Abstract
Long-term function of kidney allografts depends on multiple variables, one of which may be the compatibility in size between the graft and the recipient. Here, we assessed the long-term consequences of the ratio of the weight of the kidney to the weight of the recipient (KwRw ratio) in a multicenter cohort of 1189 patients who received a transplant between 1995 and 2006. The graft filtration rate increased by a mean of 5.74 ml/min between the third and sixth posttransplantation months among patients with a low KwRw ratio (<2.3 g/kg; P < 0.0001). In this low KwRw ratio group, the graft filtration rate remained stable between 6 months and 7 years but then decreased at a mean rate of 3.17 ml/min per yr (P < 0.0001). In addition, low KwRw ratios conferred greater risk for proteinuria, more antihypertensive drugs, and segmental or global glomerulosclerosis. Moreover, a KwRw ratio <2.3 g/kg associated with a 55% increased risk for transplant failure by 2 years of follow-up. In conclusion, incompatibility between graft and recipient weight is an independent predictor of long-term graft survival, suggesting that avoiding kidney and recipient weight incompatibility may improve late clinical outcome after kidney transplantation.
The effect of nephron reduction has long been described in animal models as well as in humans1,2 and is thought to be a potential “nonimmunologic” risk factor for chronic graft dysfunction after kidney transplantation.3,4 The paradigm generally considered to account for the deleterious effect of nephron reduction on graft function is that of “adaptive” hyperfiltration of the remaining glomeruli, ultimately leading to glomerulosclerosis.5–7 In accordance with this hypothesis, individuals who have undergone nephrectomy have been shown to develop high BP and proteinuria decades after the nephrectomy,8–11 as in the case of older recipients with a higher body mass index12; however, renal insufficiency only appears in the case of a 75% reduction in kidney mass and after at least 10 years of follow-up.9 Kidney transplantation has been proposed as an accelerated model of nephron reduction resulting from the accumulation of several unfavorable factors. For example, repeated injuries, from initial brain death of the donor13 to ischemia-reperfusion injury,14 negatively affect the transplant. Moreover, superimposed immunologic and nonimmunologic events further decrease the initial nephron mass of a transplant and serve only to exacerbate the consequences of hyperfiltration related to its single kidney status.
Given that kidney weight (Kw) and glomerular volume (but not nephron number) correlate with body surface area (BSA),15 several studies have already analyzed the effect of donor and recipient BSA mismatches.7,16–19 The effect of kidney graft size and recipient weight (Rw)20,21 has also been studied; however, the direct impact of matching the Kw itself (which correlates with both glomerular volume and nephron number)15 to the Rw has been studied only in relatively small cohorts of <300 patients and only in living donors,22,23 where the graft does not incur the same accumulating injuries as those from deceased donors.
We previously reported on the results of a first study24 focusing on the impact of graft weight on clinical outcome; however, within the relatively short survey period of the latter study (mean 32 months; range 8 days to 94 months), no impact on short-term graft survival was observed. Because renal failure has been described a decade after nephron reduction,3,10,25 we reappraised our historical cohort to which an additional 47 patients were included (whole population n = 1189) at a mean of 6.2 years from transplantation (range 8 days to 13 years). We now report that the magnitude of the Kw and Rw incompatibility is significantly associated not only with sustained “adaptive” hyperfiltration and early proteinuria but also with an increased risk for hypertension requiring more medication, a higher incidence of segmental or global glomerulosclerosis, and a significantly poorer long-term transplant survival.
Results
Demographic Analysis
Of the whole population of 1060 kidney recipients included in the statistical analysis, 938 (88.4%) had received a first kidney graft, 62% were male recipients with a mean age of 45.6 ± 13.1 years, and 68% were male donors with a mean age of 39.8 ± 15.3 years. No statistical difference of demographic characteristics was observed according to the KwRw ratio threshold of 2.3 g/kg, except for an expectedly higher number of male donors and female recipients26 in the highest ratio (≥2.3 g/kg; Table 1) and a higher donor creatinemia that correlated with heavier male donors.
Table 1.
Demographic characteristics according to the KwRw ratio < or ≥2.3 g/kg
| Characteristic | Kw ≥2.3 g/kg |
Kw <2.3 g/kg |
χ2 | P | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Recipient gender | 22.60 | <0.0001 | ||||
| female | 347 | 86.5 | 54 | 13.5 | ||
| male | 490 | 74.4 | 169 | 25.6 | ||
| Donor gender | 42.90 | <0.0001 | ||||
| female | 227 | 67.0 | 112 | 33.0 | ||
| male | 608 | 84.6 | 111 | 15.4 | ||
| Recipient age (years) | 1.98 | 0.1590 | ||||
| <55 | 614 | 80.0 | 153 | 20.0 | ||
| ≥55 | 223 | 76.1 | 70 | 23.9 | ||
| Donor age (years) | 0.02 | 0.8732 | ||||
| <55 | 680 | 79.0 | 181 | 21.0 | ||
| ≥55 | 153 | 78.5 | 42 | 21.5 | ||
| Donor creatinine (μmol/L) | 4.68 | 0.0304 | ||||
| <120 | 643 | 77.8 | 183 | 22.2 | ||
| ≥120 | 181 | 84.6 | 33 | 15.4 | ||
| Cold ischemia time (hours) | 1.63 | 0.2019 | ||||
| <36 | 697 | 79.6 | 179 | 20.4 | ||
| ≥36 | 134 | 75.5 | 44 | 24.5 | ||
| DGF (days) | 1.16 | 0.2815 | ||||
| <6 | 580 | 78.0 | 163 | 22.0 | ||
| ≥6 | 240 | 81.0 | 56 | 19.0 | ||
| No. of transplants | 1.21 | 0.2705 | ||||
| 1 | 736 | 78.5 | 202 | 21.5 | ||
| ≥2 | 101 | 82.8 | 21 | 17.2 | ||
| HLA Inc. | 0.58 | 0.4480 | ||||
| <5 | 721 | 79.2 | 189 | 20.8 | ||
| ≥5 | 100 | 76.3 | 31 | 23.7 | ||
| Anti-T PRA (%) | 1.21 | 0.2722 | ||||
| 0 | 632 | 78.1 | 177 | 21.9 | ||
| >0 | 197 | 81.4 | 45 | 18.6 | ||
| Acute rejection | 0.18 | 0.6703 | ||||
| 0 | 615 | 78.6 | 167 | 21.4 | ||
| >0 | 222 | 79.9 | 56 | 20.1 | ||
PRA, panel reactive antibodies.
Correlation of the KwRw Ratio with Kidney Graft Function
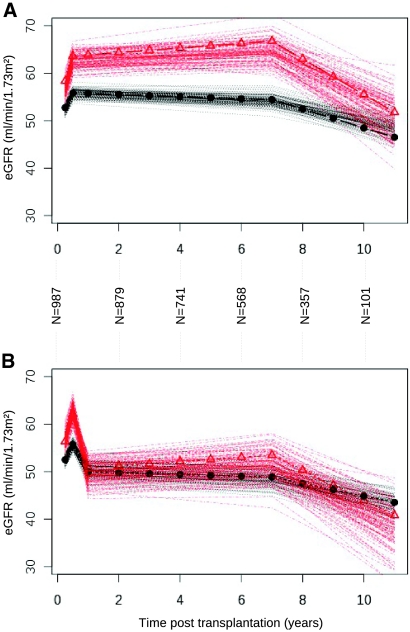
We first analyzed the relationship between the KwRw ratio and the estimated GFR (eGFR) so as to test the hypothesis that hyperfiltration is linked to the level of Kw and Rw incompatibility. The mean eGFR was 79.13 ml/min at 3 months of follow-up. Three slopes were estimated in terms of graft function evolution during the study follow-up period: 3 to 6 months, 6 months to 7 years, and >7 years. Figure 1A shows that the change in eGFR kinetics estimated by multivariate mixed linear regression was statistically different between the two KwRw ratio groups in the absence of acute rejection episodes. Indeed, we found that patients with a KwRw ratio <2.3 g/kg dramatically increased their eGFR by a mean of 5.74 ml/min between 3 and 6 months (P < 0.0001) before reaching a plateau at 6 months. No major additional changes in the eGFR values were observed between 6 months and 7 years (P = 0.0759); however, after 7 years, the eGFR dropped dramatically at a mean rate of 3.17 ml/min per yr (P < 0.0001). In contrast, for recipients with a KwRw ratio ≥2.3 g/kg, the eGFR increased significantly less than for the small KwRw ratios (3.22 ml/min between 3 and 6 months; P < 0.0001). After 7 years, the eGFR decreased at a much slower rate than that observed for small weight ratios (1.34 ml/min per yr; P < 0.0001). Several other classical independent risk factors identified by the multivariate analysis as significantly influencing graft function are described in Table 2.
Figure 1.
Modifications of the eGFR over time (multivariate mixed linear regression) on eGFR modification according to the KwRw ratio. (A) Without acute rejection episodes. Plots are calculated from the parameters estimated in the multivariate mixed linear regression. The number of patients at each time point and confidence intervals for each curve and each ratio of KwRw are provided. Each curve represents the confidence intervals of the eGFR obtained from bootstrap resampling methods (100 iterations). ▵, KwRw ratio <2.3 g/kg; •, KwRw ratio ≥2.3 g/kg. (B) Impact of acute rejection episodes on the modifications of the eGFR over time. The extent of graft function deterioration in the long-term is dramatically influenced by superimposed rejection episodes. Patients with KwRw ratios <2.3 g/kg presented a more pronounced decrease in graft function (11.53 ml/min) than those with ratios >2.3 g/kg (5.68 ml/min; P < 0.0001) in the presence of acute rejection episodes at 6 months. Symbols are the same as in A.
Table 2.
Results of multiple regression analysis on kidney graft function
| Parameter | Coefficient | SE | Wald Test | P |
|---|---|---|---|---|
| Donor age (years/10) | −3.47 | 0.35 | −9.77 | <0.0001 |
| Recipient age (years/10) | −3.92 | 0.40 | −9.71 | <0.0001 |
| DGF (>6 days) | −5.77 | 1.11 | −5.19 | <0.0001 |
| Cold ischemia time (>36 hours) | −4.57 | 1.31 | −3.47 | 0.0005 |
| Recipient gender (male) | 6.91 | 0.99 | 7.07 | <0.0001 |
| Donor gender (male) | 5.31 | 1.06 | 5.01 | <0.0001 |
| KwRw (<2.3 g/kg)a | ||||
| eGFR changes per year (3 to 6 months) | 22.96 | 3.17 | 7.24 | <0.0001 |
| eGFR changes per year (6 months to 7 years) | 0.44 | 0.25 | 1.77 | 0.0759 |
| eGFR changes per year (≥7 years) | −3.17 | 0.65 | −4.84 | <0.0001 |
| ARE | −11.53 | 2.05 | −5.63 | <0.0001 |
| KwRw (≥2.3 g/kg)a | ||||
| CrCl changes per year (3 to 6 months) | 12.89 | 1.35 | 9.49 | <0.0001 |
| CrCl changes per year (6 months to 7 years) | −0.18 | 0.12 | −1.45 | 0.1477 |
| CrCl changes per year (≥7 years) | −1.34 | 0.28 | −4.77 | <0.0001 |
| ARE | −5.68 | 0.94 | −6.02 | <0.0001 |
Patients with a KwRw ratio <2.3 g/kg (small kidneys attributed to large recipients) increase their eGFR by a mean of 5.74 ml/min between 3 and 6 months (P < 0.0001). Between 6 months and 7 years, no significant changes in eGFR values are observed (P = 0.0759). After 7 years, the eGFR drops at a mean of 3.17 ml/min per year (P < 0.0001). In contrast, for recipients with a KwRw ratio ≥2.3 g/kg (large kidneys attributed to small recipients), the eGFR increases significantly less than for the small ratios, with only 3.22 ml/min between 3 and 6 months (P < 0.0001) and a less rapid decrease after 7 years (1.34 ml/min per year; P < 0.0001). CrCl, creatinine clearance.
aBecause the interaction between the KwRw ratio and the posttransplantation and the interaction between the KwRw ratio and the AREs are significant, the results are presented according to each group of KwRw ratio (even though no subgroup analysis was performed).
Association of Acute Rejection Episodes and KwRw Ratio with Graft Function
We also observed an interaction between the KwRw ratio and acute rejection episodes (AREs). In fact, during AREs within the first 6 months, patients with KwRw ratios <2.3 g/kg presented a more pronounced loss of graft function (11.53 ml/min) than those with KwRw ratios ≥2.3 g/kg (5.68 ml/min; P < 0.0001; Figure 1B). These data emphasize that factors having a serious additional detrimental effect on graft eGFR, such as AREs, exhaust the functional reserve of the graft.
Correlation of the KwRw Ratio with Daily Proteinuria
Fifty-four percent of patients with a KwRw ratio <2.3 g/kg had proteinuria >0.5 g/24 h at 1 year compared with 43% with a KwRw ratio ≥2.3 g/kg (P < 0.01). The results of the multivariate analysis presented in Table 3 show that male recipients (relative risk [RR] 1.23; P = 0.036), an incremental increase in donor age of 10 years (RR 1.34; P = 0.012), delayed graft function (DGF; RR 1.49; P < 0.0001), ARE (RR 1.51; P = 0.0005), and a KwRw ratio <2.3 g/kg (RR 1.35; P = 0.0062) were independent risk factors for a 1-year proteinuria >0.5 g/24 h.
Table 3.
Results of the Cox model analysis on the occurrence of daily proteinuria
| Parameter | Coefficient | SE | RR (95% CI) | P |
|---|---|---|---|---|
| KwRw ratio | −0.30 | 0.11 | 1.35 (1.08 to 1.66) | 0.0062 |
| ARE | 0.41 | 0.12 | 1.51 (1.19 to 1.89) | 0.0005 |
| Donor age | 0.29 | 0.12 | 1.34 (1.07 to 1.68) | 0.0120 |
| DGF | 0.40 | 0.10 | 1.49 (1.22 to 1.83) | <0.0001 |
| Recipient gender | 0.21 | 0.10 | 1.23 (1.01 to 1.49) | 0.0360 |
Recipients with low KwRw ratios (<2.3 g/kg) have a 1.35-fold greater independent risk for proteinuria above the 0.5 g/24 h threshold than patients with higher KwRw ratios (≥2.3 g/kg; P = 0.0062). CI, confidence interval.
Patients with Low KwRw Ratios Take Antihypertensive Drugs More Frequently and in Greater Numbers
A subgroup of the 468 patients of the Nantes Centre cohort, for whom corresponding data were prospectively collected, were used to study a possible correlation between hypertension and KwRw ratio. At the time of the study analysis, 90% of patients with a KwRw ratio <2.3 g/kg received at least one antihypertensive drug versus 81% in the high ratio group (P = 0.04). Moreover, 75% of patients with a KwRw ratio <2.3 g/kg received at least two antihypertensive drugs versus 60.2% in the high ratio group (P = 0.0074). Conversely, no significant difference was observed in the distribution of angiotensin converting enzyme inhibitor (ACEI; 53.7%) or angiotensin 2 receptor antagonist (A2RA; 43.1%) drugs between the two groups of KwRw ratios, showing that patients with a high KwRw ratio were not specifically overprotected by such drugs (Table 4).
Table 4.
Results of the hypertension analysis according to the KwRw ratio
| Parameter | <2.3 g/kg (%) | ≥2.3 g/kg (%) | P |
|---|---|---|---|
| Antihypertensive drugs | 90.0 | 82.1 | 0.0435 |
| ≥2 antihypertensive drugs | 75.0 | 60.2 | 0.0074 |
| ≥1 ACEI or A2RA | 53.7 | 43.1 | 0.0685 |
Patients with a low KwRw ratio were statistically more frequently treated for hypertension and received more antihypertensive drugs than patients with a high KwRw ratio. In addition, patients with a high KwRw ratio did not take more ACEI or A2RA drugs than those with a low KwRw ratio.
Patients with a Low KwRw Ratio Present More Glomerulosclerosis in Their Kidney Biopsies
Graft biopsy analysis was performed for 980 patients who underwent indication biopsies at five of the six study centers (data were unavailable from one center for technical reasons). Of the 980 patients, 14.3% (141) had biopsies for abnormal proteinuria and/or decreased graft function at least 1 year after transplantation. Among them, 7.8% presented lesions of segmental or global glomerulosclerosis; 17.1% of these had a KwRw ratio <2.3 g/kg versus 4.7% who had a KwRw ratio ≥2.3 g/kg (P = 0.027); however, given that this study was not randomized, the P value has to be interpreted with caution. Despite the widely known limitation of retrospective studies, the higher incidence of these lesions in the biopsies of patients with a low KwRw ratio also suggests that glomerulosclerosis could have significantly contributed to shortening graft survival.
KwRw Ratio Is a Risk Factor for Kidney Graft Loss
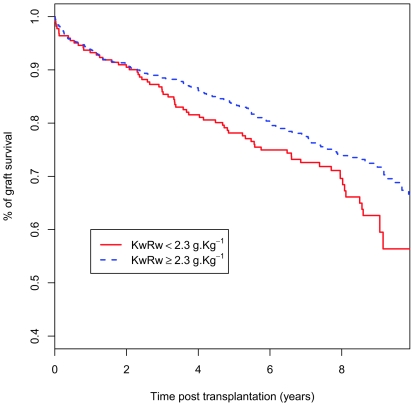
Finally, we analyzed a possible correlation between a low KwRw ratio and graft survival, which amounted to 80% at 5 years and 65% at 10 years. Several classical independent risk factors were identified by the Cox multivariate analysis27 as significantly reducing graft survival (Table 5). Interestingly, this analysis showed that a KwRw ratio <2.3 g/kg is an independent risk factor for transplant failure after 2 years of follow-up with a 1.55-fold increase in risk (P = 0.016; Figure 2).
Table 5.
Results of the “modified” Cox model analysis taking into account the two periods of time before and after 2 years of follow-up
| Parameter | Coefficient | SE | RR (95% CI) | P |
|---|---|---|---|---|
| KwRw 0 to 24 months | −0.02 | 0.27 | 0.98 (0.57 to 1.68) | 0.9300 |
| KwRw after 24 months | −0.42 | 0.17 | 1.51 (1.03 to 2.12) | 0.0160 |
| AREs | 0.64 | 0.15 | 1.90 (1.43 to 2.53) | <0.0001 |
| Recipient age | 0.17 | 0.05 | 1.19 (1.07 to 1.32) | 0.0012 |
| Donor creatinemia | 0.31 | 0.15 | 1.36 (1.01 to 1.83) | 0.0400 |
| DGF | 0.48 | 0.14 | 1.62 (1.23 to 2.14) | 0.0006 |
| Retransplantation | 0.68 | 0.19 | 1.97 (1.37 to 2.84) | 0.0003 |
| Cold ischemia time | 0.31 | 0.16 | 1.36 (1.00 to 1.85) | 0.0510 |
The KwRw ratio seems to be an independent risk factor for long-term graft survival. Small KwRw ratios (<2.3 g/kg) have a 1.55-fold greater risk for transplant failure after 2 years of follow-up.
Figure 2.
Correlation between the KwRw ratio and the long-term graft survival. Graft survival for the whole population is 80% at 5 years and 65% at 10 years. The correlation between the KwRw ratio and graft survival is not evenly distributed throughout the follow-up period and appears only after 2 years after transplantation (P = 0.016).
Taken together, our data suggest that in humans, Kw and Rw incompatibility during transplantation engenders the same sequence of events observed in the hyperfiltration paradigm described in animal models28 and observed in the context of severe nephron reduction in humans.2
Discussion
In this report, we reappraised our historical cohort24 with a much longer survey period. We first tested whether a KwRw ratio incompatibility is an independent risk factor for subsequent hyperfiltration. We showed that a KwRw ratio <2.3 g/kg results in a much greater adaptive increase in graft GFR than a higher ratio (≥2.3 g/kg) during the first 6 months. This is followed by a plateau between 6 months and 7 years and a sudden drop in eGFR thereafter. Such eGFR kinetics likely explain the seeming lack of effect of kidney weight (or size) on long-term graft survival in previous short-term studies.17,22,29 It is of importance to note that all patients were maintained on calcineurin inhibitors (CNIs) throughout the follow-up regardless of the KwRw ratio. This rules out the hypothesis that the drop after 7 years observed in the low ratio group could reflect the cessation of CNIs. This observation fits with the description of glomeruli enlargement during the first few months after transplantation associated with a better graft function but finally with a poor death-censored graft survival.30 In addition, the long-term association between the KwRw ratio and graft survival is in agreement with previous observations in humans, in whom a loss of kidney mass of ≥75% was shown to result in renal insufficiency only after 10 years of functional overload.9,11 Furthermore, we show here that the adaptive eGFR is impaired after a rejection crisis and that graft function decreases sooner within the context of a low KwRw ratio. This observation provides a new paradigm for understanding how rejection episodes affect long-term graft function in kidneys with functional overload.
It has been shown that 25% of patients have proteinuria 6 months after transplantation.31 In addition, observations have confirmed that kidney transplants are particularly vulnerable to the adverse effects of proteinuria.32 In our cohort, we showed that the risk for proteinuria >0.5g/d at 1 year increased significantly in patients with a KwRw ratio <2.3 g/kg, fitting with previous observations that nephron underdosing is a major risk factor for proteinuria.33 We also showed that patients with a low KwRw ratio received antihypertensive drugs more frequently and more intensively than the others, again fitting with the overload hypothesis.34 Of importance, no difference was found between the two groups in the proportion of patients receiving A2RA or ACEI, corroborating that the KwRw ratio per se plays a role in the development of proteinuria and that the high KwRw group was not “artificially protected” by these drugs. In addition, the subgroup analysis of the kidney biopsies showing significantly more lesions of segmental or global glomerulosclerosis in the group with low KwRw ratio suggests that glomerular protein leakage resulting from overfiltration of transplants performed within the context of small KwRw ratios could lead to chronic inflammation, interstitial fibrosis and glomerulosclerosis,1,35,36 and finally to a decrease in graft function and graft loss.37–39 Given that interstitial fibrosis and chronic graft inflammation can be generated by both immunologic and nonimmunologic parameters, we focused exclusively on glomerulosclerosis in our study, even though we are aware that glomerulosclerosis40 is not specific to glomerular hyperfiltration.
The clinical importance of the KwRw ratio as an independent factor influencing graft survival is roughly comparable to that of the presence of an acute rejection episode or of DGF after transplantation.41 Taken as a whole, these data suggest that recording KwRw may help to improve late clinical outcome and may be relevant in the context of poor long-term graft survival42,43 when the early benefit of preventing acute rejection is censored.
Concise Methods
Patients
Between April 1995 and January 2006, the weights of 1189 kidney grafts and their corresponding recipients were prospectively and consecutively collected before transplantation. Our institution in Nantes and five other French transplantation centers participated in the study. A total of 129 patients were excluded from the analysis for the following reasons: Missing data (n = 45), recipient age <18 years (because of the growth-related changes in recipient size after transplantation; n = 20) and simultaneous kidney and pancreas grafts (so as to maintain a homogeneous population in terms of donor and recipient age and cold ischemia time; n = 64). Analyses were thus performed on the 1060 remaining patients for whom full information was available (including seven living-related donors). The mean follow-up period was 6.2 years, ranging from 8 days to 13 years. Sixty-three percent of patients received induction therapy with antithymocyte globulins. All patients received CNIs associated with mycophenolate mofetil (51.4%) or azathioprine (26.8%) and/or steroids (94%). Clinical and biologic data for donors and recipients were prospectively collected, double-checked, and centralized within an anonymous and coded study data bank in accordance with the University Hospital ethical committee requirements.
Kidney Weight
The same electronic weighing scales (Maul, France) were located in the operating room. In compliance with common procedure recommendations, kidney grafts were first prepared and then weighed by the surgeon.
Parameters Studied
Donor data included age, gender, creatinemia (μmol/L), and Kw (in g). Recipient data at the time of inclusion were age, gender, Rw (Kg), KwRw ratio (in g/kg), number of retransplantations, HLA-A-B-DR incompatibilities, highest level of anti-T panel reactive antibodies, and cold ischemia time (in minutes). In addition, the following data were collected from recipients at the time of each follow-up (3, 6, and 12 months and then every year): Weight, creatinemia, eGFR (estimated by the Modification of Diet in Renal Disease [MDRD] formula), and daily proteinuria assessed on 24-hour urine collection (g/24 h). DGF,41 AREs, and the time of death or definitive graft failure were also recorded.
In addition, two subgroup analyses were conducted. The first concerned the biopsies from five of the six centers (data were unavailable from one center for technical reasons). The second addressed the level of antihypertensive treatment, information that had been prospectively computerized in the Nantes cohort only (n = 468) at each outpatient examination after hospital discharge.
Kidney Biopsy Analysis
Kidney biopsy analysis was performed for indication at each center on the 141 patients who exhibited abnormal proteinuria and/or decreased graft function. Biopsy analysis was restricted to those performed at least 1 year after transplantation and those nearest in time to the definitive graft failure. The biopsies were analyzed by two pathologists who were blinded to clinical parameters and KwRw ratios. Specific attention was paid to lesions linked to hyperfiltration (e.g., segmental or global glomerulosclerosis).40
Hypertension Analysis
Given that hypertension is a major clinical sign of glomerular hyperfiltration,2 the proportion of patients receiving at least one antihypertensive drug at the time of the analysis and the severity of the hypertension as assessed by the proportion of patients who were taking two or more antihypertensive drugs were analyzed. Finally, the distribution of patients receiving nephroprotective drugs (A2RA or ACEI) was also studied. Data were collected by an investigator who was blinded to the study parameters.
Statistical Analysis
Correlation of the KwRw Ratio with Graft Survival
Three metrics were first tested using the Hothorn and Zeileis method44: Kw alone, KwRw ratio, and Kw to recipient BSA ratio. The principle of this method is to determine the metric and the corresponding cutoff that maximizes the difference between both survival curves on the basis of the log-rank graft survival curves (including patient death). According to this procedure, the KwRw ratio with an optimal cutoff of 2.3 g/kg was determined. This threshold was then used for all subsequent analyses of the study for homogeneous procedures. Kaplan-Meier survival curves were plotted, and the log-rank method was used to test for significant differences in survival distributions (P < 0.20, univariate analysis). We used an extension of the semiparametric Cox model,27 which takes into account the possible nonproportionality of covariates. Indeed, it seemed that the hazard ratio of the KwRw ratio was not constant during the posttransplantation period (minus log minus of the survival function). The covariates were retained in the multivariate regression according to a backward procedure (Wald test, P < 0.05). The statistical analyses were performed using the R software package (http://www.r-project.org) with the survival library. χ2 and t tests were used for the subgroup analyses of hypertension and kidney biopsies, respectively.
Correlation of the KwRw Ratio with Graft Function
To evaluate the relationship between the KwRw and the eGFR by the MDRD formula over time, we performed a mixed-effect linear regression taking into account the repetition of graft function measurements (eGFR) for each patient in the longitudinal data set.45 The optimal modeling of the eGFR evolution was obtained using three slopes (between 3 and 6 months, 6 months and 7 years, and >7 years after transplantation) that minimized the Bayesian Information Criteria.46 The relationship between eGFR evolution and KwRw ratio was tested to assess the interactions between time after transplantation and the KwRw ratio. The statistical analyses were performed with the lme library, and the inference assessments were based on the Wald test. The confidence intervals of the eGFR were obtained using bootstrap resampling methods (100 iterations).47
Association of the KwRw Ratio with Daily Proteinuria
An appraisal was carried out on the time between transplantation and the occurrence of proteinuria (first measurement >0.5 g/24 h). In addition, the same statistical methods as those performed for the graft survival analysis were used.
Disclosures
None.
Acknowledgments
We thank Joanna Ashton-Chess for editing the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Brenner BM: Nephron adaptation to renal injury or ablation. Am J Physiol 249: F324–F337, 1985. [DOI] [PubMed] [Google Scholar]
- 2. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. J Am Soc Nephrol 12: 1315–1325, 2001. 11373357 [Google Scholar]
- 3. Chertow GM, Milford EL, Mackenzie HS, Brenner BM: Antigen-independent determinants of cadaveric kidney transplant failure. JAMA 276: 1732–1736, 1996. [PubMed] [Google Scholar]
- 4. Terasaki PI: Deduction of the fraction of immunologic and non-immunologic failure in cadaver donor transplants. Clin Transpl 449–452, 2003. [PubMed] [Google Scholar]
- 5. Azuma H, Nadeau K, Mackenzie HS, Brenner BM, Tilney NL: Nephron mass modulates the hemodynamic, cellular, and molecular response of the rat renal allograft. Transplantation 63: 519–528, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Brenner BM, Milford EL: Nephron underdosing: A programmed cause of chronic renal allograft failure. Am J Kidney Dis 21: 66–72, 1993. [DOI] [PubMed] [Google Scholar]
- 7. Provoost AP, Brenner BM: Long-term follow-up of humans with single kidneys: The need for longitudinal studies to assess true changes in renal function. Curr Opin Nephrol Hypertens 2: 521–526, 1993. [PubMed] [Google Scholar]
- 8. Kasiske BL, Ma JZ, Louis TA, Swan SK: Long-term effects of reduced renal mass in humans. Kidney Int 48: 814–819, 1995. [DOI] [PubMed] [Google Scholar]
- 9. Novick AC, Gephardt G, Guz B, Steinmuller D, Tubbs RR: Long-term follow-up after partial removal of a solitary kidney. N Engl J Med 325: 1058–1062, 1991. [DOI] [PubMed] [Google Scholar]
- 10. Ramcharan T, Matas AJ: Long-term (20–37 years) follow-up of living kidney donors. Am J Transplant 2: 959–964, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Zucchelli P, Cagnoli L, Casanova S, Donini U, Pasquali S: Focal glomerulosclerosis in patients with unilateral nephrectomy. Kidney Int 24: 649–655, 1983. [DOI] [PubMed] [Google Scholar]
- 12. Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ: Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratschke J, Wilhelm MJ, Kusaka M, Basker M, Cooper DK, Hancock WW, Tilney NL: Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation 67: 343–348, 1999. [DOI] [PubMed] [Google Scholar]
- 14. Azuma H, Nadeau K, Takada M, Mackenzie HS, Tilney NL: Cellular and molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation 64: 190–197, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992. [DOI] [PubMed] [Google Scholar]
- 16. el-Agroudy AE, Hassan NA, Bakr MA, Foda MA, Shokeir AA, Shehab el-Dein AB: Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am J Nephrol 23: 294–299, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Gaston RS, Hudson SL, Julian BA, Laskow DA, Deierhoi MH, Sanders CE, Phillips MG, Diethelm AG, Curtis JJ: Impact of donor/recipient size matching on outcomes in renal transplantation. Transplantation 61: 383–388, 1996. [DOI] [PubMed] [Google Scholar]
- 18. Kasiske BL, Snyder JJ, Gilbertson D: Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol 13: 2152–2159, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Moreso F, Seron D, Anunciada AI, Hueso M, Ramon JM, Fulladosa X, Gil-Vernet S, Alsina J, Grinyo JM: Recipient body surface area as a predictor of posttransplant renal allograft evolution. Transplantation 65: 671–676, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Miles AM, Sumrani N, John S, Markell MS, Distant DA, Maursky V, Hong JH, Friedman EA, Sommer B: The effect of kidney size on cadaveric renal allograft outcome. Transplantation 61: 894–897, 1996. [DOI] [PubMed] [Google Scholar]
- 21. Nicholson ML, Metcalfe MS, White SA, Waller JR, Doughman TM, Horsburgh T, Feehally J, Carr SJ, Veitch PS: A comparison of the results of renal transplantation from non-heart-beating, conventional cadaveric, and living donors. Kidney Int 58: 2585–2591, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Douverny JB, Baptista-Silva JC, Pestana JO, Sesso R: Importance of renal mass on graft function outcome after 12 months of living donor kidney transplantation. Nephrol Dial Transplant 22: 3646–3651, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Kim YS, Moon JI, Kim DK, Kim SI, Park K: Ratio of donor kidney weight to recipient bodyweight as an index of graft function. Lancet 357: 1180–1181, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Giral M, Nguyen JM, Karam G, Kessler M, Hurault de Ligny B, Buchler M, Bayle F, Meyer C, Foucher Y, Martin ML, Daguin P, Soulillou JP: Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol 16: 261–268, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Hakim RM, Goldszer RC, Brenner BM: Hypertension and proteinuria: Long-term sequelae of uninephrectomy in humans. Kidney Int 25: 930–936, 1984. [DOI] [PubMed] [Google Scholar]
- 26. Neugarten J, Kasiske B, Silbiger SR, Nyengaard JR: Effects of sex on renal structure. Nephron 90: 139–144, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Therneau T, Grambsch P: Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health), Berlin, Springer, 2001. [Google Scholar]
- 28. Mackenzie HS, Azuma H, Rennke HG, Tilney NL, Brenner BM: Renal mass as a determinant of late allograft outcome: Insights from experimental studies in rats. Kidney Int Suppl 52: S38–S42, 1995. [PubMed] [Google Scholar]
- 29. Vianello A, Calconi G, Amici G, Chiara G, Pignata G, Maresca MC: Importance of donor/recipient body weight ratio as a cause of kidney graft loss in the short to medium term. Nephron 72: 205–211, 1996. [DOI] [PubMed] [Google Scholar]
- 30. Azevedo F, Alperovich G, Moreso F, Ibernon M, Goma M, Fulladosa X, Hueso M, Carrera M, Grinyo JM, Seron D: Glomerular size in early protocol biopsies is associated with graft outcome. Am J Transplant 5: 2877–2882, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Hohage H, Kleyer U, Bruckner D, August C, Zidek W, Spieker C: Influence of proteinuria on long-term transplant survival in kidney transplant recipients. Nephron 75: 160–165, 1997. [DOI] [PubMed] [Google Scholar]
- 32. Benigni A, Bruzzi I, Mister M, Azzollini N, Gaspari F, Perico N, Gotti E, Bertani T, Remuzzi G: Nature and mediators of renal lesions in kidney transplant patients given cyclosporine for more than one year. Kidney Int 55: 674–685, 1999. [DOI] [PubMed] [Google Scholar]
- 33. Mackenzie HS, Tullius SG, Heemann UW, Azuma H, Rennke HG, Brenner BM, Tilney NL: Nephron supply is a major determinant of long-term renal allograft outcome in rats. J Clin Invest 94: 2148–2152, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bochicchio T, Sandoval G, Ron O, Perez-Grovas H, Bordes J, Herrera-Acosta J: Fosinopril prevents hyperfiltration and decreases proteinuria in post-transplant hypertensives. Kidney Int 38: 873–879, 1990. [DOI] [PubMed] [Google Scholar]
- 35. Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS: Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Ruggenenti P, Perna A, Remuzzi G: Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Fernandez-Fresnedo G, Plaza JJ, Sanchez-Plumed J, Sanz-Guajardo A, Palomar-Fontanet R, Arias M: Proteinuria: A new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant 19[Suppl 3]: iii47–iii51, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Halimi JM, Laouad I, Buchler M, Al-Najjar A, Chatelet V, Houssaini TS, Nivet H, Lebranchu Y: Early low-grade proteinuria: Causes, short-term evolution and long-term consequences in renal transplantation. Am J Transplant 5: 2281–2288, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Roodnat JI, Mulder PG, Rischen-Vos J, van Riemsdijk IC, van Gelder T, Zietse R, IJzermans JN, Weimar W: Proteinuria after renal transplantation affects not only graft survival but also patient survival. Transplantation 72: 438–444, 2001. [DOI] [PubMed] [Google Scholar]
- 40. Heptinstall R: Pathology of the Kidney, Philadelphia, Brown and Co., 1983. [Google Scholar]
- 41. Giral-Classe M, Hourmant M, Cantarovich D, Dantal J, Blancho G, Daguin P, Ancelet D, Soulillou JP: Delayed graft function of more than six days strongly decreases long-term survival of transplanted kidneys. Kidney Int 54: 972–978, 1998. [DOI] [PubMed] [Google Scholar]
- 42. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004. [DOI] [PubMed] [Google Scholar]
- 44. Hothorn T, Zeileis A: Generalized maximally selected statistics. Biometrics 64: 1263–1269, 2008. [DOI] [PubMed] [Google Scholar]
- 45. Pinheiro J, Bates D: Mixed Effects Models in S and S-plus, New York, Springer Verlag, 2000. [Google Scholar]
- 46. Schwarz G: Estimating the dimension of a model. The Annals of Statistics 6: 461–464, 1978. [Google Scholar]
- 47. Efron B, Tibshirani R: An Introduction to the Bootstrap, New York, Chapman and Hall, 1993. [Google Scholar]