Abstract
The majority of patients with chronic kidney disease have hypertension, which is an independent risk factor for progression of kidney disease and cardiovascular disease. Therefore, hypertension should be stringently controlled to a blood pressure level of <130/80 mm Hg. Achieving this goal, which usually requires two or more antihypertensive agents, slows the progression of kidney disease and reduces the risk of cardiovascular disease. All antihypertensive treatments for patients with chronic kidney disease should include a renin-angiotensin-aldosterone system (RAAS) inhibitor (an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker). Initial therapy with fixed-dose RAAS inhibitor–based combinations should be considered, because this approach has been shown to bring significantly more patients to target blood pressure levels, compared with stepped-care treatment or sequential monotherapy. Fixed-dose combination therapy may also improve patient adherence to treatment by reducing the number of pills taken daily and the number of office visits for dosage adjustments. Recent clinical data suggest that the combination of a RAAS inhibitor and a dihydropyridine calcium channel blocker may provide more cardiovascular benefit than the generally recommended combination of a RAAS inhibitor and a diuretic in patients at high risk for cardiovascular events.
An estimated 26 million adults in the USA, based on estimated glomerular filtration rates (eGFRs), are at various stages of chronic kidney disease (CKD) (Table 1) (1). These patients are at high risk for cardiovascular disease (CVD) (2), and, regardless of whether or not traditional CVD risk factors (including hypertension, diabetes, dyslipidemia) are present, their risk for a major cardiovascular event increases progressively as their renal function declines (2), ranging from 1.4-fold for patients with an eGFR of 45 to 59 mL/min/1.73 m2 to 3.4-fold for patients with an eGFR <15 mL/min/1.73 m2, relative to individuals with an eGFR ≥60 mL/min/1.73 m2 (2). CVD is the major cause of death among patients with CKD (3).
Table 1.
Kidney Disease Outcomes Quality Initiative classification of chronic kidney disease∗
| Stage | Description | Estimated GFR (mL/min/1.73 m2) |
| 1 | Kidney damage + normal or elevated GFR | ≥90 |
| 2 | Kidney damage + mildly decreased GFR | 60–89 |
| 3 | Moderately decreased GFR | 30–59 |
| 4 | Severely decreased GFR | 15–29 |
| 5 | Kidney failure | <15 (or dialysis) |
∗Defined as kidney damage confirmed by kidney biopsy showing pathological abnormalities or markers of damage or an estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2 for ≥3 months. Adapted with permission from the National Kidney Foundation (1).
Hypertension is present in 50% to 80% of patients with CKD (2, 4) and is a major independent contributor to increased risk of CVD in this population (5). It is also an independent risk factor for progression of kidney disease (6). The prevalence of hypertension increases with decreasing eGFR; reported rates are approximately 36%, 48%, 60%, and 84% for patients with stage 1, stage 2, stage 3, and stage 4–5 CKD, respectively, compared with 23% for individuals without CKD (7). Because of the key pathogenic role of hypertension in the progression of CKD and the development of CVD, effective management of hypertension is critical to improving clinical outcome. Accordingly, it is essential to achieve optimal blood pressure (BP) control in patients with CKD.
ROLE OF HYPERTENSION IN THE PROGRESSION OF RENAL DISEASE
Hypertension is a key pathogenic factor in the deterioration of renal function. Its presence accelerates the decline in eGFR and increases the onset of end-stage renal disease (ESRD) and the risk of CVD (6). Hypertension has been identified as an independent risk factor for ESRD in men (8) and women (9); the risk of ESRD increases progressively with increasing severity of hypertension. The Multiple Risk Factor Intervention Trial (N = 332,544) showed that, relative to men with systolic BP <120 mm Hg and diastolic BP <80 mm Hg, the risk for ESRD increased by 3-, 6-, 11-, and 22-fold in men with stage 1 (mild), stage 2 (moderate), stage 3 (severe), and stage 4 (very severe) hypertension, respectively (8).
Hypertension-related mechanisms in the progression of renal disease
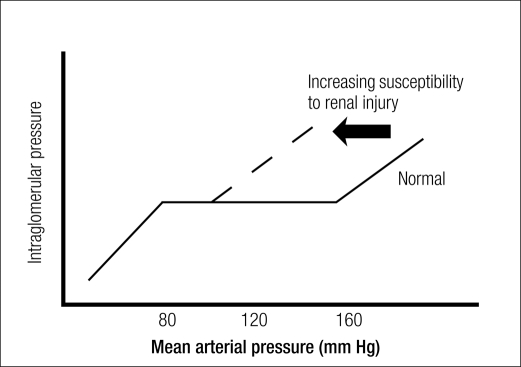
Hypertension-related mechanisms in the progression of renal damage involve the magnitude of increase in systemic BP and the degree to which the elevation in systemic BP is transmitted to the renal microvasculature (i.e., degree of impairment of renal autoregulation) (10). In the healthy kidney, renal autoregulation maintains a constant level of renal blood flow and intraglomerular capillary pressure despite fluctuations in systemic BP between 80 and 170 mm Hg (10). This is accomplished through a myogenic reflex inherent to the kidney, wherein the preglomerular vasculature constricts or dilates in response to increases or decreases in systemic BP. When systemic BP increases, the afferent arteriole constricts, thereby limiting transmission of increased pressure to glomerular capillaries (10). In damaged kidneys, the myogenic reflex is blunted, renal autoregulation becomes impaired, and the ability to prevent transmission of systemic BP changes into the glomerular circulation is partially or totally lost (10). Consequently, intraglomerular pressure begins to change directly with changes in systemic arterial pressure (Figure) (10, 11), in some cases, to the extent that a linear relationship exists between intraglomerular pressure and change in arterial pressure (a pressure-passive relationship) (10). Preclinical data indicate that glomerular capillary hypertension is closely associated with the development of glomerular sclerosis and progressive kidney failure (12). The presence of other factors associated with endothelial dysfunction of the preglomerular vasculature and impaired renal autoregulation (Table 2) (11) may compound the risk of hypertension-induced renal injury.
Figure.
Increases in intraglomerular pressure with increases in systemic mean arterial pressure in the setting of impaired renal autoregulation such as may be seen in patients with renal injury, compared with the setting of normal renal autoregulation. Reprinted with permission from Palmer, 2004 (11).
Table 2.
Conditions associated with endothelial dysfunction of the preglomerular circulation and impaired renal autoregulation∗
| • | African American ethnicity |
| • | Chronic kidney disease |
| • | Diabetes mellitus |
| • | Advancing age |
| • | Low birth weight, intrauterine growth retardation |
| • | Hypercholesterolemia |
| • | Hyperuricemia |
| • | Obesity |
∗Adapted with permission from Palmer, 2004 (11).
Proteinuria, a useful marker of kidney damage associated with hypertension, is itself a risk factor for the progression of renal disease (13, 14). The Irbesartan Diabetic Nephropathy Trial demonstrated that for each doubling of baseline proteinuria level, the risk of progression to kidney failure (defined as doubling of baseline serum creatinine level, serum creatinine level of 530 μmol/L [6.0 mg/dL], or development of ESRD) doubled (13). The accumulation of filtered proteins in proximal tubular cells triggers proinflammatory, profibrogenic, and cytotoxic pathways that contribute to tubulointerstitial injury and renal scarring (15). Thus, hypertension promotes progression of renal disease by worsening glomerular injury and increasing proteinuria, and proteinuria in turn promotes further renal damage.
Reduction of renal damage risk through lower blood pressure
The most effective strategies for lowering intraglomerular pressure are aggressive lowering of the BP and inhibition of the renin-angiotensin-aldosterone system (RAAS) (10). In patients with CKD, establishing and maintaining optimal BP control is the most important initial step in reducing urinary protein excretion (i.e., preventing or slowing progression of kidney disease) (1). A metaanalysis of 11 randomized controlled trials evaluating angiotensin-converting enzyme inhibitor- (ACEI)-based regimens (N = 1860) found that systolic BPs of 110 to 129 mm Hg and proteinuria <2.0 g/d were associated with the lowest risk for kidney disease progression in patients with nondiabetic CKD (14). The Irbesartan Diabetic Nephropathy Trial showed that the optimal reduction in risk for progression to kidney failure was conferred by lowering systolic BP to between 120 and 130 mm Hg (16).
GOALS OF ANTIHYPERTENSIVE THERAPY IN CKD
The goals of antihypertensive therapy set by the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guidelines include reducing BP, slowing the progression of kidney disease, and reducing CVD risk (1). The National Kidney Foundation and the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommend a target BP of <130/80 mm Hg for patients with CKD (1, 17). Achieving this target BP usually takes multiple antihypertensive drugs (1, 17) but can slow the progression of kidney disease and reduce CVD risk in this patient population (1). An even lower systolic BP (e.g., 110–119 mm Hg, or 125 mm Hg) may prevent progression of kidney disease in patients with proteinuria >1 g/d (14, 18). However, lowering systolic BP to <110 mm Hg may increase the risk of kidney disease progression (14) and should be avoided.
Poor control of hypertension in CKD patients
Hypertension is adequately managed in only 11% of patients with stage 1–2 CKD and only 20% of patients with stage 3–4 CKD (7). Analysis of data from 10,813 participants with CKD enrolled in the Kidney Early Evaluation Program found that despite high rates of awareness (80%) and treatment (70%) of hypertension, only 13% of participants were at the recommended target BP (<130/80 mm Hg) (19).
Physician-related factors contributing to inadequate hypertension control include excessive reliance on monotherapy and reluctance to increase drug doses or add additional antihypertensive agents to the treatment regimen. The main patient-related factor is nonadherence to prescribed treatment (20). The latter can be improved by reducing the number of daily doses (21) or prescribing a single-tablet fixed-dose combination antihypertensive product. Significantly greater treatment adherence has been observed for hypertensive patients receiving a single-capsule fixed-dose combination product than for patients receiving the combination components separately (22).
RAAS INHIBITOR THERAPY
As recommended by JNC 7, patients with diabetic kidney disease or nondiabetic kidney disease with a spot urine total protein-to-creatinine ratio ≥200 mg/g, with or without hypertension, should receive a RAAS inhibitor (ACEI or angiotensin receptor blocker [ARB]) (17), unless there is a specific contraindication to their use. ACEIs (14, 23) and ARBs (13, 24, 25) have been proven to be effective in slowing the progression of kidney disease. For this indication, ACEI and ARB therapy were shown to be clinically equivalent in the DETAIL (Diabetics Exposed to Telmisartan and Enalapril) study in 250 type 2 diabetic subjects with early nephropathy (26).
The Irbesartan Diabetic Nephropathy Trial and the RENAAL (Reduction of Endpoints in NIDDM [noninsulin-dependent diabetes mellitus] with the Angiotensin II Antagonist Losartan) trial demonstrated that, independent of BP reduction, ARB therapy was superior to non-ACEI, non-ARB, or calcium channel blocker (CCB) (amlodipine) therapy in slowing the progression of nephropathy (24, 25). A metaanalysis of 11 randomized controlled trials in patients with nondiabetic CKD (N = 1860) demonstrated a mean relative risk reduction of 33% for progression of kidney disease with ACEI-based treatment, compared with non–ACEI-based treatments (14).
ACEI (23) and ARB (27–29) therapy has been shown to significantly reduce the risk of cardiovascular morbidity and mortality in patients at high risk for cardiovascular events. Their clinical equivalence in doing so in such patients was demonstrated by ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) comparing telmisartan and ramipril (N = 25,620) (28), and the Valsartan in Acute Myocardial Infarction trial comparing valsartan and captopril (N = 14,703) (29).
INITIAL FIXED-DOSE COMBINATION THERAPY
Most CKD patients require two or more antihypertensive drugs to achieve their target BP (1, 17). Based on JNC 7 and the National Kidney Foundation recommendations for ACEI or ARB therapy as first-line antihypertensive treatment in patients with CKD, combination antihypertensive therapy in this population should include one of these RAAS inhibitors (1, 17). Clinical evidence supports the use of a RAAS inhibitor–based fixed-dose combination product as initial antihypertensive therapy. In the Strategies of Treatment in Hypertension: Evaluation (STRATHE) study, initial fixed low-dose RAAS inhibitor–based combination therapy was superior to either sequential monotherapy (atenolol replaced by losartan and then by amlodipine) or stepped-care treatment (valsartan 40 mg titrated to 80 mg, coadministered with hydrochlorothiazide if needed) in achieving the target BP (30). Fifty-six percent of patients in the low-dose combination group achieved their target BP without experiencing treatment-related adverse events, compared with 42% in the sequential monotherapy (P = 0.001) and the stepped-care (P = 0.004) groups. An additional disadvantage of the stepped-care approach is the requirement for multiple office visits over several months to determine the optimal dosing regimen. Furthermore, because hypertensive CKD patients are likely to require a combination of antihypertensive drugs, starting with fixed-dose combination therapy would appear logical.
In other studies, target BPs were achieved sooner (31) and by significantly more patients receiving initial fixed-dose RAAS inhibitor–based combination therapy than those receiving initial monotherapy (30, 32–35). In a randomized, double-blind, placebo-controlled study (N = 214), hypertensive type 2 diabetic patients receiving fixed-dose combination therapy (benazepril/amlodipine) achieved the predetermined target BP (<130/85 mm Hg) in a mean of 5.3 weeks versus 6.4 weeks for (enalapril) monotherapy (P = 0.001) (31). At 12 weeks, 70% of patients receiving fixed-dose combination therapy were at the currently recommended target BP (<130/80 mm Hg), compared with 31% receiving monotherapy (31).
Rationale for combination therapy
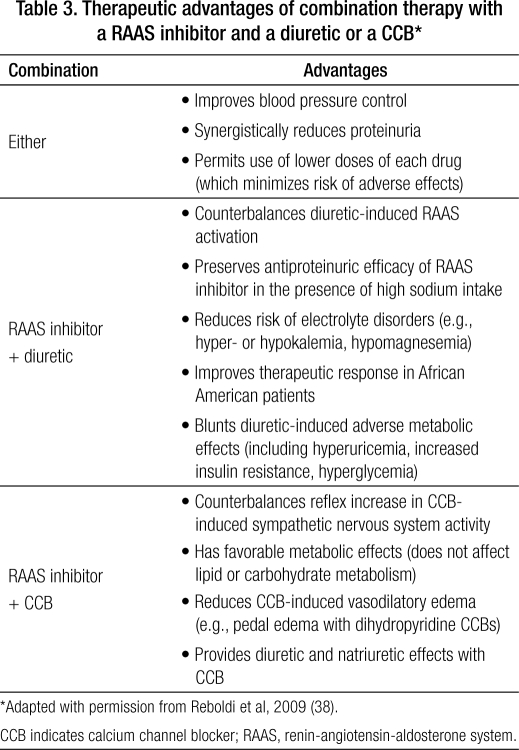
Rational drug combinations are characterized by synergistic or additive BP-lowering efficacy via complementary mechanisms (e.g., RAAS-inhibiting drugs plus a diuretic or a CCB) (32, 33, 36–38), and a mechanism of action of one component that offsets the other's adverse effects (Table 3) (33, 34, 38, 39). With respect to the latter, combining a RAAS inhibitor (ACEI or ARB) with a diuretic attenuates RAAS stimulation triggered by diuretic-induced salt excretion and reduced plasma volume (38). Combining a RAAS inhibitor with a dihydropyridine CCB attenuates the reflex vasoconstriction and tachycardia resulting from increased sympathetic nervous system activity reflexive to CCB-induced systemic vasodilation (38). Compared with treatment with multiple single agents, initial fixed-dose combination therapy offers greater BP-lowering efficacy (30, 32, 37, 39) and simplified dosing regimens (fewer pills), which improves treatment compliance (22).
Table 3.
Therapeutic advantages of combination therapy with a RAAS inhibitor and a diuretic or a CCB∗
Combining an ARB with a dihydropyridine CCB (amlodipine) can reduce peripheral edema (39), a common adverse effect of this CCB class believed to be caused by preferential arteriolar vasodilation and subsequent increased capillary permeability, leading to fluid hyperfiltration and dependent edema (40). In a randomized, placebo-controlled subgroup analysis of 1078 patients with moderate or severe hypertension, the incidence of peripheral edema was significantly lower with telmisartan 40 mg/amlodipine 10 mg (7%) and telmisartan 80 mg/amlodipine 10 mg (9.5%), than with amlodipine 10 mg monotherapy (17.2%; P < 0.0001 for both comparisons) (34).
WHICH RAAS INHIBITOR–BASED COMBINATION?
Although the Kidney Disease Outcomes Quality Initiative working group recommends the combination of a RAAS inhibitor and a diuretic for most patients (1), recent findings of the ACCOMPLISH (Avoiding Cardiovascular events through COMbination therapy in Patients Living with Systolic Hypertension) trial suggest that RAAS inhibitor/CCB fixed-dose combination therapy may be a better treatment choice for patients at high risk for cardiovascular events, including patients with impaired renal function (18% of the study population had eGFR <60 mL/min/1.73 m2; 6% had proteinuria or elevated serum creatinine) (41).
In the ACCOMPLISH trial, despite similar reductions in systolic and diastolic BP (41), single-tablet, fixed-dose RAAS inhibitor/CCB (benazepril/amlodipine) combination therapy was superior to single-tablet, fixed-dose RAAS inhibitor/diuretic (benazepril/hydrochlorothiazide) combination therapy in reducing the composite risk of cardiovascular morbidity and mortality (41). Relative to RAAS inhibitor/diuretic therapy, RAAS inhibitor/CCB therapy was associated with a 20% lower risk of cardiovascular morbidity and mortality (P < 0.001) and a 22% lower risk of fatal and nonfatal myocardial infarction (P = 0.04) (41). After 6 months of single-tablet fixed-dose RAAS inhibitor/CCB or RAAS inhibitor/diuretic combination therapy, significant reductions from baseline in systolic BP were observed (P < 0.001 for total study population and for the CKD subgroup), and 40% of the patients with CKD were at the recommended target BP (<130/80 mm Hg) (42). This finding supports the initial use of fixed-dose combination therapy in hypertensive patients with CKD, even those with mild (stage 1) hypertension.
The greater reduction in CVD risk observed in the ACCOMPLISH trial with RAAS inhibitor/CCB versus RAAS inhibitor/diuretic combination in the setting of similar BP reductions (41) suggests that the combination of RAAS inhibition and calcium channel blockade may confer an additive cardioprotective effect. This favorable effect may be due to altering the mechanisms involved in the pathogenesis of vascular damage, including endothelial dysfunction, decreased levels of nitric oxide, and resultant inflammation (38, 43–45).
In a rat myocardial ischemia model, although monotherapy with benazepril or amlodipine caused significant increases in nitric oxide levels in cardiac interstitial fluid (P < 0.05 versus untreated ischemia), coadministration of the two drugs caused further increases in nitric oxide levels (P < 0.001 versus untreated ischemia) (43). Combined RAAS inhibitor/CCB treatment and monotherapy with each drug also significantly reduced cardiac interstitial fluid levels of the proinflammatory cytokine tumor necrosis factor α (P < 0.01 for monotherapy; P < 0.001 for combination therapy versus no treatment) (43). In contrast, hydrochlorothiazide treatment had no significant effect on cardiac interstitial fluid levels of nitric oxide or tumor necrosis factor α (43). In two studies in patients with hypertension, combination RAAS inhibitor (benazepril)/CCB (amlodipine) therapy produced significantly greater increases in arterial compliance (P < 0.05 versus enalapril monotherapy) and arterial distensibility (P = 0.008 versus amlodipine monotherapy; P = 0.03 versus benazepril monotherapy) than RAAS inhibitor or CCB monotherapy (44, 45).
With respect to renoprotective effects, RAAS inhibitor/CCB combination therapy has been shown to reduce urinary albumin excretion to a significantly greater extent than RAAS inhibitor (fosinopril) or CCB (amlodipine) monotherapy in hypertensive diabetic patients (46).
Combination ACEI/ARB
Although dual blockade of the RAAS with combined ACEI/ARB therapy may appear to be a rational mechanism for enhancing renal and cardiovascular outcomes, there is no conclusive clinical trial evidence of the long-term renal and cardiovascular benefit of ACEI/ARB therapy in hypertensive CKD patients (38). A secondary analysis of the ONTARGET study failed to show a significant reduction in risk of renal impairment with combination ramipril/telmisartan therapy versus ramipril or telmisartan monotherapy; rather, the combination was associated with a significant 33% increase in risk of renal impairment, compared with ramipril monotherapy (P < 0.001) (28). In addition, the ONTARGET trial found no benefit of combination ramipril/telmisartan therapy over ramipril monotherapy in reducing the risk of CVD (28). Nonetheless, combination ACEI/ARB therapy may be an option for some patients who fail to achieve adequate BP control after optimization of RAAS inhibitor/diuretic or RAAS inhibitor/CCB combination therapy.
OTHER TREATMENT CONSIDERATIONS
In addition to reducing BP to the recommended target level (systolic BP between 110 and 130 mm Hg) (1, 14) and implementation of RAAS inhibitor–based combination therapy, other aspects of treatment should be considered (Table 4) (1, 47). If strategies to minimize hyperkalemia (Table 5) (48) fail to maintain serum potassium concentrations below 5.6 mEq/L, the RAAS inhibitor should be discontinued and another class of antihypertensive drug used.
Table 4.
Additional treatment considerations in the management of hypertension and proteinuria in patients with CKD∗
| • Restrict dietary sodium intake to <2.4 g/d (100 mmol/d) |
| • Restrict dietary protein to ≤1.4 g/kg/d for CKD stages 1–2 or 0.6 to 0.8 g/kg/d for CKD stages 3–4 |
| • Use effective thiazide diuretic therapy for CKD stages 2–3 or use loop diuretics when reestimated GFR is <30 mL/min/1.73 m2 for CKD stages 4–5 |
| • Use moderate to high doses of ACEIs or ARBs |
| • Modify antihypertensive therapy in patients with a spot urine total protein-to-creatinine ratio >0.5 to 1 mg/g |
| • Take steps to minimize risk of hyperkalemia induced by ACEI or ARB |
∗In addition to reducing systolic blood pressure to 110 to 130 mm Hg and using renin-angiotensin-aldosterone system inhibitor–based combination therapy (1, 49).
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; GFR, glomerular filtration rate.
Table 5.
Strategies to minimize risk of hyperkalemia caused by renin-angiotensin-aldosterone system inhibitors in patients with chronic kidney disease∗
| • Wherever possible, discontinue drugs that can impair renal potassium excretion (e.g., nonsteroidal antiinflammatory drugs, including selective cyclooxygenase-2 inhibitors) |
| • Prescribe a low-potassium diet; advise patients to avoid use of salt substitutes that contain potassium |
| • Prescribe thiazide diuretics (loop diuretics if estimated GFR is <30 mL/min) |
| • Prescribe sodium bicarbonate to correct metabolic acidosis; decrease dose of ACEI or ARB |
| • Measure serum potassium level 1 week after initiating ACEI or ARB therapy or after increasing the dose |
| • If patient is taking some combination of an ACEI, an ARB, and an aldosterone-receptor blocker, discontinue one and recheck serum potassium level |
| • Do not exceed a 25-mg daily dose of spironolactone when used in combination with an ACEI or an ARB; avoid this combination of drugs when the GFR is <30 mL/min |
∗Adapted with permission from Palmer, 2004 (48).
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate.
CONCLUSIONS
In patients with CKD, hypertension is a common comorbid condition that increases the risk of progression of CKD and the risk of cardiovascular complications. Reduction of BP to <130/80 mm Hg, slowing the progression of kidney disease, and reducing CVD risk are goals of antihypertensive therapy. However, this BP goal is not attained by the majority of CKD patients.
All patients with CKD and hypertension should receive a RAAS inhibitor. Stringent control of hypertension using a RAAS inhibitor–based treatment regimen is an evidence-based approach to slow the progression of CKD and reduce CVD risk. Most CKD patients require multiple antihypertensive drugs to reduce BP to target level. Clinical evidence indicates that initial fixed-dose RAAS inhibitor–based combination therapy is more effective and more efficient than stepped-care therapy or sequential monotherapy for lowering BP to target levels. The fixed-dose combination approach also allows the use of lower doses of each drug in the combination product, which reduces the risk of adverse events and simplifies treatment, thereby promoting patient adherence to treatment. Recent clinical data support initial fixed-dose RAAS inhibitor/CCB combination antihypertensive therapy in CKD patients.
References
- 1.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease: executive summary. Am J Kidney Dis. 2004;43(5 Suppl 1):S16–S41. [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation. The facts about chronic kidney disease (CKD) Available at http://www.kidney.org/kidneydisease/ckd/index.cfm; accessed August 3, 2009.
- 4.Wong ND, Lopez VA, L'Italien G, Chen R, Kline SE, Franklin SS. Inadequate control of hypertension in US adults with cardiovascular disease comorbidities in 2003–2004. Arch Intern Med. 2007;167(22):2431–2436. doi: 10.1001/archinte.167.22.2431. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Jurkovitz CT, Pergola PE, McGill JB, Brown WW, Collins AJ, Chen SC, Li S, Singh A, Norris KC, Klag MJ, Bakris GL, KEEP Investigators Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP) Arch Intern Med. 2007;167(11):1122–1129. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT, Jr, National Kidney Foundation Task Force on Cardiovascular Disease Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? Am J Kidney Dis. 1998;32(5):853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 7.US Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008. Available at http://www.usrds.org/adr.htm; accessed August 4, 2009.
- 8.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 9.Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41(6):1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 10.Palmer BF. Impaired renal autoregulation: implications for the genesis of hypertension and hypertension-induced renal injury. Am J Med Sci. 2001;321(6):388–400. doi: 10.1097/00000441-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Palmer BF. Disturbances in renal autoregulation and the susceptibility to hypertension-induced chronic kidney disease. Am J Med Sci. 2004;328(6):330–343. doi: 10.1016/s0002-9629(15)33943-4. [DOI] [PubMed] [Google Scholar]
- 12.Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis. 1998;32(1):1–22. doi: 10.1053/ajkd.1998.v32.pm9669419. [DOI] [PubMed] [Google Scholar]
- 13.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2):281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, AIPRD Study Group Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139(4):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 15.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17(11):2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 16.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JB, Mangili R, Moore J, Jr, Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16(10):3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123(10):754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, Klag MJ, Bakris GL. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008;121(4):332–340. doi: 10.1016/j.amjmed.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens (Greenwich) 2008;10(1 Suppl 1):20–26. doi: 10.1111/j.1524-6175.2007.08028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164(7):722–732. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril HCl versus comparable component-based therapy. Congest Heart Fail. 2003;9(6):324–332. doi: 10.1111/j.1527-5299.2003.03269.x. [DOI] [PubMed] [Google Scholar]
- 23.Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]
- 24.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 25.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 26.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J, Diabetics Exposed to Telmisartan and Enalapril Study Group Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351(19):1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 27.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, LIFE Study Group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 28.ONTARGET Investigators. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, K⊘ber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM, Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 30.Mourad JJ, Waeber B, Zannad F, Laville M, Duru G, Andréjak M, investigators of the STRATHE trial Comparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approach. J Hypertens. 2004;22(12):2379–2386. doi: 10.1097/00004872-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Bakris GL, Weir MR, Study of Hypertension and the Efficacy of Lotrel in Diabetes (SHIELD) Investigators Achieving goal blood pressure in patients with type 2 diabetes: conventional versus fixed-dose combination approaches. J Clin Hypertens (Greenwich) 2003;5(3):202–209. doi: 10.1111/j.1524-6175.2002.2041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salerno CM, Demopoulos L, Mukherjee R, Gradman AH. Combination angiotensin receptor blocker/hydrochlorothiazide as initial therapy in the treatment of patients with severe hypertension. J Clin Hypertens (Greenwich) 2004;6(11):614–620. doi: 10.1111/j.1524-6175.2004.03808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamerson KA, Nwose O, Jean-Louis L, Schofield L, Purkayastha D, Baron M. Initial angiotensin-converting enzyme inhibitor/calcium channel blocker combination therapy achieves superior blood pressure control compared with calcium channel blocker monotherapy in patients with stage 2 hypertension. Am J Hypertens. 2004;17(6):495–501. doi: 10.1016/j.amjhyper.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Littlejohn TW, 3rd, Majul CR, Olvera R, Seeber M, Kobe M, Guthrie R, Oigman W, Study investigators Telmisartan plus amlodipine in patients with moderate or severe hypertension: results from a subgroup analysis of a randomized, placebo-controlled, parallel-group, 4 × 4 factorial study. Postgrad Med. 2009;121(2):5–14. doi: 10.3810/pgm.2009.03.1972. [DOI] [PubMed] [Google Scholar]
- 35.Lacourcière Y, Martin K. Comparison of a fixed-dose combination of 40 mg telmisartan plus 12.5 mg hydrochlorothiazide with 40 mg telmisartan in the control of mild to moderate hypertension. Am J Ther. 2002;9(2):111–117. doi: 10.1097/00045391-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Chrysant SG, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30(4):587–604. doi: 10.1016/j.clinthera.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Neutel JM, Franklin SS, Lapuerta P, Bhaumik A, Ptaszynska A. A comparison of the efficacy and safety of irbesartan/HCTZ combination therapy with irbesartan and HCTZ monotherapy in the treatment of moderate hypertension. J Hum Hypertens. 2008;22(4):266–274. doi: 10.1038/sj.jhh.1002293. [DOI] [PubMed] [Google Scholar]
- 38.Reboldi G, Gentile G, Angeli F, Verdecchia P. Choice of ACE inhibitor combinations in hypertensive patients with type 2 diabetes: update after recent clinical trials. Vasc Health Risk Manag. 2009;5(1):411–427. doi: 10.2147/vhrm.s4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogari R, Zoppi A, Derosa G, Mugellini A, Lazzari P, Rinaldi A, Fogari E, Preti P. Effect of valsartan addition to amlodipine on ankle oedema and subcutaneous tissue pressure in hypertensive patients. J Hum Hypertens. 2007;21(3):220–224. doi: 10.1038/sj.jhh.1002140. [DOI] [PubMed] [Google Scholar]
- 40.Pedrinelli R, Dell'Omo G, Mariani M. Calcium channel blockers, postural vasoconstriction and dependent oedema in essential hypertension. J Hum Hypertens. 2001;15(7):455–461. doi: 10.1038/sj.jhh.1001201. [DOI] [PubMed] [Google Scholar]
- 41.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ, ACCOMPLISH Trial Investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 42.Jamerson K, Bakris GL, Dahlöf B, Pitt B, Velazquez E, Gupte J, Lefkowitz M, Hester A, Shi V, Kjeldsen SE, Cushman W, Papademetriou V, Weber M, ACCOMPLISH Investigators Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Press. 2007;16(2):80–86. doi: 10.1080/08037050701395571. [DOI] [PubMed] [Google Scholar]
- 43.Siragy HM, Xue C, Webb RL. Beneficial effects of combined benazepril-amlodipine on cardiac nitric oxide, cGMP, and TNF-alpha production after cardiac ischemia. J Cardiovasc Pharmacol. 2006;47(5):636–642. doi: 10.1097/01.fjc.0000211750.01326.b3. [DOI] [PubMed] [Google Scholar]
- 44.Neutel JM, Smith DH, Weber MA. Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass. Am J Hypertens. 2004;17(1):37–42. doi: 10.1016/j.amjhyper.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Winer N, Folker A, Murphy JA, Hung E, Bard M, Perkelvald A, Sowers JR, Bakris GL. Effect of fixed-dose ACE-inhibitor/calcium channel blocker combination therapy vs. ACE-inhibitor monotherapy on arterial compliance in hypertensive patients with type 2 diabetes. Prev Cardiol. 2005;8(2):87–92. doi: 10.1111/j.1520-037x.2005.3535.x. [DOI] [PubMed] [Google Scholar]
- 46.Fogari R, Preti P, Zoppi A, Rinaldi A, Corradi L, Pasotti C, Poletti L, Marasi G, Derosa G, Mugellini A, Voglini C, Lazzari P. Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients. Am J Hypertens. 2002;15(12):1042–1049. doi: 10.1016/s0895-7061(02)03017-0. [DOI] [PubMed] [Google Scholar]
- 47.MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis. 2006;48(1):8–20. doi: 10.1053/j.ajkd.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 48.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351(6):585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]