Abstract
Angiotensin II converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) presumably stimulate renin secretion by interrupting angiotensin II feedback inhibition. The increase in cytosolic calcium caused by activation of Gq-coupled AT1 receptors may mediate the renin-inhibitory effect of angiotensin II at the cellular level, implying that ACEI and ARB may work by reducing intracellular calcium. Here, we investigated whether angiotensin II blockade acts predominantly through Gs-mediated stimulation of adenylyl cyclase (AC) by testing the effect of ACEI and ARB in mice with juxtaglomerular cell-specific deficiency of the AC-stimulatory Gsα. The ACEI captopril and quinaprilate and the ARB candesartan significantly increased plasma renin concentration (PRC) to 20 to 40 times basal PRC in wild-type mice but did not significantly alter PRC in Gsα-deficient mice. Captopril also completely abrogated renin stimulation in wild-type mice after co-administration of propranolol, indomethacin, and L-NAME. Treatment with enalapril and a low-NaCl diet for 7 days led to a 35-fold increase in PRC among wild-type mice but no significant change in PRC among Gsα-deficient mice. Three different pharmacologic inhibitors of AC reduced the stimulatory effect of captopril by 70% to 80%. In conclusion, blockade of angiotensin II stimulates renin synthesis and release indirectly through the action of ligands that activate the cAMP/PKA pathway in a Gsα-dependent fashion, including catecholamines, prostaglandins, and nitric oxide.
Angiotensin II regulates renin secretion through a homeostatic mechanism that has been called the “short feedback loop,” with renin synthesis and secretion inhibited by increases and stimulated by reductions of angiotensin II concentration.1 The cellular mechanisms underlying the feedback effects of angiotensin II are not entirely clear. There is good experimental support for the notion that the acute inhibition is mediated, at least in part, by a direct type 1 angiotensin II receptor (AT1A)-dependent effect of the peptide on cell calcium.1,2 Angiotensin II inhibits renin release in kidney slices3 and in isolated juxtaglomerular granular (JG) cells, where the effect is blocked by losartan.4,5 In the isolated JG cell, angiotensin II increases intracellular calcium levels, with increases correlating with the reduction of renin release.5
On the other hand, it is unclear whether the stimulatory arm of the short feedback loop (i.e., the increase of renin in response to a reduction of angiotensin II) can be explained by the reverse of the same mechanism. In the intact animal, acute administration of angiotensin II converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) causes a prompt and large increase in plasma renin concentration (PRC), reflecting stimulation of renin secretion. Chronic inhibition of ACE or AT1A receptors leads to very marked and maintained increases in renal renin mRNA and renin content associated with recruitment of renin-generating cells in more proximal parts of the afferent arterioles.6 A similar potent upregulation of renin expression is found in ACE- or AT1A-receptor-deficient mice.7,8 There is no good in vitro model of the stimulatory effect of angiotensin II blockade on cell calcium or renin release. Furthermore, monitoring JG cell calcium in the intact animal is not practical, and therefore data that would speak directly to the question whether the application of ACEI or ARB causes measurable reductions of JG cell calcium are not available. Such an effect is by no means certain because cytosolic calcium in the JG cell is likely to be influenced by several other ligands that couple via G proteins to phospholipase C as well as by the cellular mechanisms that control calcium exchange across the plasma membrane and across boundaries of intracellular organelles.
The experiments presented here were performed to further investigate the mechanisms by which angiotensin II blockade stimulates renin secretion. The growing body of data suggesting that renin may have direct, angiotensin-independent effects through the renin receptor9 gives added urgency to understanding the biologic mechanisms of this phenomenon, because the high circulating levels of renin produced by ACEI or ARB treatment may have pathophysiological effects. Thus, in the study presented here we asked the specific question whether the stimulation of renin release by ACEI and ARB requires the presence of Gsα as an adenylyl cyclase (AC) activator. We found that in mice in which AC-dependent generation of cAMP in JG cells is suppressed by a cell-specific knockout of Gsα, the acute and chronic stimulatory effects of ACEI or ARB were virtually abolished. These observations support the hypothesis that angiotensin blockade in vivo enhances renin release indirectly through influencing levels of one or more ligands that act through the Gsα-dependent pathway for activation of AC in JG cells. Because the effect of juxtaglomerular Gsα deletion to abrogate renin stimulation by ACEI is fully mimicked by combined administration of indomethacin, propranolol, and L-NAME, we conclude that prostaglandins, catecholamines, and nitric oxide (NO) are the main factors in stimulating the cAMP/protein kinase A pathway during angiotensin II blockade.
Results
Response of PRC to Renin Stimuli
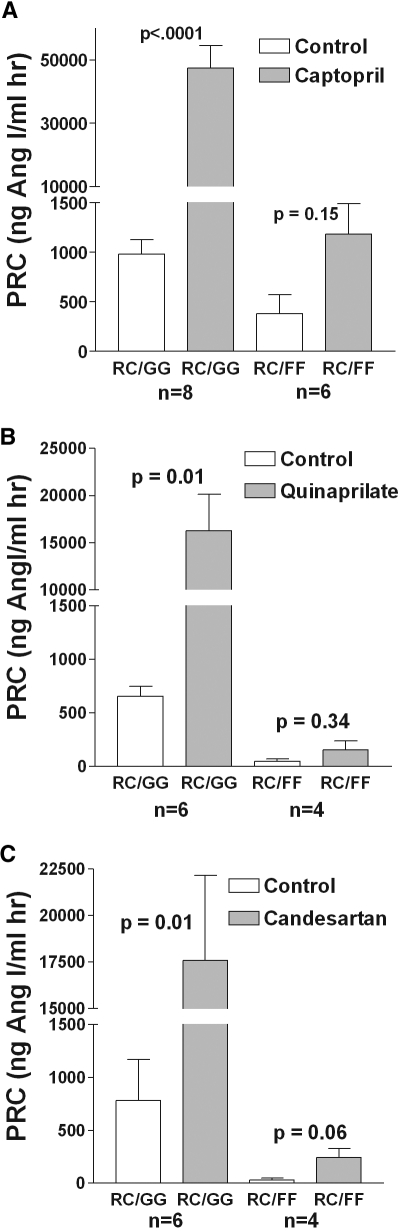
To determine the effect of Gsα on the regulation of renin release by angiotensin II, we measured PRC in conscious mice with JG cell-specific deletion of Gsα before and after blockade of angiotensin II production by captopril or quinaprilate, and of angiotensin II action by candesartan (Figure 1A through 1C). Acute administration of captopril (30 mg/kg) significantly increased PRC in wild-type animals from 981.5 ± 145 to 47,461 ± 7072 ng Ang I/ml hr (n = 8; P < 0.0001 by paired t test). In contrast to this approximately 50-fold increase of PRC in wild-type mice, the effect of captopril in Gsα-deficient animals did not reach the 5% significance level (380 ± 191 versus 1183 ± 305 ng Ang I/ml hr, n = 6; P = 0.15 by paired t test). Similarly, quinaprilate and candesartan at 50 μg/mouse increased PRC in animals with intact Gsα about 25-fold: quinaprilate from 651 ± 95 to 16,277 ± 3854 ng Ang I/ml hr (n = 6; P = 0.01) and candesartan from 780 ± 387 to 17,587 ± 4556 ng Ang I/ml hr (n = 6; P = 0.01). This contrasts markedly with findings in the Gsα-deficient mice in which quinaprilate and candesartan caused only a slight increase of PRC that again was statistically NS (P = 0.34 and P = 0.059 by paired t test; n = 5 for quinaprilate and candesartan).
Figure 1.
Stimulation of renin by inhibition of angiotensin II formation or action is Gsα-dependent. (A) PRC before (open bars) and 60 minutes after an intraperitoneal injection of 30 mg/kg body weight captopril (shaded bars) in wild-type mice (RC/GG, left) and in mice with JG cell-specific deletion of Gsα (RC/FF, right). (B) PRC before and after 50 mg quinaprilate in the same strains of mice. (C) PRC before and after 50 mg candesartan in the same strains of mice. Significances are given for comparisons with PRC before stimulation (paired t test).
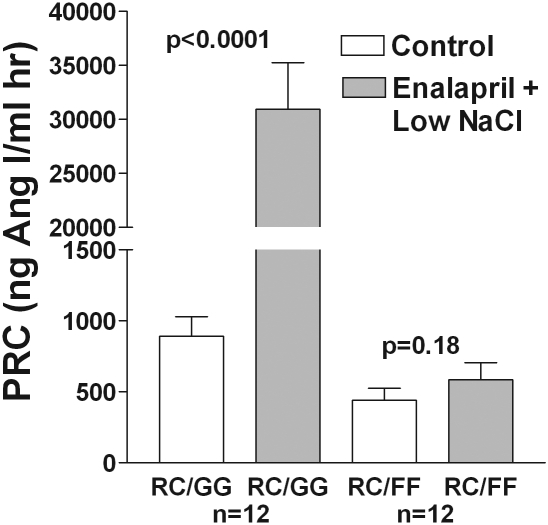
Figure 2 shows that chronic treatment with a low-salt diet [0.03% sodium chloride (NaCl) w/w] and administration of the ACE inhibitor enalapril (10 mg/kg per d) via drinking water for 7 days significantly increased PRC in wild-type mice. Again, there was no significant effect of this treatment in Gsα-deficient animals (P = 0.18 by paired t test). Efficacy of the prolonged treatment with ACEI was examined in anesthetized mice by determining the blood pressure (BP) response to angiotensin I. In wild-type and Gsα-deficient animals pretreated for 7 days with enalapril, we observed no significant responses of BP to angiotensin I, whereas BP increased dose-dependently in nontreated animals. All four groups showed BP increases to angiotensin II.
Figure 2.
Elevation of renin secretion by chronic ACE inhibition and low salt is Gsα-dependent. PRC (mean ± SEM) in conscious mice without and with Gsα recombination in control (open bars) and during administration of a low-salt diet (0.03% NaCl w/w) and the ACE inhibitor enalapril (10 mg/kg per d) via drinking water for 7 days (shaded bars). Significances are given for comparisons with PRC before stimulation (paired t test).
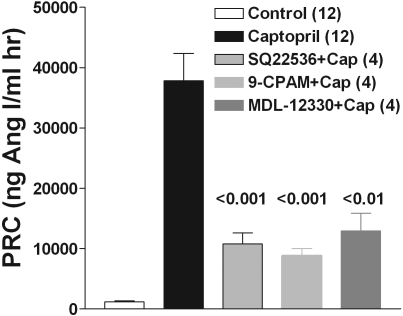
Administration of three structurally different inhibitors of AC, all at 10 mg/kg, significantly reduced the renin-stimulating effect of captopril (Figure 3) to 26% of the control response with SQ 22536, to 21% with 9-CPAM, and to 32% with MDL-12330, which confirms that activation of AC is a major mechanism behind captopril action.
Figure 3.
Stimulation of renin by ACE inhibition requires activation of adenylyl cyclase (AC). Effect of three structurally different inhibitors of AC on the response of plasma renin to captopril (mean ± SEM). Inhibitors were given intraperitoneally at 10 mg/kg and captopril at 30 mg/kg. Significances are indicated for comparison with the captopril-treated animals (ANOVA with Bonferroni post hoc test). Numbers in brackets are numbers of animals. 9-CPAM, 9-cyclopentyladenine monomethanesulfonate; MDL-12330, MDL-12,330A hydrochloride.
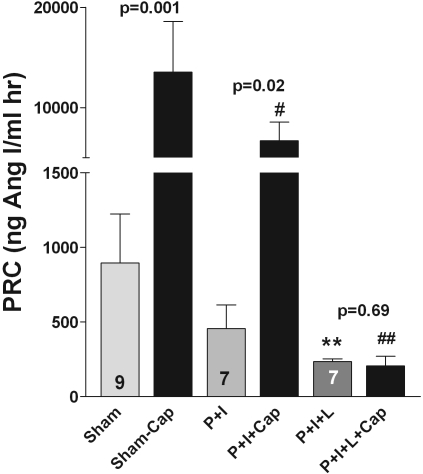
Because we propose that the Gsα dependence of the renin stimulatory effect of angiotensin II blockade reflects the roles of β-adrenergic agonists, prostaglandins, and NO, simultaneous inhibition of action or formation of these agents by co-administration of propranolol, indomethacin, and L-NAME should block the stimulation of renin release by ACEI or ARB. It was shown earlier that all three interventions diminish basal plasma renin levels when applied individually.10–12 Co-administration of indomethacin (5 mg/kg intraperitoneally) and propranolol (5 mg/kg intraperitoneally) reduced but did not eliminate the effect of captopril on PRC (Figure 4). In contrast, simultaneous injection of propranolol (5 mg/kg), indomethacin (5 mg/kg), and L-NAME (25 mg/kg intraperitoneally; P+I+L) completely eliminated the rise of PRC (ng Ang I/ml hr) by captopril (sham: 896.2 ± 329, sham+captopril 13,536 ± 5058, P = 0.001, n = 7; P+I: 457 ± 158.6, P+I+captopril: 6722.8 ± 1836.5, n = 7, P = 0.046; P+I+L: 234.5 ± 19.3, P+I+L+captopril: 207.4 ± 63.4, n = 9; P = 0.69). Relative expression of renal renin mRNA was reduced by approximately 35% at the time of captopril injection (relative expression levels control: 104.6% ± 18, P+I+L: 67.5% ± 9.7; n = 4; P = 0.14). In 4 C57Bl/6 mice equipped with BP transmitters, we examined the effect of P+I+L on arterial BP (MAP). MAP averaged 109 ± 5.5 mmHg in the 2 hours before and 107 ± 6.4 mmHg in the 2 hours after P+I+L. Furthermore, captopril given 15 minutes after the combined drug administration did not alter BP in the subsequent hour
Figure 4.
Prostaglandins, catecholamines, and NO mediate the stimulatory effect of ACE inhibitors on renin secretion. Effect of captopril (Cap, 30 mg/kg) on PRC after sham injection (sham+Cap); after injection of indomethacin (5 mg/kg) and propranolol (5 mg/kg, P+I+Cap); and after injection of indomethacin, propranolol, and L-NAME (25 mg/kg, P+I+L+Cap). Significances are given for the captopril effect in each group. **P < 0.01 versus sham; #P < 0.05 and ##P < 0.01 versus sham+captopril.
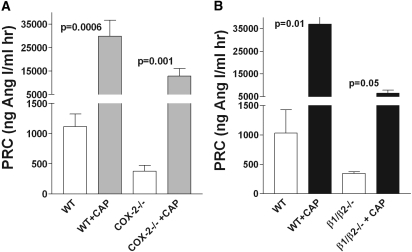
The other two low-renin animal models, cyclooxygenase-2 (COX-2) knockout mice (Figure 5A) and β1/β2-adrenergic receptor knockout mice (Figure 5B), which reduce Gsα-cAMP signaling through diminished receptor activation, were found to retain significant stimulatory responses to captopril.
Figure 5.
PRC (mean ± SEM) increases in response to acute stimulation with captopril in conscious mice deficient in COX-2 or β1/β2-adrenergic receptors. (A) PRC before (open bars) and 60 minutes after an intraperitoneal injection of 30-mg/kg body weight captopril (shaded bars) in wild-type (n = 10) and COX-2 knockout mice (n = 10). (B) PRC before (open bars) and 60 minutes after an intraperitoneal injection of 30-mg/kg body weight captopril (shaded bars) in wild-type (n = 3) and β1/β2-adrenergic receptor knockout mice (n = 4). Significances are given for comparisons with PRC before stimulation (paired t test).
Discussion
The purpose of the study presented here was to further explore the mechanisms responsible for the stimulation of renin by inhibitors of formation and action of angiotensin II in intact animals. The main observation is that cell-specific deletion of Gsα from JG cells is associated with near absence of the stimulatory effect on renin release normally elicited by acute or chronic inhibition of ACE or AT1A receptors, indicating that Gsα is required for the stimulation of renin release caused by blockade of the AT1A receptor interaction with its ligand. The implication of this finding is that an acute or chronic decrease in the occupation of AT1A receptors in vivo leads to Gsα-mediated stimulation of AC in JG cells.
We have previously demonstrated that the strain of mice used in this study, a cross between mice with floxed Gsα and Cre recombinase expressed under control of the endogenous renin promoter (RC/FF mice), has a marked reduction of Gsα expression in JG cells.13 JG cell-specific deletion of Gsα was associated with a 70% reduction of renal renin mRNA expression and an associated fall in PRC to 10% to 20% of normal. Furthermore, the response of renin release to known stimulators including furosemide, isoproterenol, and hydralazine was greatly reduced, suggesting their dependence on Gsα signaling. The study presented here shows that the stimulation of renin release by angiotensin II blockade also has a requirement for Gsα-signaling.
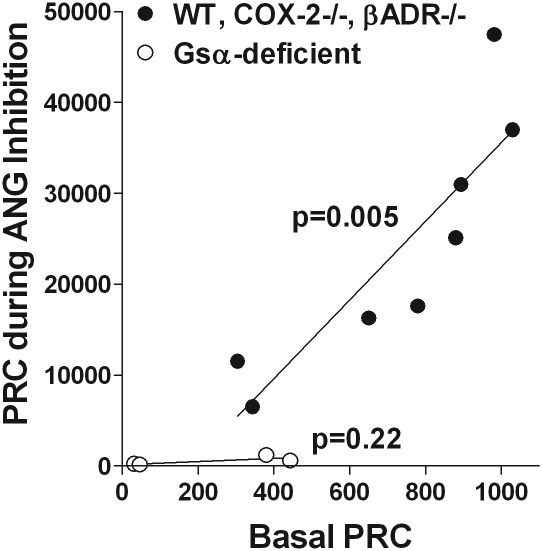
Previous studies have suggested that the magnitude of the renin release response to diverse stimulating interventions is a direct function of the pre-existing levels of renin expression. For example, basal renin expression was found to be reduced in mice with deletions of COX-2 or β-adrenergic receptors.14,15 In both strains of mice, the responses to all tested stimulators of renin release were markedly reduced. In the study presented here, we confirmed reduced basal PRC levels in both strains of mice and reduced absolute response magnitudes to captopril stimulation. Thus, the curtailed response to ACEI or ARB in the RC/FF mice may be a reflection of their low basal renin values. Although the low basal renin will limit the magnitude of the release response, our previous studies in isolated JG cells from RC/FF mice have shown that the response of renin secretion to forskolin, a direct stimulator of AC, was reduced by only 50% compared with wild-type cells, much less than the 80% to 90% reduction of the renin response to Gsα-dependent stimulators such as prostaglandin E2 (PGE2) or isoproterenol.13 This observation indicates that the Gsα-depleted cells maintain a sizable ability to release renin provided that the Gsα signaling pathway can be bypassed. Consideration of the quantitative relation between basal plasma renin and the response magnitude to captopril also suggests that the reduced renin release is related to Gsα deficiency. As shown in Figure 6, the relationship between mean PRC values after captopril and mean basal PRC seems to be the same in wild-type mice and in COX-2- and β1/β2-adrenergic receptor-deficient mice, low renin models in which Gsα signaling is intact. In contrast, data from Gsα-deficient mice treated with captopril lie outside of this relationship on a line with a slope that is not significantly different from zero, although basal levels overlap. Complete prevention of the stimulatory effect of captopril was also observed after acute co-administration of indomethacin, propranolol, and L-NAME. Because in this situation renin expression was only moderately reduced, inhibiting the effect of ligands transduced by Gsα can cause inhibition of renin release without prior depletion of cellular renin stores. Finally, pharmacologic inhibition of AC caused a 70% to 80% reduction in the stimulatory effect of ACE inhibition, confirming primacy of the cAMP pathway in mediating the effects of angiotensin II blockade. As a caveat, it should be pointed out that chronic Gsα deficiency might cause secondary changes in the development or distribution of JG cells that may make them unresponsive to angiotensin II withdrawal.
Figure 6.
Mean basal PRC correlates with mean PRC during acute and chronic inhibition of angiotensin II in mice with intact Gsα (●), but not in Gsα-deficient mice (○). P values indicate significances of slopes.
Recent studies by two independent laboratories have shown that a decrease of cytosolic calcium stimulates renin release by removal of calcium-dependent inhibition of AC and a concomitant increase of cellular cAMP. Although all ACs are inhibited by calcium, only AC5 and AC6 are affected by submicromolar concentration changes of calcium.16 JG cells have been shown to express these isoforms of AC, and their role in control of AC activity and cAMP formation in cultured cells has been documented by using specific antagonists and small-interfering RNA approaches.17,18 However, the presence of calcium-regulated ACs in JG cells may not satisfactorily explain the observations in the study presented here. Because the interaction between calcium and the catalytic subunit of AC is believed to be direct,19,20 lowering calcium should activate AC5 and/or AC6 regardless of whether or not Gsα signaling is intact. Furthermore, although AT1A blockade prevented the rise of cytosolic calcium caused by angiotensin II administration in vascular smooth muscle and glomerular mesangial cells, it did not reduce calcium below basal levels.21,22 Given that cytosolic levels of calcium are well regulated, a measurable reduction after withdrawal of the effect of a single agonist may not be expected in vivo. Another possible direct effect of angiotensin II at the level of the JG cell would be an inhibitory relationship between Gq- and Gs-coupled receptors. However, only the opposite, a synergistic interaction between Gq and Gs, has been observed in some cell types.23,24 Indirect mediation of the effects of angiotensin II blockade is supported by the earlier observations that absence of AT1A in JG cells does not abrogate the stimulatory effects of ACE or AT1A deficiencies on renin expression.25,26
We suspect that the strong stimulation of renin by angiotensin II blockade in vivo may be mostly indirect and involve participation of other cell types and the concerted action of ligands that require Gsα signaling. In fact, several complex pathways have been identified along which angiotensin II can exert inhibitory effects on renin release and expression. Previous reports have shown that inhibition or genetic deletion of COX-2 markedly attenuates the stimulatory effect of angiotensin II blockade on renin.15,27,28 Thus, a reduction in angiotensin II signaling, probably at the level of the macula densa and/or thick ascending limb cells, appears to lessen COX-2 inhibition. This is consistent with the observation that COX-2 expression is markedly upregulated in angiotensin-II-blocked or -deficient mice.27,29,30 A 5-fold upregulation of COX-2 and of urinary PGE2 excretion has also been described in the Gsα-deficient mice used in this study.13 Increased COX-2-mediated generation of PGE2 could then stimulate renin release through the prostaglandin E receptor 4, which signals through a Gsα-coupled receptor, therefore this mechanism would be disrupted in Gsα-deficient mice. Nevertheless, the observation that captopril retains some modest stimulatory effect in COX-2-deficient animals indicates the participation of additional non-PGE2 pathways.21
Upregulation of neuronal NO synthase in mice with AT1A receptor or angiotensinogen deletions indicates that angiotensin II exerts a suppressing effect on neuronal NO synthase expression.31,32 Increased levels of NO may stimulate COX-2 and thereby indirectly enhance renin expression and release in a Gsα-dependent fashion.33 This action would be magnified by the effect of NO to inhibit phosphodiesterase 3 and thereby to stabilize cAMP.34,35 Finally, angiotensin II removal is usually associated with a decrease of MAP and reflex activation of the sympathetic nervous system. That the effect of both of these changes are mediated by ligands that signal through Gsα is supported by our previous finding that the effects of a BP reduction by hydralazine and of the β-adrenergic agonist isoproterenol on plasma renin are drastically curtailed in Gsα-deficient mice.13 The proposal that the stimulatory effect of ACEI and ARB on the renin system is the result of an activation of several pathways that all converge on cellular cAMP is fully supported by the observation that simultaneous inhibition of the prostaglandin, catecholamine, and NO inputs is necessary to mimic the effect of Gsα deletion.
The data presented here show that the stimulatory effects of inhibitors of angiotensin II generation and action on renin synthesis and release are largely obliterated in mice with JG cell-specific deletion of Gsα. Thus, ACEI and ARB appear to affect renin secretion indirectly through mechanisms that utilize ligands acting through a Gsα-dependent pathway. Catecholamines, prostaglandins, and NO seem to act in a concerted fashion as mediators of ACEI- and ARB-stimulated renin secretion.
Concise Methods
Animals
Mice with tissue-specific deletion of Gsα in renin-producing cells were generated from crosses of mice in which the endogenous renin promoter was used to control the expression of Cre recombinase in juxtaglomerular cells and mice in which exon 1 of the Gsα gene was flanked by LoxP sites.1 Mice were derived from colonies of both strains maintained at the National Institutes of Health (NIH). COX-2-deficient mice and β1/β2-adrenergic receptor-deficient mice (β1/β2ADR−/−) were obtained from Jackson Laboratories. The Animal Care and Use Committee of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/NIH approved animal studies.
Treatment
For acute responses, captopril (30 mg/kg), quinaprilate (50 μg/mouse), or candesartan (50 μg/mouse) were given intraperitoneally. In the co-administration studies, propranolol (5 mg/kg) and L-NAME (25 mg/kg) were intraperitoneally injected in 0.3 ml saline followed by intraperitoneal injection of indomethacin (5 mg/kg). After 10 minutes, blood was collected by submandibular puncture for basal PRC. Captopril was injected 5 minutes later (30 mg/kg intraperitoneally) and a second blood sample was collected after 60 min from the submandibular plexus of the other side. For chronic treatment, mice were fed a low-salt diet (0.03% NaCl w/w) for 7 days and received enalapril in the drinking water (about 10 mg/kg per d). Three structurally different AC inhibitors (SQ 22536, 9-CPAM, and MDL-12330; Sigma-Aldrich Company) were used at 10 mg/kg to assess their effect on captopril-induced renin release. The effectiveness of the chronic enalapril treatment was verified in control and enalapril-treated wild-type and Gsα-deficient mice during anesthesia by determining angiotensin I (25, 50, 100 ng) and angiotensin II (25, 50, 100 ng) effects on BP. BP telemetry has been described in detail earlier.21
Blood Collection and Renin Determination
Blood was taken from conscious mice by tail vein puncture and collection into a 75-μl hematocrit tube that contained 1 μl 125 mM EDTA in its tip after 1-hour acute treatment or 7- day ACEI chronic treatment. In the drug co-administration studies, blood was collected by puncturing the submandibular plexus and collecting the emerging blood as described above. PRC was determined as described previously.8
Statistical Analysis
The t test was used for statistical comparisons between two groups or paired comparisons.
Disclosures
None.
Acknowledgments
This work was supported by intramural funds from NIDDK, NIH grant HL 66242 (R.A.G.), and by the National Natural Science Foundation of China (30770861) and Scientific Research Foundation for Excellent Returned Scholars, Ministry of Human Resources of China (L.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Hackenthal E, Paul M, Ganten D, Taugner R: Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Keeton TK, Campbell WB: The pharmacologic alteration of renin release. Pharmacol Rev 32: 81–227, 1980. [PubMed] [Google Scholar]
- 3. Michelakis AM: The effect of angiotensin on renin production and release in vitro. Proc Soc Exp Biol Med 138: 1106–1108, 1971. [DOI] [PubMed] [Google Scholar]
- 4. Kurtz A, Della Bruna R, Scholz H, Baier W: Amiloride enhances the secretion but not the synthesis of renin in renal juxtaglomerular cells. Pflugers Arch 419: 32–37, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Ichihara A, Suzuki H, Murakami M, Naitoh M, Matsumoto A, Saruta T: Interactions between angiotensin II and norepinephrine on renin release by juxtaglomerular cells. Eur J Endocrinol 133: 569–577, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM: Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol 259: F660–F665, 1990. [DOI] [PubMed] [Google Scholar]
- 7. Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA: Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension 29: 216–221, 1997. [DOI] [PubMed] [Google Scholar]
- 8. Sugaya T, Nishimatsu S, Tanimoto K, Takimoto E, Yamagishi T, Imamura K, Goto S, Imaizumi K, Hisada Y, Otsuka A, et al. : Angiotensin II type 1a receptor-deficient mice with hypotension and hyperreninemia. J Biol Chem 270: 18719–18722, 1995. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen G, Danser AH: Prorenin and (pro)renin receptor: A review of available data from in vitro studies and experimental models in rodents. Exp Physiol 93: 557–563, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Castrop H, Schweda F, Mizel D, Huang Y, Briggs J, Kurtz A, Schnermann J: Permissive role of nitric oxide in macula densa control of renin secretion. Am J Physiol Renal Physiol 286: F848–F857, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J: Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol 290: F1016–F1023, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Schricker K, Hamann M, Kurtz A: Nitric oxide and prostaglandins are involved in the macula densa control of the renin system. Am J Physiol Renal Physiol 269: F825–F830, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J: Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol 292: F27–F37, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J: Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension 50: 103–109, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J: Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol 292: F415–F422, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Hanoune J, Defer N: Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41: 145–174, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F: The calcium paradoxon of renin release: Calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res 99: 1197–1206, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH: Adenylyl cyclase isoform v mediates renin release from juxtaglomerular cells. Hypertension 49: 618–624, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Boyajian CL, Garritsen A, Cooper DM: Bradykinin stimulates Ca2+ mobilization in NCB-20 cells leading to direct inhibition of adenylyl cyclase. A novel mechanism for inhibition of cAMP production. J Biol Chem 266: 4995–5003, 1991. [PubMed] [Google Scholar]
- 20. Chabardes D, Imbert-Teboul M, Elalouf JM: Functional properties of Ca2+-inhibitable type 5 and type 6 adenylyl cyclases and role of Ca2+ increase in the inhibition of intracellular cAMP content. Cell Signal 11: 651–663, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Fuller AJ, Hauschild BC, Gonzalez-Villalobos R, Awayda MS, Imig JD, Inscho EW, Navar LG: Calcium and chloride channel activation by angiotensin II-AT1 receptors in preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol 289: F760–F767, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tepel M, Heidenreich S, Zhu Z, Walter M, Nofer JR, Zidek W: Captopril inhibits the agonist-induced increase of cytosolic free Ca2+ in glomerular mesangial cells. Kidney Int 46: 696–702, 1994. [DOI] [PubMed] [Google Scholar]
- 23. Ostrom RS, Naugle JE, Hase M, Gregorian C, Swaney JS, Insel PA, Brunton LL, Meszaros JG: Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem 278: 24461–24468, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Selbie LA, Hill SJ: G protein-coupled-receptor cross-talk: The fine-tuning of multiple receptor-signaling pathways. Trends Pharmacol Sci 19: 87–93, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Matsusaka T, Nishimura H, Utsunomiya H, Kakuchi J, Niimura F, Inagami T, Fogo A, Ichikawa I: Chimeric mice carrying “regional” targeted deletion of the angiotensin type 1A receptor gene. Evidence against the role for local angiotensin in the in vivo feedback regulation of renin synthesis in juxtaglomerular cells. J Clin Invest 98: 1867–1877, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC: Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest 103: 953–961, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC: Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol 280: F449–F456, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto T, Kihara M, Sato K, Matsushita K, Tanimoto K, Toya Y, Fukamizu A, Umemura S: Expression of cyclooxygenase-2 in the juxtaglomerular apparatus of angiotensinogen gene-knockout mice. Nephron Physiol 102: 1–8, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Wolf K, Castrop H, Hartner A, Goppelt-Strube M, Hilgers KF, Kurtz A: Inhibition of the renin-angiotensin system upregulates cyclooxygenase-2 expression in the macula densa. Hypertension 34: 503–507, 1999. [DOI] [PubMed] [Google Scholar]
- 31. Kihara M, Umemura S, Kadota T, Yabana M, Tamura K, Nyuui N, Ogawa N, Murakami K, Fukamizu A, Ishii M: The neuronal isoform of constitutive nitric oxide synthase is up-regulated in the macula densa of angiotensinogen gene-knockout mice. Lab Invest 76: 285–294, 1997. [PubMed] [Google Scholar]
- 32. Kihara M, Umemura S, Sugaya T, Toya Y, Yabana M, Kobayashi S, Tamura K, Kadota T, Kishida R, Murakami K, Fukamizu A, Ishii M: Expression of neuronal type nitric oxide synthase and renin in the juxtaglomerular apparatus of angiotensin type-1a receptor gene-knockout mice. Kidney Int 53: 1585–1593, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC: Nitric oxide regulates renal cortical cyclooxygenase-2 expression. Am J Physiol Renal Physiol 279: F122–F129, 2000. [DOI] [PubMed] [Google Scholar]
- 34. Kurtz A, Gotz KH, Hamann M, Kieninger M, Wagner C: Stimulation of renin secretion by NO donors is related to the cAMP pathway. Am J Physiol Renal Physiol 274: F709–F717, 1998. [DOI] [PubMed] [Google Scholar]
- 35. Kurtz A, Gotz KH, Hamann M, Wagner C: Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci U S A 95: 4743–4747, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]