- Lowe C.F, Horne P.J, Harris F.D.A, Randle V.R.L. Naming and categorization in young children: Vocal tact training. Journal of the Experimental Analysis of Behavior. 2002;78:527–549. doi: 10.1901/jeab.2002.78-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenkron B. Generalization of delayed identity matching in retarded children. Journal of the Experimental Analysis of Behavior. 1988;50:163–172. doi: 10.1901/jeab.1988.50-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenkron B. Joint control and the generalization of selection-based verbal behavior. The Analysis of Verbal Behavior. 1991;9:121–126. doi: 10.1007/BF03392866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenkron B. Some logical functions of joint control. Journal of the Experimental Analysis of Behavior. 1998;69:327–354. doi: 10.1901/jeab.1998.69-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenkron B. Joint control and the selection of stimuli from their description. The Analysis of Verbal Behavior. 2006;22:129–151. doi: 10.1007/BF03393035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice C, Green G, Luce S, editors. Behavioral intervention for young children with autism: A manual for parents and professionals. Austin, TX: Pro-Ed; 1996. [Google Scholar]
- Michael J. Skinner's elementary verbal relations: Some new categories. The Analysis of Verbal Behavior. 1982;1:1–3. doi: 10.1007/BF03392791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist C, Conners F.A, Roskos-Ewoldsen B. Phonological and visuo-spatial working memory in individuals with intellectual disability. American Journal on Mental Retardation. 2003;108:403–413. doi: 10.1352/0895-8017(2003)108<403:PAVWMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Skinner B.F. Verbal behavior. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Sundberg M.L. VB-MAPP: Verbal behavior milestones assessment and placement program. Concord, CA: AVB Press; 2008. [Google Scholar]
- Sundberg M.L, Partington J.W. Teaching language to children with autism or other developmental disabilities. Pleasant Hill, CA: Behavior Analysts; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J.C. The role of joint control in the manded selection responses of both vocal and non-vocal children with autism. The Analysis of Verbal Behavior. 2006;22:191–207. doi: 10.1007/BF03393039. [DOI] [PMC free article] [PubMed] [Google Scholar]