Abstract
Agonists of the sphingosine-1-phosphate receptor (S1PR) attenuate kidney ischemia-reperfusion injury (IRI). Previous studies suggested that S1P1R-induced lymphopenia mediates this protective effect, but lymphocyte-independent mechanisms could also contribute. Here, we investigated the effects of S1PR agonists on kidney IRI in mice that lack T and B lymphocytes (Rag-1 knockout mice). Administration of the nonselective S1PR agonist FTY720 or the selective S1P1R agonist SEW2871 reduced injury in both Rag-1 knockout and wild-type mice. In vitro, SEW2871 significantly attenuated LPS- or hypoxia/reoxygenation-induced apoptosis in cultured mouse proximal tubule epithelial cells, supporting a direct protective effect of S1P1R agonists via mitogen-activated protein kinase and/or Akt pathways. S1P1Rs in the proximal tubule mediated IRI in vivo as well: Mice deficient in proximal tubule S1P1Rs experienced a greater decline in renal function after IRI than control mice and their kidneys were no longer protected by SEW2871 administration. In summary, S1PRs in the proximal tubule are necessary for stress-induced cell survival, and S1P1R agonists are renoprotective via direct effects on the tubule cells. Selective agonists of S1P1Rs may hold therapeutic potential for the prevention and treatment of acute kidney injury.
Ischemia-reperfusion injury (IRI) is a leading cause of acute kidney injury (AKI), which is associated with prolonged hospitalization and high morbidity and mortality. The pathogenesis of AKI is complex and involves direct effects on vascular endothelial cells, tubule cells, and immune cells.1,2 Kidney tubular epithelial cells express both TLR-2 and TLR-4, which are thought to respond to endogenous “danger signals” to initiate AKI.3 These danger signals activate immune cells, leading to inflammation induced by cytokines, chemokines, and classic innate effector immune cells, such as neutrophils and macrophages.
Lymphocytes are important mediators of experimental IRI in the kidney as well as other organs.4–6 Lymphocytes contribute to the pathogenesis of IRI, and their absence, such as in mice deficient in B (μMT)7 or T cells (nu/nu)6,8 or both (Rag-1 knockout [KO]),6 confers protection from kidney IRI.
Sphingosine-1-phosphate (S1P), a sphingolipid that is produced by phosphorylation of sphingosine by sphingosine kinases, is the natural ligand for a family of five G protein–coupled receptors (GPCRs; S1P1 to 5Rs) and evokes diverse cellular signaling responses.9–11 Phosphorylated FTY720 (FTY720-P), the active form of the drug, is a nonselective agonist that activates S1P1,3 to 5Rs.12,13 The protective efficacy of FTY720 via direct activation of S1P1Rs results in a reversible redistribution of lymphocytes (B and T cells) from the circulation to secondary lymph tissue and thus away from sites of inflammation.12,14 Furthermore, activation of S1P1Rs elicits various tissue-protective effects in normal and disease states. More importantly, S1P1R is critical in mouse vascular development15 (i.e., global S1P1R gene disruption is embryonically lethal at midgestation as a result of a failure of vascular maturation15,16). FTY720-P–treated cultured oligodendrocyte progenitor cells are protected from apoptotic cell death through activation of mitogen-activated protein kinase/extracellular regulated kinase (MEK/ERK) and phosphatidylinositol-3-kinase (PI3K)/Akt signaling.17 The downstream MEK/ERK and PI3K/Akt signaling pathways, which are activated by various GPCRs, have been implicated in promoting cell survival under various conditions along with pertussis toxin–sensitive coupling of S1P1R activation.18,19
We and others have demonstrated that selective activation of the S1P1Rs with S1P1R agonists reduces kidney IRI20–22; however, it is not known whether the tissue-protective effects of these S1P1R agonists are mediated by the canonical effect of S1P1R agonists to induce lymphopenia or by stimulating S1P1Rs on other target tissues or both. Previously, we demonstrated that mice treated with FTY720 were protected from kidney IRI; VPC44116, a selective S1P1R antagonist, reversed the protective effects of FTY720 but did not reverse the lymphopenia induced by FTY720.20 This observation led us to hypothesize that S1P1R agonists may have additional direct effects on kidney resident cells independent of lymphocytes to reduce injury after kidney IRI.
This study explored the mechanisms involved in S1P1R-mediated tissue protection from IRI in a mouse model of AKI. We demonstrated that S1P1R agonists protect kidneys from IRI, independent of B and T lymphocytes, through direct activation of S1P1Rs expressed on renal proximal tubule epithelial cells (PTECs) and, further, that the S1P1R agonist protective effect requires PTEC S1P1Rs as demonstrated by using Cre-floxed transgenic mice. Furthermore, S1P1R agonists directly block apoptosis and induce cell survival pathways via activation of the Akt and/or mitogen-activated protein kinase (MAPK) pathways.
Results
Selective S1P1R Activation Reduces Kidney IRI in Rag-1 KO Mice
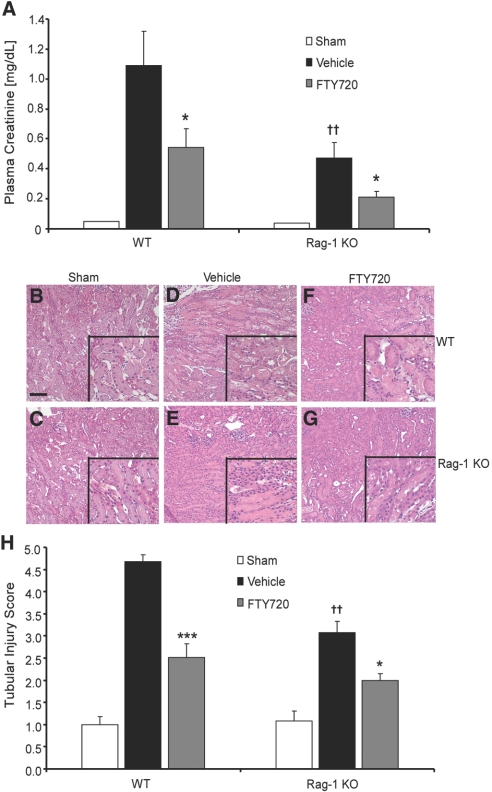
To investigate whether S1P1R activation reduces kidney IRI independent of T and B cells, we administered FTY720 to wild-type (WT) and Rag-1 KO mice and subjected mouse kidneys to 28 minutes of ischemia followed by 24 hours of reperfusion. Vehicle-treated WT mice had increased plasma creatinine levels after kidney IRI, but Rag-1 KO mice were partially protected from injury. FTY720 attenuated kidney IRI in WT mice and further reduced plasma creatinine in the partially protected Rag-1 KO mice (Figure 1A). Similar results were found in light microscopic analysis of hematoxylin- and eosin-stained kidney outer medulla. Kidney IRI led to tubular necrosis, loss of brush border villi, and obstruction of proximal tubules in vehicle-treated WT and Rag-1 KO mice (Figure 1, D and E) compared with sham-operated mice (Figure 1, B and C); however, FTY720 reduced the severity of injury in both WT and Rag-1 KO kidneys (Figure 1, F and G). Baseline kidney morphology was similar in sham-operated Rag-1 KO and WT mice, but Rag-1 KO mice had less tubule damage after IRI compared with WT mice. Quantification of the medullary tubule injury parallels functional data (Figure 1H).
Figure 1.
FTY720 reduces kidney IRI in Rag-1 KO mice. (A) Effects of FTY720 on plasma creatinine in WT and Rag-1 KO mice exposed to kidney IRI. Mice were treated with FTY720 24 hours and 1 hour before ischemia. There were no differences between baseline plasma creatinine levels in sham-operated WT (0.10 ± 0.02; n = 2) and Rag-1 KO (0.09 ± 0.03; n = 2) mice. Data are means ± SEM; n = 2 to 8 for each group (n = 2 for sham-operated mice, n = 8 for WT-vehicle, n = 8 for WT-FTY720, n = 6 for Rag-1 KO-vehicle, and n = 6 for Rag-1 KO-FTY720). ††P < 0.01 versus WT-vehicle; *P < 0.05 versus each respective vehicle treatment. (B through G) Hematoxylin and eosin (H&E) staining of kidney sections from WT and Rag-1 KO IRI; insets show a ×2.5 magnified image. (B) WT-sham. (C) Rag-1 KO sham. (D) WT vehicle. (E) Rag-1 KO vehicle. (F) WT FTY720. (G) Rag-1 KO FTY720. Bar = 100 μm. (H) Semiquantitative scoring of tubular injury in H&E-stained kidney sections using a scale of 0 to 5 as described in the Concise Methods section. Data are means ± SEM; n = 2 to 5 for each group. ***P < 0.001, *P < 0.05 versus respective vehicle treatment; ††P < 0.001 versus WT-vehicle.
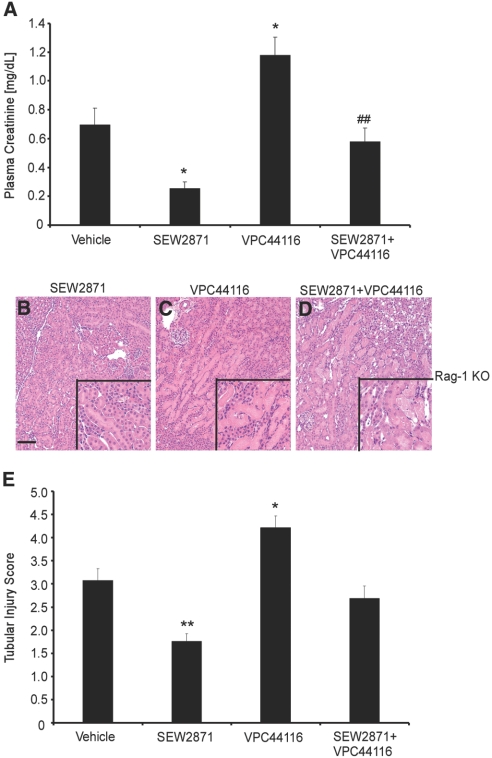
To test the role in tissue protection of direct activation of S1P1Rs independent of lymphocytes, we treated Rag-1 KO mice with S1P1R selective compounds. SEW2871, an S1P1R agonist, or VPC44116, a selective S1P1R antagonist, was administered to Rag-1 KO mice 1 hour before IRI. The rise in plasma creatinine in vehicle-treated Rag-1 KO mice after IRI was partially reversed by SEW2871. Co-administration of SEW2871 and VPC44116 abolished the partial protection observed with SEW2871. Moreover, VPC44116 enhanced injury compared with vehicle-treated mice (Figure 2A). Consistent with these functional studies, morphologic analysis revealed comparable changes in the severity of injury by SEW2871 (Figure 2B), VPC44116 (Figure 2C), and SEW2871+VPC44116 (Figure 2D) that was substantiated by semiquantitative scoring (Figure 2E).
Figure 2.
Selective activation of S1P1R mediates tissue protection from kidney IRI in Rag-1 KO mice. (A) Effects of SEW2871 and VPC44116 administered to Rag-1 KO mice 1 hour before kidney IRI. Data are means ± SEM; n = 3 to 7 per group. *P < 0.05 versus vehicle; ##P < 0.01 versus SEW2871. (B through D) H&E staining of mouse kidney sections from Rag-1 KO mice pretreated with SEW2871 (B), VPC44116 (C), SEW2871+VPC44116 (D) before IRI; insets show a ×2.5 magnified image. Bar = 100 μm. (E) Tubular injury score. Data are means ± SEM; n = 5 to 7 for treated groups, and n = 3 for sham and SEW2871+VPC44116. **P < 0.01, *P < 0.05 versus vehicle.
Profile of Infiltrating Leukocytes in Kidney after IRI in SEW2871- and VPC44116-Treated Rag-1 KO Mice
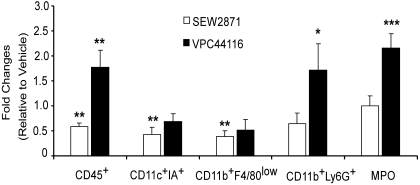
We investigated the effect of lymphocyte-independent S1P1R stimulation on leukocyte infiltration after kidney IRI in Rag-1 KO mice. FACS analysis of total CD45+ cells, neutrophils (CD11b+GR-1+ [Ly6G]), macrophages (CD11b+F4/80low), and activated dendritic cells (CD11c+IA+) revealed that the percentages of all cells increased in kidney 24 hours after IRI (vehicle pretreatment) compared with sham (data not shown). SEW2871 blocked the increase in total CD45+ cells and activated dendritic cells and macrophages, whereas VPC44116 treatment increased the total number of CD45+ cells compared with vehicle and SEW2871. The increase in neutrophils in kidneys of vehicle-treated Rag-1 KO mice after IRI (3.9-fold relative to sham) was further enhanced with VPC44116 treatment (6.7-fold relative to sham and 1.7-fold relative to vehicle). Similarly, VPC44116 (5.2-fold relative to sham and 2.2-fold relative to vehicle) more than doubled the increased myeloperoxidase (MPO) activity, another measure of polymorphonuclear leukocyte infiltration, produced by IRI (2.4-fold relative to sham) in kidneys from Rag-1 KO mice (Figure 3).
Figure 3.
Selective activation of S1P1Rs attenuates leukocyte infiltration in kidney after IRI in Rag-1 KO mice. The effects of SEW2871 and VPC44116 on leukocyte infiltration in Rag-1 KO mouse kidneys 24 hours after IRI were examined. FACS analysis of total live (7AAD−) leukocytes (CD45+) and subsets of CD45+ cells expressed as percentage of total CD45+ cells as determined by cell count per gram of kidney weight. Fold changes relative to cell numbers of each population in vehicle-treated mice are shown; n = 4 to 5. ***P < 0.001, **P < 0.01, *P < 0.05 versus vehicle.
LPS Increases Relative mRNA Levels of S1P1Rs in Kidney PTECs
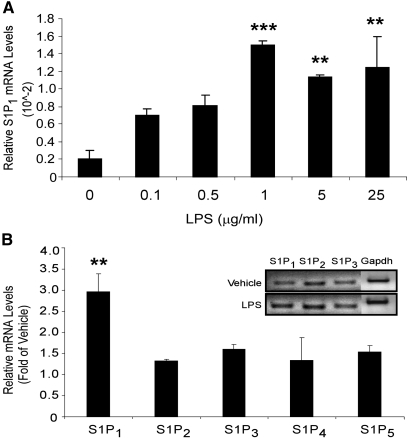
Our studies of Rag-1 KO mice demonstrated that the protective effects of S1P1R stimulation are mediated partially independent of lymphocyte S1P1Rs. To examine the role of PTEC S1PRs in kidney cell injury further, we used an LPS-induced tubule cell injury model in a well-characterized mouse proximal tubule cell line, TKPTS. We first sought to determine whether S1PR expression in TKPTS cells changes after injury, because we found previously that kidney S1PRs increase in WT mice after kidney IRI.20 We used LPS, a TLR-4 ligand, to induce apoptosis in vitro in TKPTS cells. TKPTS cells expressed mRNAs encoding all S1PRs (S1P1 to 5). On LPS stimulation, only S1P1R mRNA levels showed a significant dosage-dependent increase, whereas other subtypes (S1P2 to 5Rs) remained unchanged or had a modest increase in mRNA (Figure 4).
Figure 4.
LPS induces expression of S1P1R in cultured kidney epithelial cells. (A) S1P1R expression in TKPTS cells 24 hours after LPS doses of 0.1 to 25.0 μg/ml; n = 3 to 4 replicates. **P < 0.01, ***P < 0.001 versus 0 LPS. (B) mRNA expression of S1PR subtypes in TKPTS cells in response to 1 μg/ml LPS treatment for 24 hours. Inset is a representative gel of S1PRs PCR products from vehicle- and LPS-treated cells. S1P4R and S1P5R expression levels were too low to detect and therefore were not included in gel analysis. **P < 0.01 versus vehicle-treated cells. Results in A and B are from representative experiments that were repeated three times.
Selective Activation of S1P1Rs Reduces Injury through Direct Effects on PTECs by Activating MAPK Pathways
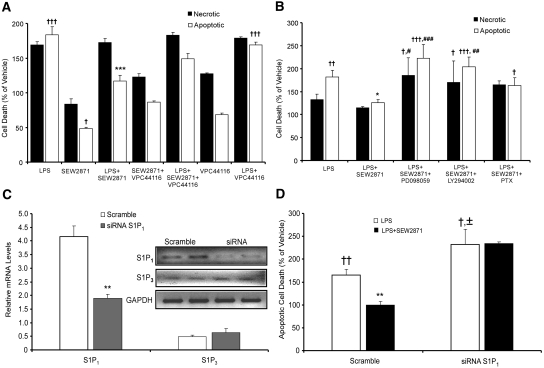
LPS increased apoptosis in a dosage-dependant manner with significant cell death noted 24 hours after treatment with a concentration of 1 μg/ml (data not shown); this concentration and time were therefore selected for subsequent injury experiments. Cell death was determined by counting 7-AAD−AnV+ (apoptotic)23 and 7-AAD+AnV+ (necrotic)24 cells by FACS, and results were confirmed by measuring DNA laddering (data not shown). FTY720-P alone reduced apoptosis (data not shown), and SEW2871 alone reduced cell death (apoptosis and necrosis). LPS increased apoptosis, an effect that was reduced by treatment with FTY720-P (data not shown), and the LPS-induced increase in necrosis and apoptosis was reduced by SEW2871 (Figure 5A). Treatment with VPC44116 blocked the effect of SEW2871 alone and the protective effect of SEW2871 on LPS-induced cell death but had no effect on LPS alone (Figure 5A).
Figure 5.
S1P1R agonists attenuate LPS-induced injury in cultured kidney epithelial cells through MAPK and Akt pathways and knockdown of S1P1R expression abolishes protection by S1P1R agonist. Necrotic and/or apoptotic cell death measured by FACS analysis of Annexin V and 7-AAD labeling 24 hours after treatment of TKPTS cells. (A) TKPTS cells were treated with SEW2871 (1 μM) and/or with VPC44116 (10 μM) 1 hour before treatment with or without LPS (1 μg/ml); n = 3 to 4. †††P < 0.001, †P < 0.05 relative to vehicle; ***P < 0.001 relative to LPS. (B) Cells were pretreated with the ERK inhibitor PD098059 (5 μM), Akt inhibitor LY294002 (5 μM), or pertussis toxin (PTX; 100 ng/ml) 1 hour before SEW2871 and LPS. †††P < 0.001, ††P < 0.01, and †P < 0.05 relative to vehicle; *P < 0.05 relative to LPS; ##P < 0.01, #P < 0.05 relative to LPS+SEW2871. Results are from a representative experiment that was performed three times with n = 3 to 4 replicates of each treatment. (C) Relative mRNA levels of S1P1R (reduced by approximately 50%) and S1P3R (unchanged) after transfection of TKPTS cells with 25 nM scrambled sequence or S1P1R siRNA; n = 3. Inset shows RT-PCR products for S1P1R, S1P3R, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from a representative gel. **P < 0.01 relative to siRNA-scramble transfected cells. Experiment was performed three times. (D) Apoptotic cell death in siRNA-transfected cells after LPS treatment (1 μg/ml) for 24 hours with and without 1 hour of pretreatment with SEW2871 (1 μM). ††P < 0.01, †P < 0.05 relative to vehicle; **P < 0.01 relative to LPS; ±P < 0.05 relative to LPS in scramble.
We next examined the cell signaling pathways leading to improved cell survival with SEW2871. SEW2871 increased activation of the MAPK (ERK) or PI3K (Akt) pathways in TKPTS cells by increasing phosphorylation of ERK and Akt in a time-dependent manner (data not shown). Pretreatment of cells with LY294002 or PD098059 abolished the protective effect of SEW2871. Protection by SEW2871 was also abolished by previous treatment with pertussis toxin, suggesting that SEW2871 signals through Gi/oα proteins (Figure 5B).
S1P1Rs Expressed on Kidney Epithelial Cells Are Necessary for the Protective Effects of SEW2871 from LPS and Hypoxia-Reoxygenation
To investigate the requirement of the epithelial cell S1P1Rs for the protective effects of SEW2871, we attenuated the level of S1P1R expression with small interfering RNA (siRNA) before injury with LPS. The S1P1R subtype was specifically knocked down (60 to 80% decrease relative to transfection with scrambled sequence; Figure 5C), and no changes in S1P3R mRNA were observed in the S1P1R siRNA–transfected cells. TKPTS cells transfected with S1P1R or scrambled siRNA were subjected to injury with 1 μg/ml LPS (24 hours). The LPS-induced increase in apoptotic cell death was greater in S1P1R siRNA–transfected cells than in scramble siRNA–transfected cells. SEW2871 protected cells from LPS-induced (Figure 5D) apoptotic cell death in TKPTS cells transfected with scrambled but not S1P1R siRNA. Using a different model of tissue injury, exposure of TKPTS cells to hypoxia-reoxygenation (H/R) produced results that were strikingly similar to those of the LPS studies. Vehicle-treated TKPTS cells subjected to H/R underwent apoptosis at 156 ± 12% the rate of unmanipulated cells (normoxia), whereas preincubation with SEW2871 (0.5 hours before H/R) protected cells from H/R-induced apoptosis (115 ± 12% the rate of unmanipulated cells; P < 0.05; n = 3).
PTEC Deficiency of S1P1R In Vivo Leads to Enhanced Injury and Renders S1P1R Agonists Ineffective
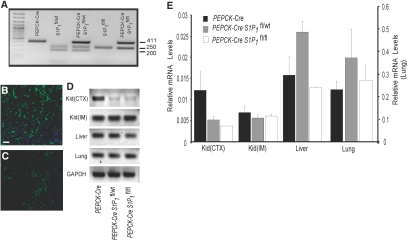
Our findings that S1P1R agonists protect PTECs from LPS- and H/R-induced cell death support our hypothesis that direct activation of S1P1Rs on kidney resident cells, independent of lymphocytes, may contribute to reduced injury after kidney IRI. To investigate, in vivo, the direct involvement of PTEC S1P1Rs in kidney IRI and because global S1P1R deficiency is embryonic lethal,15 we used conditional KO mice to delete S1P1Rs in kidney PTECs. To delete specifically the S1P1Rs from PTEC, S1P1fl/fl mice were bred with a transgenic mouse with Cre-recombinase under the control of the phosphoenolpyruvate carboxykinase promoter (PEPCK-Cre).25 Crosses of PEPCK-Cre mice with S1P1fl/fl mice yielded offspring harboring one floxed S1P1 and one wild-type (PEPCK-CreS1P1fl/wt) allele or two floxed S1P1 alleles (PEPCK-CreS1P1f1/f1) that reached adulthood with no apparent abnormalities. PCR confirmed the genotype of S1P1R null mice (PEPCK-CreS1P1fl/fl and PEPCK-CreS1P1fl/wt) and the control transgenic mice (S1P1fl/wt, S1P1fl/fl, and PEPCK-Cre) generated from the breeding (Figure 6A). PEPCK-Cre mice were also bred with ROSA26YFP/+ mice26 to assess the specificity of Cre recombinase expression. Immunofluorescence confirmed the anticipated expression of Cre recombinase in the kidney (Figure 6B) and only sparse expression in liver (Figure 6C). S1P1R mRNA levels were measured by real-time reverse transcriptase–PCR (RT-PCR) of kidney cortex, kidney inner medulla, liver, and lung from PEPCK-Cre, PEPCK-CreS1P1fl/wt, and PEPCK-CreS1P1fl/fl. A representative gel of PCR products (Figure 6D) and the quantified relative mRNA levels (Figure 6E) show a marked decrease in S1P1R mRNA in cortical samples of kidney with minimal to no changes in kidney inner medulla, liver, and lung of hetero- or homozygous KOs relative to the PEPCK-Cre.
Figure 6.
Generation of PEPCK-Cre-S1P1 floxed mice results in reduced copy number of S1P1Rs in kidney cortex. (A) Genotyping of PEPCK-Cre and S1P1 floxed mice. Agarose gel analysis of PCR products amplified from tail DNA. Lane 1, molecular weight markers; lane 2, PEPCK-Cre; lane 3, S1P1fl/wt; lane 4, PEPCK-CreS1P1fl/wt; lane 5, S1P1fl/fl; lane 6, PEPCK-CreS1P1fl/fl. Size of predicted PCR products (number of bp) is shown at right side of gel. (B and C) Immunofluorescent localization of YFP reporter (GFP immunoreactivity, green) indicative of Cre recombinase expression in kidney (B) and liver (C) sections of PEPCK-CreRosa26YFP/wt mice. Nuclei were labeled with DAPI, blue. Bar = 40 μm. (D) Representative gel of S1P1R mRNA levels in different tissues from PEPCK-Cre, PEPCK-CreS1P1fl/wt, and PEPCK-CreS1P1fl/fl mice (lanes from left to right). Regions of cortex (CTX) and inner medulla/papilla (IM) were dissected from kidney. (E) Expression of S1P1R (mRNA levels relative to GAPDH, determined by real-time RT-PCR) in kidney cortex (Kid-CTX) and medulla (Kid-IM), liver, and lung from PEPCK-Cre, PEPCK-CreS1P1fl/wt, and PEPCK-CreS1P1fl/fl mice.
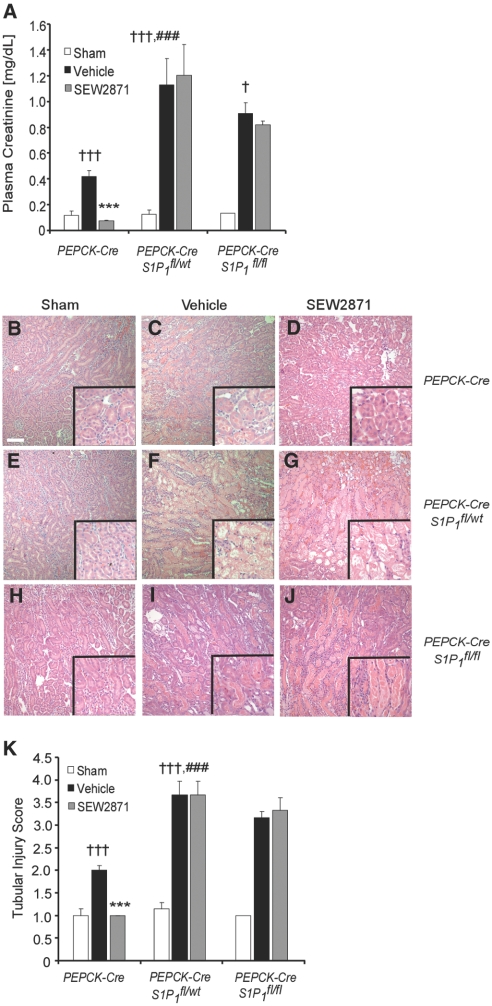
The effects of IRI with vehicle or SEW2871 treatment on plasma creatinine or kidney histology were comparable in S1P1fl/fl, S1P1fl/wt, and PEPCK-Cre mice; therefore, only PEPCK-Cre results are shown. IR produced significantly more injury as measured by plasma creatinine in PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl than in the control PEPCK-Cre mice (Figure 7A). SEW2871 treatment protected the kidneys of PEPCK-Cre mice from IR. In contrast, SEW2871 had no protective effect in PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl mice (Figure 7A). Morphologic analysis revealed changes in the severity of injury comparable to the functional studies (Figure 7, B through J) that were verified by semiquantitative scoring (Figure 7K). Hemodynamic changes were not observed in PEPCK-CreS1P1fl/wt, because BP and heart rate (136.25 ± 2.01 mmHg and 351.00 ± 6.44 bpm, respectively; n = 4) were not different from PEPCK-Cre mice (140.40 ± 3.04 mmHg and 367.00 ± 8.51 bpm, respectively; n = 5). SEW2871 reduced peripheral lymphocyte counts to a similar extent in PEPCK-Cre (SEW2871 0.26 ± 0.03 K/μl and vehicle 3.20 ± 1.10 K/μl; n = 5) and PEPCK-CreS1P1fl/wt (SEW2871 0.34 ± 0.05 K/μl and vehicle 3.17 ± 0.80 K/μl; n = 4).
Figure 7.
S1P1Rs are necessary for PTEC survival during kidney IRI. (A) Plasma creatinine levels of PEPCK-Cre, PEPCK-CreS1P1fl/wt, and PEPCK-CreS1P1fl/fl mice pretreated with vehicle or SEW2871 1 hour before mild kidney ischemia (26 minutes) and 24 hours of reperfusion. †††P < 0.001 versus respective sham; ***P < 0.001, *P < 0.05 versus respective vehicle; ### P < 0.001 versus vehicle-treated PEPCK-Cre mice. (B through J) Representative H&E staining of mouse kidney sections after sham surgery or IRI (treatments as in A) from PEPCK-Cre sham (B), PEPCK-Cre vehicle (C), PEPCK-Cre SEW2871 (D), PEPCK-CreS1P1fl/wt sham (E), PEPCK-CreS1P1fl/wt vehicle (F) PEPCK-CreS1P1fl/wt SEW2871, (G), PEPCK-CreS1P1fl/fl sham (H), PEPCK-CreS1P1fl/fl vehicle (I), and PEPCK-CreS1P1fl/fl SEW2871 mice (J). (K) Semiquantitative tubular injury score of kidneys from sham-, vehicle-, and SEW2871-treated PEPCK-Cre, PEPCK-CreS1P1fl/wt, and PEPCK-CreS1P1fl/fl mice. Data are means ± SEM; n = 3 to 7. †††P < 0.001 versus respective sham; ***P < 0.001; ###P < 0.001 versus vehicle-treated control mice.
Discussion
In this study, we found that S1P1R agonists protect kidneys from IRI by direct actions on kidney tubule cells rather than through their canonical effect to induce lymphopenia, as previously hypothesized. We base our conclusion on the finding that S1PR agonists attenuated kidney IRI in Rag-1 KO mice, suggesting that these agonists may target kidney PTECs directly in mediating tissue protection. The lymphocyte-independent protective effect was also demonstrated by the ability of S1P1R agonists to reduce cell death in vitro after LPS- or H/R-induced injury of TKPTS cells. Lastly, we demonstrated the role of PTEC S1P1Rs in vivo using mice deficient in PTEC S1P1Rs. Kidneys of PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl mice subjected to IR demonstrated enhanced injury compared with control PEPCK-Cre mice. Furthermore, the protective effect of SEW2871 in PEPCK-Cre was abolished in PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl mice. These studies demonstrate that PTEC S1P1Rs are necessary to attenuate injury when kidneys are subjected to IR and to mediate the protective effect of S1P1R agonists and, hence, support an emerging concept that S1P1R activation has a tissue-specific cell survival advantage that can be exploited independent of lymphocytes.
FTY720, acting on lymphocyte S1P1Rs, evokes long-lasting lymphopenia.27 FTY720 diminishes disease severity in numerous models, such as diabetes,28–31 multiple sclerosis,32 IR,33–41 and even clearance of viral infection42 and has shown positive results in phase II trials of patients with remitting relapsing multiple sclerosis. FTY720 and SEW2871 protect kidneys from IRI, an effect presumed to be due to their ability to induce lymphopenia20,36,43 through S1P1R activation.
We conducted a series of experiments to prove that S1P1R activation attenuated kidney IRI through mechanisms that do not involve S1P1R-mediated lymphopenia. We demonstrated that SEW2871 acts directly on PTECs, independently of lymphocytes, to reduce kidney IRI via activation of Akt/ERK1/2 signaling pathways and furthermore that PTEC S1P1Rs are necessary for cell survival after kidney IRI. First, kidneys from Rag-1 KO mice were protected from IRI in this study compared with injured WT mice as we have demonstrated previously.6 FTY720 and SEW2871 further reduced injury in Rag-1 KO mice, suggesting that the tissue-protective effect of FTY720 and SEW2871 is at least in part independent of lymphocytes. Second, S1P1R agonists were shown directly to reduce cytotoxic effects of LPS or H/R in TKPTS cells. The observations that VPC44116, a selective S1P1R antagonist, and S1P1R siRNA blocked the effects of SEW2871 indicated that SEW2871 mediated tissue protection via S1P1Rs expressed on PTECs. Third, IRI was greater in bitransgenic PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl than in PEPCK-Cre mice, and SEW2871 was no longer effective in reducing injury in these KOs as observed in PEPCK-Cre mice. The magnitude of injury after IR and the ineffectiveness of SEW2871 were observed equally in PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl mice. One would have predicted a difference between PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl on the basis of differences in S1P1R copy number. We speculate that S1P1R level in PTECs of both PEPCK-CreS1P1fl/wt and PEPCK-CreS1P1fl/fl may have been reduced to a level insufficient to protect kidneys from IRI and to mediate the protective effect of SEW2871. The precise S1P1R expression in PTECs could not be ascertained because antibodies to detect low levels of endogenous S1P1Rs are not available. In addition, our isolation methods separated only cortex from inner medulla; therefore, the RNA isolated from cortex consisted primarily but not exclusively of PTECs. The concept that PEPCK-CreS1P1fl/wt PTECs contained inadequate S1P1Rs to mediate protection form IRI is supported by our observation that global S1P1 heterozygote (S1P1ko/wt) mice whose kidneys were subjected to IR demonstrated more severe injury than litter mate WT (S1P1wt/wt) controls (A.B. and M.D.O., personal observation). These results suggest that a threshold for S1P1R number is necessary for S1P1R-dependent protection from kidney IRI.
Our data demonstrate that S1P1R agonists block cell death by direct effects on PTECs. The S1P1R agonist FTY720 has been demonstrated to have direct cytoprotective effects in oligodendrocyte progenitor cells.44 Treatment of these cells blocks apoptosis, an effect that is due to S1PR-induced activation of downstream ERK and Akt signaling pathways.44 Activation of the Akt pathway is cytoprotective, whereas blocking this pathway leads to apoptosis. Our results support these observations and demonstrate that Akt/ERK pathways are likely mediators of tissue protection after S1P1R activation. In this study, S1P1R activation with SEW2871 resulted in a time-dependent increase in Akt/ERK phosphorylation in proximal tubule TKPTS cells. Currently, there is no direct evidence that S1P1R/Akt activation protects renal tissue from ischemic injury, but Akt activation in the kidney had been demonstrated to be antiapoptotic45–47; however, in the heart, PI3Ks have been shown to reduce mitochondrial membrane transition pore opening and caspase activation during reperfusion in cardiomyocytes,48 events that possibly contribute to ischemic cell death. Furthermore, Akt activation in cardiomyocytes protects from hypoxia by reducing contracture and normalizing calcium handling.49 In PTECs, phosphorylation of ERK1/2 and Akt is cytoprotective.46 Akt and ERK1/2 activation in PTECs in response to S1P1R stimulation may have similar protective effects. Our preliminary data suggest that pretreatment of TKPTS cells with SEW2871 before H/R reduces cytochrome c release and caspase-3 activation compared with vehicle-treated H/R cells. In summary, we can conclude that activation of the Akt/ERK1/2 pathway is necessary for the cytoprotective role of S1P1Rs in ischemic injury.
Our in vitro and in vivo studies demonstrate conclusively that PTEC S1P1Rs are critical for cell survival after IRI; however, we cannot exclude the possibility that S1P1R agonists mediated non–lymphocyte-dependent protection from IRI via direct effects on the endothelium. The endothelium separates circulating vascular components from the interstitium, and disruption of the endothelium results in increased vascular permeability with subsequent inflammation and organ dysfunction. S1P maintains endothelial cell barrier integrity primarily by binding to S1P1R.50 Demonstrating the significance of endothelial cell S1P1Rs will require an inducible system to delete S1P1Rs from endothelial cells because global S1P1R gene disruption is embryonically lethal as a result of a failure in vascular maturation.16
In summary, our results demonstrate the following: (1) S1P1Rs, which are normally expressed in PTECs, are necessary for cell survival after IRI; (2) S1P1R agonists attenuate kidney IRI through direct activation of S1P1Rs expressed on PTEC; (3) the protective effect of S1P1R agonists in kidney IRI is independent of their canonical effects to induce lymphocyte sequestration and peripheral lymphopenia; and (4) the protection observed with FTY720 and SEW2871 is likely due to their ability to inhibit cell death of kidney PTECs by a mechanism that may involve the Akt/ERK pathways. We conclude that current and more selective S1P1R agonists may be useful for the prevention and treatment of human AKI.
Concise Methods
Materials
The following reagents were used: LPS (Sigma Chemical Co., St. Louis, MO); SEW2871 (Cayman Chemical, Ann Arbor, MI); Annexin V-FITC and 7-AAD (BD Pharmingen/BD Biosciences, San Jose, CA); and antibodies for Akt, pAkt, ERK and pERK (Cell Signaling Technology, Boston, MA). Inhibitors of MAPK (PD98059) and PI3K (Akt; LY294002) were from Calbiochem-Novabiochem (EMD Biosciences, San Diego, CA). SuperSignal West Pico, MPER lysis buffer, and protease inhibitors were from Pierce Biotechnology (Rockford, IL).
Kidney IRI
All animals were handled and procedures were performed in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Virginia Institutional Animal Care and Use Committee. Male C57BL/6 (WT) mice and B6.129S7-Rag1tm1Mom/J (Rag-1 KO) mice (7 to 8 weeks of age; Jackson Laboratories, Bar Harbor, ME) were used. For the kidney IR protocol, bilateral flank incisions were made under anesthesia. Kidney pedicles were exposed and cross-clamped for 28 minutes, clamps were removed, and kidneys were allowed to reperfuse for 24 hours as described previously by our laboratory.20 Kidney pedicles of PEPCK-Cre and PEPCK-CreS1P1 floxed mice were cross-clamped for 26 minutes for mild ischemia, clamps were removed, and kidneys were allowed to reperfuse for 24 hours.
Compound Administration
FTY720 (Novartis, Basel, Switzerland), SEW2871, and VPC4411651 were prepared in a 3% fatty acid–free BSA (Sigma)/1× PBS solution. FTY720 (48 μg/kg), SEW2871 (10 mg/kg), VPC44116 (10 mg/kg), or vehicle (3% fatty acid–free BSA/PBS solution) was administered intraperitoneally 1 hour before ischemia. In contrast to in vivo studies, in which the prodrug FTY720 is activated through phosphorylation by endogenous sphingosine kinases, in vitro studies necessitate the use of the phosphorylated, active form of FTY720, FTY720-P.
Generation of the PEPCK-Cre-S1P1 Floxed Mice
S1P1fl/wt mice carrying the S1P1 gene modified by two floxed (fl) sequences flanking exon 2 were provided by Dr. Richard L. Proia (National Institutes of Health).16 For specific deletion of the S1P1Rs from kidney PTECs, S1P1fl/wt mice were bred with a transgenic mouse with Cre-recombinase under the control of the phosphoenolpyruvate carboxykinase promoter (PEPCK-Cre).25 The PEPCK-Cre transgenic mice were generated using a mutated version of the PEPCK promoter, which reduces PEPCK expression in the liver by 60% and increases PEPCK expression in the kidney by 10-fold in transgenic mice.25 S1P1fl/fl or S1P1fl/wt mice carrying the PEPCK-Cre gene (PEPCK-CreS1P1fl/fl or PEPCK-CreS1P1fl/wt) and control mice (S1P1fl/fl, S1P1fl/wt, or PEPCK-Cre) were genotyped by PCR using PEPCK-Cre-specific primers: Cre-F 5′-AGGTGTAGAGAAGGCACTTAGC-3′ and Cre-R 5′-CTAATCGCCATCTTCCAGCAGG-3′, which generated a 411-bp fragment.52 For identification of the S1P1 WT (S1P1wt) and floxed alleles (S1P1fl) by PCR, the following primers were used: P1 5′-GAGCGGAGGAAGTTAAAAGTG and P2 5′-CCTCCTAAGAGATTGCAGCAA. P1 and P2 amplify an approximately 250-bp fragment for the S1P1 floxed allele and a 200-bp fragment for the S1P1 WT allele. P3 5′-GATCCTAAGGCAATGTCCTAGAATGGGACA was used to amplify the excised product, an approximately 180-bp fragment. Mice were genotyped by PCR analysis using DNA from tail biopsies. PEPCK-Cre mice were bred with ROSA26YFP/+ floxed reporter mice26 for determination of specificity of Cre recombinase expression in kidney and liver.
Assessment of Kidney Function and Histology
Plasma creatinine was determined using a colorimetric assay according to the manufacturer's protocol (Sigma).20 Anticoagulated blood was analyzed for leukocyte counts (HEMAVET 850; CDC Technologies, Oxford, CT). Kidneys were fixed in 0.2% sodium periodate–1.4% dl-lysine–4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4; 4% PLP) and embedded in paraffin. Kidney sections (4 μm) were stained with hematoxylin and eosin and viewed by light microscopy (Zeiss AxioSkop), and images were taken using a SPOT-RT Camera (software version 3.3; Diagnostic Instruments, Sterling Heights, MI) under ×200 magnification. For quantification of tubular injury score, sections were assessed by counting the percentage of tubules that displayed cell necrosis, loss of brush border, cast formation, and tubule dilation as follows: 0 = normal; 1 = <10%; 2 = 10 to 25%; 3 = 26 to 50%; 4 = 51 to 75%; 5 = >75%. Five to 10 fields from each outer medulla were evaluated and scored.
For localization of Cre recombinase (reported as yellow fluorescence protein [YFP] expression) in PEPCK-CreROSA26YFP/+ mice, kidneys were fixed in 1% PLP overnight, incubated in 30% sucrose for 48 hours at 4°C, and embedded and frozen in Optimal Cutting Temperature compound (OCT; Ted Pella). Frozen sections (10 μm) were permeabilized with 0.3% Triton X-100, and nonspecific binding was blocked with 10% horse serum and anti-mouse CD16/32 (10 μg/ml; clone 2.4G2; StemCell Technologies). Sections were labeled with FITC-conjugated rabbit anti–green fluorescence protein (which also recognizes YFP; 2 μg/ml; Invitrogen Corp., Carlsbad, CA) for 1 hour at room temperature. After washing, specimens were mounted with ProLong Gold Antifade reagent with DAPI (Molecular Probes). Images were acquired using the Zeiss Axiovert 200 microscopy system with ApoTome imaging (Carl Zeiss MicroImaging) and processed using Zeiss AxioVision 4.6 software.
Quantitative Real-Time RT-PCR
Total RNA was extracted (from whole kidney, dissected portions of kidney [cortex or the region of inner medulla and papilla], liver, and lung), and single-stranded cDNA was synthesized as described previously.20 Primers were obtained from Integrated DNA Technologies (Coralville, IA), and S1PR primer sequences were as described previously,20 except for S1P5: Forward TTGCTATTACTGGATGTCGCGTGC and reverse AGATGATGGGATTCAGCAGCGACT. Quantitative RT-PCR was performed using a MyIQ Single Color Real-Time PCR Detection System iCycler (Bio-Rad Laboratories, Hercules, CA). Sample values were calculated with normalization to glyceraldehyde-3-phosphate dehydrogenase as described previously.20
Cell Culture
The immortalized mouse proximal tubule cell line (TKPTS), a gift from Dr. Bello-Reuss,53 was maintained in complete DMEM/F12 medium (Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. For siRNA experiments, TKPTS cells were seeded in medium without antibiotics the day before transfection. Two ready-to-use validated double-stranded 21-nucleotide siRNAs for S1P1 (D-051684-01 and D-051684-04; 25 nM) were transfected into TKPTS cells along with ON-TARGETplus Nontargeting siRNA (D-002810-01; Dharmacon, Lafayette, CA) using oligofectamine (Invitrogen) diluted in Opti-MEM I (Invitrogen) overnight.
FACS Analysis (Rag-1 KO Kidney and TKPTS Apoptosis)
Flow cytometry was used to analyze kidney leukocyte content 24 hours after reperfusion in Rag-1 KO mice. In brief, kidneys were extracted, minced, digested, and then passed through a filter and a cotton column as described previously.54 After blocking nonspecific Fc binding with anti-mouse CD16/32 (2.4G2), fresh kidney suspensions were incubated with anti-mouse CD45-FITC (30-F11) for determination of total leukocyte cell numbers. CD45-labeled samples were then labeled with various combinations of anti-mouse F4/80-APC (BM8), GR-1-APC (Ly6G), CD11b-PE, CD11c-APC, and IA-PE (MHCII). 7-AAD (BD Biosciences) was added 15 minutes before analysis of the sample to separate live from dead cells. Subsequent flow cytometry data acquisition was performed on FACS Calibur (Becton Dickinson, San Jose, CA). Data were analyzed by FlowJo 6.4 (Tree Star, Ashland, OR). All of the antibodies were purchased from eBioscience (San Diego, CA) and were used at a concentration of 5 μg/ml. For measurement of apoptosis, TKPTS cells were washed with cold PBS and resuspended in 1× binding buffer (BD Pharmingen, cat. no. 556454); 100 μl of cells were incubated with 5 μl of FITC-conjugated Annexin V and 3 μl of 7-AAD for 15 minutes at room temperature in the dark, the reaction was stopped by addition of 400 μl of 1× binding buffer, and cells were analyzed by FACS.
MPO Activity
MPO activity, a measure of polymorphonuclear leukocyte infiltration, was determined in kidney homogenates as described previously.20
Hypoxia-Reoxygenation (H/R)
For exposure to H/R, culture dishes were placed in a humidified, sealed hypoxic chamber (Billups-Rothenberg, Del Mar, CA) as described previously.55 Briefly, the chamber was purged with 95% N2 and 5% CO2 for 15 minutes to establish hypoxia, and then the chamber was placed in a cell culture incubator for 6 hours. Reoxygenation was achieved by removing the plates from the hypoxic chamber and placing them in a normoxic, humidified incubator (37°C, 5% CO2 and 95% O2) for 1 hour. All experiments (both normoxic and hypoxic) were performed at 37°C.
Statistical Analysis
Comparisons between treatment groups of WT and Rag-1 KO mice or between transgenic mice were examined by two-way ANOVA by using Sigma Plot (San Jose, CA), and Rag-1 KO mouse treatment groups and cell culture experiments were examined by one-way ANOVA by using InStat 3.0 software (La Jolla, CA) with post hoc test using Tukey. Data are expressed as means ± SEM. Statistical significance was identified at P < 0.05.
Disclosures
None.
Acknowledgments
This work was supported by National Institutes of Health grants R01 DK58413, T32 DK072922, and R01 GM067958 and a research fellowship from the National Kidney Foundation.
We gratefully acknowledge the University of Virginia Research Histology core for help with hematoxylin and eosin staining, Dr. V. Brinkmann (Novartis) for providing FTY720, Dr. Richard L. Proia (National Institutes of Health) for providing S1P1fl/wt mice, and all members of the Okusa laboratory.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Schrier RW, Wang W, Poole B, Mitra A: Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5–14, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonventre JV: Pathophysiology of acute kidney injury: Roles of potential inhibitors of inflammation. Contrib Nephrol 156: 39–46, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C: In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Greef KE, Ysebaert DK, Dauwe S, Persy V, Vercauteren SR, Mey D, De Broe ME: Anti-B7–1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney Int 60: 1415–1427, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol 176: 3108–3114, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Yokota N, Daniels F, Crosson J, Rabb H: Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation 74: 759–763, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Spiegel S, Milstien S: Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta 1484: 107–116, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T: Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem 277: 6667–6675, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Spiegel S, Milstien S: Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 277: 25851–25854, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H: Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–3569, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR: The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Yanagawa Y, Masubuchi Y, Chiba K: FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III: Increase in frequency of CD62L-positive T cells in Peyer's patches by FTY720-induced lymphocyte homing. Immunology 95: 591–594, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL: Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allende ML, Yamashita T, Proia RL: G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102: 3665–3667, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C: The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 323: 626–635, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T: Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998. [DOI] [PubMed] [Google Scholar]
- 19. Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y: EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun 260: 203–208, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Lien YH, Yong KC, Cho C, Igarashi S, Lai LW: S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69: 1601–1608, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD: Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol 6: 1228–1235, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C: A novel assay for apoptosis: Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184: 39–51, 1995. [DOI] [PubMed] [Google Scholar]
- 24. Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV: Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 13: 204–208, 1992. [DOI] [PubMed] [Google Scholar]
- 25. Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999. [DOI] [PubMed] [Google Scholar]
- 27. Brinkmann V, Pinschewer D, Chiba K, Feng L: FTY720: A novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol Sci 21: 49–52, 2000. [DOI] [PubMed] [Google Scholar]
- 28. Fu F, Hu S, Deleo J, Li S, Hopf C, Hoover J, Wang S, Brinkmann V, Lake P, Shi VC: Long-term islet graft survival in streptozotocin- and autoimmune-induced diabetes models by immunosuppressive and potential insulinotropic agent FTY720. Transplantation 73: 1425–1430, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Maeda A, Goto M, Zhang J, Bennet W, Groth CG, Korsgren O, Wennberg L: Immunosuppression with FTY720 and cyclosporine A inhibits rejection of adult porcine islet xenografts in rats. Transplantation 75: 1409–1414, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Maki T, Gottschalk R, Ogawa N, Monaco AP: Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation 79: 1051–1055, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Popovic J, Kover KL, Moore WV: The effect of immunomodulators on prevention of autoimmune diabetes is stage dependent: FTY720 prevents diabetes at three different stages in the diabetes-resistant biobreeding rat. Pediatr Diabetes 5: 3–9, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Menge T, Weber MS, Hemmer B, Kieseier BC, von Budingen HC, Warnke C, Zamvil SS, Boster A, Khan O, Hartung HP, Stuve O: Disease-modifying agents for multiple sclerosis: Recent advances and future prospects. Drugs 68: 2445–2468, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Anselmo D, Amersi FF, Shen XD, Gao F, Katori M, Ke B, Lassman C, Coito AJ, Brinkmann V, Busuttil RW, Kupiec-Weglinski JW, Farmer DG: FTY720: A novel approach to the treatment of hepatic ischemia-reperfusion injury. Transplant Proc 34: 1467–1468, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Gallo AP, Silva LB, Franco M, Burdmann EA, Bueno V: Tacrolimus in combination with FTY720: An analysis of renal and blood parameters. Int Immunopharmacol 6: 1919–1924, 2006. [PubMed] [Google Scholar]
- 35. Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, Lynch KR, Okusa MD: Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int 75: 167–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaudel CP, Frink M, Schmiddem U, Probst C, Bergmann S, Krettek C, Klempnauer J, van Griensven M, Winkler M: FTY720 for treatment of ischemia-reperfusion injury following complete renal ischemia; impact on long-term survival and T-lymphocyte tissue infiltration. Transplant Proc 39: 499–502, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Kaudel CP, Schmiddem U, Frink M, Bergmann S, Pape HC, Krettek C, Klempnauer J, Winkler M: FTY720 for treatment of ischemia-reperfusion injury following complete renal ischemia in C57/BL6 mice. Transplant Proc 38: 679–681, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Man K, Ng KT, Lee TK, Lo CM, Sun CK, Li XL, Zhao Y, Ho JW, Fan ST: FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant 5: 40–49, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Oliveira CM, Borra RC, Franco M, Schor N, Silva HT, Jr, Pestana JO, Bueno V: FTY720 impairs necrosis development after ischemia-reperfusion injury. Transplant Proc 36: 854–856, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Suleiman M, Cury PM, Pestana JO, Burdmann EA, Bueno V: FTY720 prevents renal T-cell infiltration after ischemia/reperfusion injury. Transplant Proc 37: 373–374, 2005. [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO: Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293: H3150–H3158, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Premenko-Lanier M, Moseley NB, Pruett ST, Romagnoli PA, Altman JD: Transient FTY720 treatment promotes immune-mediated clearance of a chronic viral infection. Nature 454: 894–898, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Lai LW, Yong KC, Igarashi S, Lien YH: A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int 71: 1223–1231, 2007. [DOI] [PubMed] [Google Scholar]
- 44. Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C: The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 232: 626–635, 2007. [DOI] [PubMed] [Google Scholar]
- 45. Kwon DS, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK: Signal transduction of MEK/ERK and PI3K/Akt activation by hypoxia/reoxygenation in renal epithelial cells. Eur J Cell Biol 85: 1189–1199, 2006. [DOI] [PubMed] [Google Scholar]
- 46. Lee HT, Kim M, Jan M, Emala CW: Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 291: F67–F78, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Letavernier E, Perez J, Joye E, Bellocq A, Fouqueray B, Haymann JP, Heudes D, Wahli W, Desvergne B, Baud L: Peroxisome proliferator-activated receptor beta/delta exerts a strong protection from ischemic acute renal failure. J Am Soc Nephrol 16: 2395–2402, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M: PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res 69: 178–185, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A: Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 50. Singleton PA, Dudek SM, Chiang ET, Garcia JG: Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 19: 1646–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Davis MD, Clemens JJ, Macdonald TL, Lynch KR: Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem 280: 9833–9841, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y: Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Ernest S, Bello-Reuss E: Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol 269: C323–C333, 1995. [DOI] [PubMed] [Google Scholar]
- 54. Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007. [DOI] [PubMed] [Google Scholar]
- 55. Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE: Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 293: L105–L113, 2007. [DOI] [PubMed] [Google Scholar]