Abstract
The continuing shortage of kidneys for transplantation requires major efforts to expand the donor pool. Donation after cardiac death (DCD) increases the number of available kidneys, but it is unknown whether patients who receive a DCD kidney live longer than patients who remain on dialysis and wait for a conventional kidney from a brain-dead donor (DBD). This observational cohort study included all 2575 patients who were registered on the Dutch waiting list for a first kidney transplant between January 1, 1999, and December 31, 2004. From listing until the earliest of death, living-donor kidney transplantation, or December 31, 2005, 459 patients received a DCD transplant and 680 patients received a DBD transplant. Graft failure during the first 3 months after transplantation was twice as likely for DCD kidneys than DBD kidneys (12 versus 6.3%; P = 0.001). Standard-criteria DCD transplantation associated with a 56% reduced risk for mortality (hazard ratio 0.44; 95% confidence interval 0.24 to 0.80) compared with continuing on dialysis and awaiting a standard-criteria DBD kidney. This reduction in mortality translates into 2.4-month additional expected lifetime during the first 4 years after transplantation for recipients of DCD kidneys compared with patients who await a DBD kidney. In summary, standard-criteria DCD kidney transplantation associates with increased survival of patients who have ESRD and are on the transplant waiting list.
Kidney transplantation results in substantial survival benefit for dialysis patients who are on the waiting list in North America, Europe, and Australia.1–4 This survival benefit extends to subgroups of high-risk recipients at the extremes of age or after failure of a previous kidney transplant5–9 and also applies to high-risk donors with old age or medical comorbidities.10
Considering the survival benefit of kidney transplantation, major efforts must be made to increase the supply of deceased-donor kidneys and to reduce the waiting times for transplantation. In recent years, transplantation of organs from donation after cardiac death (DCD) has been advocated as a means to expand the donor pool.11 In contrast to organ donation after brain death (DBD), the delay between circulatory arrest and organ preservation may cause acute ischemic injury in DCD organs. As a consequence, the incidence of delayed graft function and primary nonfunction in DCD kidney transplantation is relatively high, although survival of functioning grafts seems to be satisfactory.12–18
During the past decade, DCD has evolved into routine clinical practice that currently supplies >10% of all deceased-donor kidneys in the United States and up to 50% in the Netherlands; however, it is unknown whether patients who receive a DCD kidney live longer than patients who receive conventional therapy (i.e., continue dialysis treatment with the option of later receiving a DBD kidney). We therefore evaluated the effect of kidney transplantation from different types of deceased donors on the survival of dialysis patients who were on the waiting list in an observational cohort study including all patients who were registered on the Dutch waiting list for a first kidney transplantation between 1999 and 2004.
Results
Study Population
During the recruitment phase of the study, 2708 patients were actively listed for a first kidney transplantation (Figure 1). Of these patients, 133 were excluded because they did not receive dialysis therapy while actively listed for kidney transplantation or because they received a transplant outside Eurotransplant with an unknown transplantation date. Characteristics of the 2575 patients included in the study are described in Table 1. Of these patients, 680 underwent DBD kidney transplantation and 459 patients received a DCD transplant within the study period. Dialysis patients who were on the waiting list contributed a mean of 675 days (SD 514 days) of follow-up, accumulating to a total follow-up time of 4774 patient-years. Recipients of DBD and DCD kidneys were followed for 845 (SD 581) and 625 days (SD 483 days), respectively, accumulating to total follow-up times of 1575 and 787 patient-years. Unadjusted mortality rates were 5.0% per patient-year in the dialysis patients who were on the waiting list and 3.9 and 3.4% per patient-year for recipients of DBD and DCD kidneys, respectively.
Figure 1.
Flow chart of patient selection and follow-up in the observational cohort study.
Table 1.
Patient characteristics and unadjusted mortality of the study cohort
| Characteristic | Patients on the Waiting List |
DBD Recipients |
DCD Recipients |
|||
|---|---|---|---|---|---|---|
| n | Mean ± SD or % | n | Mean ± SD or % | n | Mean ± SD or % | |
| Age (years; mean ± SD) | 2575 | 49 ± 15 | 680 | 46 ± 18 | 459 | 51 ± 13 |
| Gender (male/female) | 2575 | 60/40% | 680 | 57/43% | 459 | 63/37% |
| Kidney disease (renovascular/other) | 2068 | 30/70% | 543 | 28/72% | 374 | 29/71% |
| Panel reactive antibodies (≤5/6 to 84/≥85%) | 2566 | 93/6/1% | 677 | 92/7/1% | 457 | 94/6/0% |
| Dialysis before placement on waiting list (days) | 2517 | 516 ± 542 | 669 | 579 ± 652 | 457 | 601 ± 575 |
| Dialysis type (hemodialysis/peritoneal) | 2517 | 56/44% | 669 | 56/44% | 457 | 57/44% |
| Time from placement on waiting list until transplantation (days) | 680 | 594 ± 499 | 459 | 713 ± 503 | ||
| Donor age (years) | 680 | 47 ± 16 | 459 | 45 ± 16 | ||
| Donor gender (male/female) | 680 | 46/54% | 459 | 55/45% | ||
| Donor criteria (SCD/ECD) | 680 | 73/27% | 459 | 78/22% | ||
| DCD type (controlled/uncontrolled) | 459 | 88/12% | ||||
| Average follow-up time (days) | 2575 | 675 ± 514 | 680 | 845 ± 581 | 459 | 625 ± 483 |
| Total follow-up time (patient-years) | 2575 | 4774 | 680 | 1575 | 459 | 787 |
| Deaths | 2575 | 239 | 680 | 61 | 459 | 27 |
| Unadjusted mortality rate (/patient-year; %) | 5.0% | 3.9% | 3.4% | |||
| Graft failure within 90 days | 678 | 6.3% | 456 | 12% | ||
SCD, standard-criteria donor.
Overall, 25% of the transplanted kidneys were recovered from expanded-criteria donors (ECDs). Among the DCD kidneys, 88% had been recovered after controlled withdrawal of treatment in the intensive care unit, whereas 12% were procured in an uncontrolled manner after failed cardiopulmonary resuscitation. DCD donors were on average 1.9 years younger (P = 0.05) and significantly more likely to be male (55 versus 46%; P = 0.01) than DBD donors. Recipients of DCD kidneys were on average 4.8 years older (P < 0.001) and significantly more likely to be male (63 versus 57%; P = 0.04) than recipients of DBD kidneys. Mean waiting time for DCD kidneys was 141 days longer than for DBD kidneys (P < 0.001). DCD and DBD kidney transplantations were not significantly different with respect to other baseline characteristics. DCD kidneys failed significantly more often than DBD kidneys in the first 3 months after transplantation (12 versus 6.3%; P = 0.001). Early graft failure occurred in 22% of ECD DCD and in 20% of uncontrolled DCD kidney transplantations.
Effect of Kidney Transplantation on Survival of Dialysis Patients on the Waiting List
Overall mortality rates of patients who received DBD and DCD kidneys were compared with the alternative therapeutic option of dialysis treatment or waiting on dialysis until standard-criteria DBD kidney transplantation, respectively, using Cox regression with sequential stratification (Table 2).19,20 In line with previous studies,1–4 standard-criteria DBD kidney transplantation was associated with a 49% mortality rate reduction compared with dialysis treatment (hazard ratio [HR] 0.51; 95% confidence interval [CI] 0.32 to 0.81; P = 0.004). We subsequently compared the survival benefit of receiving a standard-criteria DCD kidney to dialysis treatment with the option of later receiving a standard-criteria DBD kidney. The mortality rate after standard-criteria DCD kidney transplantation was 56% lower than with this conventional therapy (HR 0.44; 95% CI 0.24 to 0.80; P = 0.007). The effect of DCD kidney transplantation on mortality rate did not differ with patient age or kidney disease (P = 0.44 and P = 0.83 for interaction terms, respectively). Mortality rates after ECD DBD or DCD kidney transplantation were not significantly different from conventional therapy (P = 0.62 and P = 0.15, respectively). Sensitivity analyses excluding pediatric patients, excluding recipients of uncontrolled DCD kidneys, excluding patients with missing data, or by adjusting only for stratification covariates produced similar results.
Table 2.
Reduced mortality rate after kidney transplantation from different deceased-donor types compared with the alternative therapeutic option of dialysis treatment or waiting on dialysis until standard-criteria DBD kidney transplantation (conventional therapy)
| Deceased-Donor Type | Overall HR | 95% CI | P |
|---|---|---|---|
| SCD DBD kidney transplantation versus dialysis treatment | 0.51 | 0.32 to 0.81 | 0.004 |
| SCD DCD kidney transplantation versus conventional therapy | 0.44 | 0.24 to 0.80 | 0.007 |
| ECD DBD kidney transplantation versus conventional therapy | 1.12 | 0.71 to 1.76 | 0.62 |
| ECD DCD kidney transplantation versus conventional therapy | 0.61 | 0.31 to 1.19 | 0.15 |
Results were adjusted for age, gender, calendar year, dialysis time before placement on the waiting list, dialysis time while on waiting list, kidney disease, transplant center, and panel reactive antibodies.
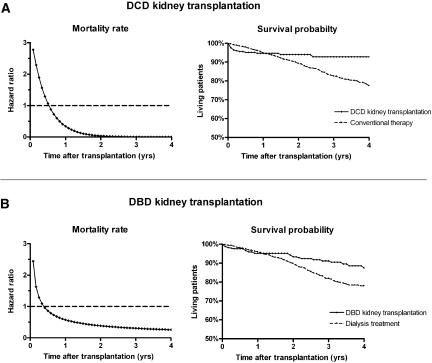
Because mortality rates after kidney transplantation may change over time,1 statistical models with time-dependent HRs were fitted. In the early posttransplantation period, kidney transplant recipients experienced higher mortality rates and lower survival probabilities than patients who were treated with conventional therapy (Figure 2). The mortality rate of transplant recipients gradually decreased, however, which in time led to a survival benefit for patients who received a kidney transplant. The mortality rate and survival probability of standard-criteria DCD kidney transplant recipients were equal to those of patients who received conventional therapy at 7 and 13 months after transplantation, respectively, after which a survival benefit was observed for transplant recipients (Figure 2A, Table 3). Within the first 4 years after transplantation, recipients of standard-criteria DCD kidneys were expected to live 2.4 months longer than patients who continued dialysis treatment with the option of later receiving a kidney from a standard-criteria DBD donor. For comparison, after standard-criteria DBD kidney transplantation, mortality rates and survival probabilities were equal to those of dialysis treatment at 5 and 9 month after transplantation, respectively, whereupon transplant recipients had the advantage (Figure 2B). The estimates for ECD DBD and DCD kidney transplantation, which were not associated with overall mortality rate reductions, are presented in Table 3.
Figure 2.
DCD and DBD kidney transplantation provide survival benefit compared to conventional therapy. (A) Survival benefit of standard-criteria DCD kidney transplantation compared with conventional therapy (i.e., continuing dialysis treatment with the option of later receiving a standard-criteria DBD kidney). (B) Survival benefit of standard-criteria DBD kidney transplantation compared with dialysis treatment. Comparison groups had spent equal times on the waiting list at time 0.
Table 3.
Survival benefit after kidney transplantation from different deceased-donor types compared with the alternative therapeutic option of dialysis treatment or waiting on dialysis until standard-criteria DBD kidney transplantation (conventional therapy)
| Deceased-Donor Type | Time to Equal Mortality Rate (months) | Time to Equal Survival (months) | Time to Equal Lifetime (months) | Additional Lifetime (months)a |
|---|---|---|---|---|
| SCD DBD kidney transplantation versus dialysis treatment | 5 | 9 | 21 | +2.1 |
| SCD DCD kidney transplantation versus conventional therapy | 7 | 13 | 24 | +2.4 |
| ECD DBD kidney transplantation versus conventional therapy | 20 | 43 | Not observed | −1.5 |
| ECD DCD kidney transplantation versus conventional therapy | 5 | 14 | 22 | +1.5 |
Results were adjusted for age, transplant center, and dialysis time while on waiting list. Statistical analysis did not allow estimation of CIs.
aAdditional lifetime within the first 4 years after kidney transplantation.
Discussion
The continuing shortage of kidneys for transplantation requires major efforts to expand the donor pool. Despite substantial increases in kidney transplantation from living donors and ECD DBD in the past decade, the supply of donor kidneys still does not meet demands.21 Liberal use of DCD kidneys may lead to considerable expansion of the donor pool but is associated with a relatively high incidence of delayed graft function and primary nonfunction.12–18,22,23 In this study, graft failure in the first 3 months after transplantation was twice as likely for DCD kidneys than for DBD kidneys; however, DCD kidneys that overcome the early posttransplantation period function as long as DBD kidneys12–18; therefore, it is unclear whether dialysis patients who are on the waiting list should accept an offer for DCD kidney transplantation or continue dialysis treatment until a conventional DBD kidney is available.
In this study, we show for the first time that standard-criteria DCD kidney transplantation was associated with a survival advantage compared with conventional therapy for patients waiting for a first kidney transplantation. The overall mortality rate was reduced by 56% after DCD kidney transplantation, which translated into a 2.4-month increase in expected lifetime during the first 4 years after transplantation. The follow-up period of 1 to 5 years from placement on the waiting list is relatively short to address long-term outcomes after transplantation; however, by extrapolating the survival curves, it seems likely that the additional lifetime gained from DCD kidney transplantation will increase with longer follow-up (Figure 2A). Our findings indicate that despite the relatively high incidence of delayed graft function and early graft loss, dialysis patients who are on the waiting list will enjoy longer life expectancy after DCD kidney transplantation compared with continuation of dialysis treatment with the option of later receiving a conventional DBD kidney.
The findings of this study should be interpreted within the limitations of observational studies that may be subject to selection bias. Patients who undergo kidney transplantation will inherently be healthier than the general dialysis population because of selection at the time of waiting list registration and acceptance of an allocated organ. We used sequential stratification analysis, a refinement of Cox regression, to avoid comparison of transplant recipients with dialysis patients who had become medically unfit for transplantation. This statistical technique sequentially compares each transplant recipient with control subjects who were actual transplant candidates at that time while maintaining intention-to-treat analysis, which is not possible with standard Cox regression.19,20 In addition, the survival benefit after DCD kidney transplantation was relatively large and remained stable when adjusted for several patient characteristics. Relative mortality rates were not affected by patient age or kidney disease and were insensitive to exclusion of potentially confounding cases. Taken together, we consider it unlikely that the effect of DCD kidney transplantation on survival has been overestimated as a result of patient selection bias.
The survival benefit of kidney transplantation over dialysis treatment was previously demonstrated in various geographic areas with different health care systems.1–4 Our study confirms these results and adds contemporary information from a large European cohort of dialysis patients who were on the waiting list. The relative mortality rate reduction of 55% in our cohort at 18 months after standard-criteria DBD kidney transplantation seems lower than previously reported (68 to 82% at 12 to 18 months after transplantation).1–4 This difference may be the result of the more rigorous sequential stratification analysis used in this study, because analysis of our data using time-dependent Cox regression produced a relative mortality rate reduction of 65% at 18 months after standard-criteria DBD kidney transplantation. Furthermore, the survival benefit of kidney transplantation in the United States extends to kidneys recovered from ECDs.10 We could not confirm these findings and instead report similar survival for dialysis patients and recipients of ECD kidneys from either DBD or DCD donors, which may be due to the relatively high mortality of patients who receive dialysis treatment in the United States.24 Despite the lack of survival benefit, ECD kidney transplantation can still be advantageous by improving quality of life and by reducing the mean waiting time to transplantation for dialysis patients.
Our study provides novel insight into the clinical outcome of DCD kidney transplantation. This is a timely subject, because the contribution of DCD to the pool of donor kidneys is growing exponentially.11 Currently, approximately 10% of deceased-donor kidneys in the United States are being recovered from DCD donors, and this proportion is expected to increase further.21 In the Netherlands, almost 50% of deceased-donor kidneys have been procured from DCD donors since the introduction of a nationwide protocol.25 This large proportion of DCD donors allowed accurate analysis of the survival benefit from liberal use of DCD kidneys for transplantation. The generalizability of our results may depend on the specific donor and recipient characteristics of other health care systems. DCD kidneys were mostly procured after controlled withdrawal of supportive treatment in the intensive care unit. Only a small proportion (12%) of DCD kidneys were procured in an uncontrolled manner after failed cardiopulmonary resuscitation. Because organs from uncontrolled DCD donors generally sustain more extensive warm ischemic injury, it would be of interest to examine these kidney transplantations separately in future studies.
In conclusion, patients who receive a standard-criteria DCD kidney live longer than patients who continue dialysis treatment with the option of later receiving a conventional DBD kidney transplantation. More widespread implementation of DCD may further increase the life expectancy of patients with ESRD by reducing the waiting times for transplantation.
Concise Methods
Study Design
The effect of kidney transplantation from various types of deceased donors on the survival of dialysis patients who were on the waiting list was evaluated in an observational cohort study including all patients who were registered on the Dutch waiting list for a first kidney transplantation between January 1, 1999, and December 31, 2004. Individuals who did not receive dialysis therapy while listed as candidates for kidney transplantation were excluded from this analysis. All patients were followed until the earliest of death, living-donor kidney transplantation, transplantation outside the Eurotransplant area, listing for multiorgan transplantation, or December 31, 2005. All kidneys were allocated through standard Eurotransplant policies that were identical for DCD and DBD grafts. Patients were informed of the possibility of DCD kidney transplantation at the time of placement on the waiting list.
Data Sources and Definitions
Dates of placement on the waiting list and transplantation were provided by Eurotransplant. The Dutch Organ Transplant Registry provided data on donor and recipient characteristics, including the dates of graft failure and death after transplantation. Characteristics of dialysis patients and dates of death during dialysis treatment were obtained from the Renine database, which registers all dialysis patients in the Netherlands. Mortality data were checked with the Central Bureau of Genealogy, which records all deaths reported to the Dutch authorities. The data sources were accessed from March 1, 2007, to September 30, 2007. Patient data were collected, stored, and used in agreement with the code of conduct “use of data in health research” from the Dutch federation of biomedical scientific societies; ethics approval was not required. Dialysis time before placement on the waiting list was missing at random in 2.3% of patients, in which case transplant center–specific means were imputed.
ECD status was defined as donation at ≥60 years or between 50 and 60 years with two additional risk factors (last serum creatinine >1.5 mg/dl, history of hypertension, cardiovascular cause of death); all other donors were considered standard-criteria donors.26 Panel reactive antibodies were categorized into three groups (≤5, 6 to 84, or ≥85%) according to the Eurotransplant definition of nonimmunized, immunized, and highly immunized transplant candidates, respectively. Primary kidney disease was classified as renovascular (including hypertensive and diabetic nephropathy) or other reasons because of the difference in life expectancy between these subgroups.27 Graft failure was defined as return to dialysis treatment or retransplantation; recipient death with a functioning transplant was not considered as graft failure.
Statistical Analysis
Continuous variables were expressed as mean ± SD and categorical variables as percentages. Baseline characteristics of DCD and DBD kidney transplantations were compared using independent samples t tests for continuous variables and Pearson χ2 tests for categorical variables.
For each donor type, the covariate-adjusted effect of kidney transplantation on survival was evaluated by sequential stratification, an extension of Cox regression required for appropriate set-up of comparison groups for time-dependent treatments that has been used in several analyses of transplant registries.19,20,28–30 This method proceeds by first matching each transplant recipient to patients within the same age group (≤19, 20 to 39, 40 to 59, and ≥60 years) and transplant center who were actual transplant candidates (i.e., not deceased, having received a transplant, de-listed, or inactivated) at the follow-up time at which the index patient received his or her kidney transplant. The median number of matches per recipient was 46 (interquartile range 20 to 91). These matched patients formed the conventional therapy group with which the index transplant recipient was being compared. To evaluate standard-criteria DBD kidney transplantation, patients in the conventional therapy group continued to be followed up after de-listing but were censored when receiving any kidney transplant. To evaluate kidney transplantation from nonstandard donors, however, follow-up of patients in the comparison group continued after receipt of a standard-criteria DBD kidney, because this is considered part of conventional therapy.
After sequentially creating matched sets (strata) of conventional therapy patients for each transplant recipient, we combined the matched sets and fitted a stratified Cox regression model. Matching adjusted for patient age, transplant center, and time on the waiting list. The analysis was further adjusted for potential confounding by patient gender, kidney disease, dialysis time before placement on the waiting list, calendar year, and panel reactive antibodies by including these covariates in the Cox model. Overall hazard ratios (HR) were computed as in standard Cox regression. To account for the repetition of patients across strata, we used a robust (sandwich) variance estimator to calculate SEs. Interactions between therapy × patient age and therapy × kidney disease were evaluated.
It was previously reported that the mortality rate after kidney transplantation decreases progressively over time1; therefore, we fitted nonproportional hazard models, which allowed the treatment effect to differ with time since transplantation. In addition, Nelson-Aalen survival curves were computed for the kidney transplantation and conventional therapy groups (10 randomly selected conventional therapy patients per stratum, excluding the approximately 5% of strata containing <10 matches). These analyses allowed estimation of the time at which mortality rates, survival probabilities, and restricted residual mean lifetimes of the kidney transplantation and conventional therapy groups were equal. Furthermore, we estimated the lifetime gained through kidney transplantation within the first 4 years after transplantation.
Sensitivity analyses were done by exclusion of pediatric patients (<16 years), by exclusion of recipients of uncontrolled DCD kidneys, by exclusion of patients with missing data on dialysis time before placement on the waiting list, and by adjusting only for covariates used for stratification (patient age, transplant center, and time since placement on the waiting list). P < 0.05 was considered statistically significant. All statistical computing was carried out by DES using SAS 9.1.3 (SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
M.G.S. was supported by a clinical research trainee grant from the Netherlands Organization for Health Research and Development. Development of the statistical methods and analysis was supported by National Institutes of Health grant R01 DK-70869 to D.E.S.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Renal Donation after Cardiac Death,” on pages 888–890.
REFERENCES
- 1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999. [DOI] [PubMed] [Google Scholar]
- 2. McDonald SP, Russ GR: Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant 17: 2212–2219, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Rabbat CG, Thorpe KE, Russell JD, Churchill DN: Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol 11: 917–922, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Oniscu GC, Brown H, Forsythe JL: Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 16: 1859–1865, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK: Renal transplantation in elderly patients older than 70 years of age: Results from the Scientific Registry of Transplant Recipients. Transplantation 83: 1069–1074, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Pereira BJ, Natov SN, Bouthot BA, Murthy BV, Ruthazer R, Schmid CH, Levey AS: Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. The New England Organ Bank Hepatitis C Study Group. Kidney Int 53: 1374–1381, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Glanton CW, Kao TC, Cruess D, Agodoa LY, Abbott KC: Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int 63: 647–653, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB: Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System. Transplantation 66: 1651–1659, 1998. [DOI] [PubMed] [Google Scholar]
- 9. Gillen DL, Stehman-Breen CO, Smith JM, McDonald RA, Warady BA, Brandt JR, Wong CS: Survival advantage of pediatric recipients of a first kidney transplant among children awaiting kidney transplantation. Am J Transplant 8: 2600–2606, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Steinbrook R: Organ donation after cardiac death. N Engl J Med 357: 209–213, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Wijnen RM, Booster MH, Stubenitsky BM, de Boer J, Heineman E, Kootstra G: Outcome of transplantation of non-heart-beating donor kidneys. Lancet 345: 1067–1070, 1995. [PubMed] [Google Scholar]
- 13. Sanchez-Fructuoso AI, Marques M, Prats D, Conesa J, Calvo N, Perez-Contin MJ, Blazquez J, Fernandez C, Corral E, Del Rio F, Nunez JR, Barrientos A: Victims of cardiac arrest occurring outside the hospital: A source of transplantable kidneys. Ann Intern Med 145: 157–164, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Weber M, Dindo D, Demartines N, Ambuhl PM, Clavien PA: Kidney transplantation from donors without a heartbeat. N Engl J Med 347: 248–255, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Cho YW, Terasaki PI, Cecka JM, Gjertson DW: Transplantation of kidneys from donors whose hearts have stopped beating. N Engl J Med 338: 221–225, 1998. [DOI] [PubMed] [Google Scholar]
- 16. Cooper JT, Chin LT, Krieger NR, Fernandez LA, Foley DP, Becker YT, Odorico JS, Knechtle SJ, Kalayoglu M, Sollinger HW, D'Alessandro AM: Donation after cardiac death: The University of Wisconsin experience with renal transplantation. Am J Transplant 4: 1490–1494, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Gok MA, Buckley PE, Shenton BK, Balupuri S, El-Sheikh MA, Robertson H, Soomro N, Jaques BC, Manas DM, Talbot D: Long-term renal function in kidneys from non-heart-beating donors: A single-center experience. Transplantation 74: 664–669, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Barlow AD, Metcalfe MS, Johari Y, Elwell R, Veitch PS, Nicholson ML: Case-matched comparison of long-term results of non-heart beating and heart-beating donor renal transplants. Br J Surg 96: 685–691, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Schaubel DE, Wolfe RA, Port FK: A sequential stratification method for estimating the effect of a time-dependent experimental treatment in observational studies. Biometrics 62: 910–917, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Schaubel DE, Wolfe RA, Sima CS, Merion RM: Estimating the effect of a time-dependent treatment by levels of an internal time-dependent covariate. J Am Stat Assoc 104: 49–59, 2009. [Google Scholar]
- 21. Port FK, Merion RM, Roys EC, Wolfe RA: Trends in organ donation and transplantation in the United States, 1997–2006. Am J Transplant 8: 911–921, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Daemen JW, Oomen AP, Kelders WP, Kootstra G: The potential pool of non-heart-beating kidney donors. Clin Transplant 11: 149–154, 1997. [PubMed] [Google Scholar]
- 23. Terasaki PI, Cho YW, Cecka JM: Strategy for eliminating the kidney shortage. Clin Transpl 265–267, 1997. [PubMed] [Google Scholar]
- 24. Foley RN, Hakim RM: Why is the mortality of dialysis patients in the United States much higher than the rest of the world? J Am Soc Nephrol 20: 1432–1435, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Keizer KM, de Fijter JW, Haase-Kromwijk BJ, Weimar W: Non-heart-beating donor kidneys in the Netherlands: Allocation and outcome of transplantation. Transplantation 79: 1195–1199, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ: Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74: 1281–1286, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC: Comparison of hemodialysis and peritoneal dialysis survival in the Netherlands. Kidney Int 71: 153–158, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Miles CD, Schaubel DE, Jia X, Ojo AO, Port FK, Rao PS: Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant 7: 1140–1147, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM: The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant 8: 419–425, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, Fisher RA, Freise CE, Ghobrial RM, Shaked A, Fair JH, Everhart JE: Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology 133: 1806–1813, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]