Abstract
Long QT syndrome (LQTS) is characterized by inherited or acquired prolonged QT interval on the surface electrocardiogram. This can lead to torsade de pointes ventricular tachycardia (TdP VT) and ventricular fibrillation. In the acquired form of the disease, medications from several classes can cause TdP VT or potentiate the electrocardiographic findings. These include class IA and III antiarrhythmics, antibiotics (macrolides and quinolones), antidepressants (tricyclics and selective serotonin reuptake inhibitors), antipsychotics (haloperidol and phenothiazines), and antiemetics (ondansetron and prochlorperazine). We present four cases of drug-induced LQTS resulting in life-threatening cardiac arrhythmias. Antiarrhythmic medications were the cause in two cases, and the other two cases involved noncardiac medications. All four patients had at least one risk factor for LQTS in addition to the offending drug, including female gender, hypokalemia, hypomagnesemia, and bradycardia. In one patient, amiodarone was administered for treatment of VT, although the correct diagnosis was actually TdP VT. In patients with polymorphic VT or ventricular fibrillation without a significant history of cardiovascular disease, drug-induced LQTS should be high in the differential diagnosis. Prompt diagnosis is key, as amiodarone, while often used to suppress VT, is potentially harmful in the setting of LQTS and TdP VT.
We present four case vignettes that illustrate various clinical presentations of drug-induced arrhythmias in the setting of long QT syndrome (LQTS). Commonly used noncardiac medications and risk factors can predispose to LQTS and subsequent life-threatening cardiac arrhythmias, as can bradycardia, which promotes torsade de pointes (TdP) in patients with LQTS. Key features of each presentation are shown in Table 1.
Table 1.
Summary of patient characteristics
| Patient |
||||
| 1 | 2 | 3 | 4 | |
| Age (years) | 38 | 74 | 89 | 42 |
| Gender | M | F | F | F |
| QT-prolonging medications | Sotalol | Ondansetron Citalopram | Quinidine | Amitriptyline Haloperidol |
| Bradycardia | + | + | 0 | 0 |
| Hypokalemia | 0 | + | + | + |
| Hypomagnesemia | 0 | + | 0 | + |
| QTc interval (msec) | 559 | 610 | 550 | 610 |
| Suspected latent congenital long QT syndrome | 0 | + | 0 | 0 |
| Torsade de pointes | + | + | + | + |
| Ventricular fibrillation | + | 0 | 0 | 0 |
| Treatment | ||||
| Magnesium | + | + | 0 | + |
| Potassium | 0 | + | + | + |
| Pacing | + | + | 0 | 0 |
CASE 1
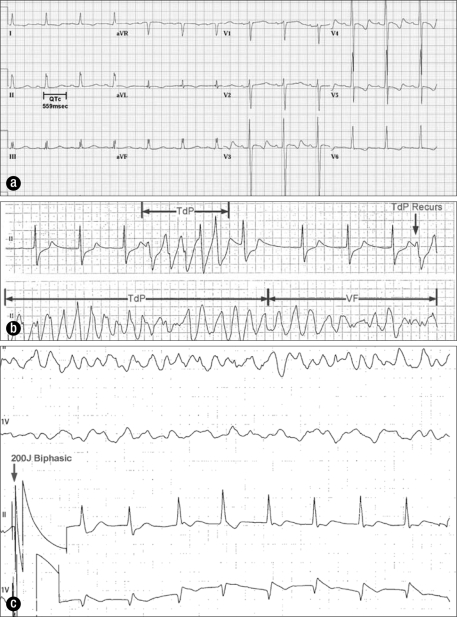
A 38-year-old man was admitted after a severe anaphylactic reaction to cefazolin. His electrocardiogram (ECG) showed a QTc interval of 559 msec (Figure 1a). He had a long history of paroxysmal atrial fibrillation, for which he had been taking sotalol 80 mg twice daily. Serum potassium and magnesium levels were within normal range. He had chronic kidney disease requiring chronic hemodialysis. As sotalol is cleared by renal excretion and contraindicated in patients on dialysis, it was promptly discontinued (1). A junctional rhythm at 50 to 60 beats per minute (bpm) developed. On hospital day 2, he had TdP degenerating to ventricular fibrillation (VF) (Figure 1b) requiring 200 joules biphasic shock for termination (Figure 1c). Intravenous magnesium was administered. Transvenous pacing at a rate of 100 bpm was initiated, and there were no recurrences of TdP.
Figure 1.
Electrocardiograms for case 1. (a) QTc prolongation (559 msec) secondary to sotalol. (b) A brief episode of torsade de pointes (TdP) followed by sustained TdP degenerating into ventricular fibrillation. (c) A 200-joule biphasic shock with restoration of junctional rhythm.
Important points:
Sotalol is cleared by renal excretion and requires careful dose adjustment in patients with impaired renal function. It should be avoided in patients on hemodialysis.
Sotalol in the setting of bradycardia resulted in TdP (1).
Transvenous pacing was the appropriate treatment until the QTc normalized (2).
CASE 2
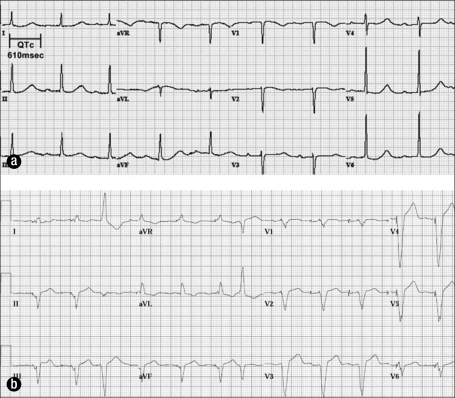
A 74-year-old woman presented to the emergency department with generalized body pain, anxiety, and nausea. She had been taking fentanyl 25 μg/h by transdermal patch for chronic pain until 3 days prior to presentation, when she ran out of her prescription. Her medication list also included carvedilol and citalopram. Her initial ECG (Figure 2a) showed sinus bradycardia at 57 bpm, first-degree atrioventricular block with a PR interval of 300 msec, and a QTc interval of 610 msec. Her serum potassium level was 3.0 mEq/L, and her serum magnesium level was 1.7 mg/dL. She was given ondansetron 4 mg intravenously for nausea.
Figure 2.
Electrocardiograms for case 2. (a) Sinus bradycardia and a QTc of 610 msec in a patient with a serum potassium level of 3.0 mEq/L. (b) After implantation of a dual-chamber pacemaker.
Seven minutes later, telemetry revealed intermittent salvos of a wide-complex tachycardia. Amiodarone was initially given for ventricular tachycardia (VT). Upon further review of the telemetry, LQTS with intermittent TdP was identified, and amioda-rone was discontinued. Two grams of magnesium sulfate were given intravenously and the arrhythmia abated. Carvedilol was discontinued, and further administration of QT-prolonging medications (ondansetron) was avoided. Potassium and magnesium levels were repleted to normal levels and monitored.
Despite these interventions, on hospital day 5, her rhythm remained sinus bradycardia at 52 bpm. The QTc interval remained prolonged at 490 msec. As she had persistent bradycardia and prolonged QTc, a dual-chamber permanent pacemaker was implanted with a lower rate limit programmed at 75 bpm to prevent recurrences of TdP (Figure 2b).
Important points:
This patient had baseline sinus node dysfunction and underlying LQTS. Carvedilol likely exacerbated the bradycardia, which can lengthen the QTc. In addition, hypokalemia and hypomagnesemia contributed to her prolonged QTc interval.
Ondansetron (Zofran), given for her acute nausea, can prolong the QTc and likely resulted in TdP.
Amiodarone, frequently given for VT, is contraindicated in patients with TdP.
In this patient with underlying latent LQTS and sinus node dysfunction, permanent pacing was indicated to prevent persistent bradycardia and pause-dependent TdP (3).
CASE 3
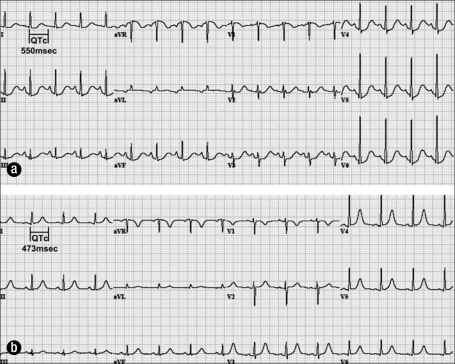
An 89-year-old woman presented with a 2-week history of nausea, poor appetite, and bloody diarrhea. Sigmoidoscopy revealed nonspecific colitis with ulceration. Her medications included quinidine 200 mg four times daily, which she had been taking chronically for 15 years. The baseline QTc interval was 550 msec (Figure 3a). Her serum potassium level was 3.4 mEq/L.
Figure 3.
Electrocardiograms for case 3. (a) QTc prolongation (550 msec) in a patient on quinidine with hypokalemia. (b) Near-normalization of QTc after discontinuation of quinidine and correction of hypokalemia.
On hospital day 1, she had a transient loss of consciousness, believed to be secondary to an arrhythmia. The patient was not monitored with telemetry at the time, although TdP was strongly suspected. Quinidine was discontinued and potassium repleted. A subsequent ECG (Figure 3b) 12 hours later revealed near-normalization of the QTc interval.
Important points:
Despite taking quinidine for 15 years without developing TdP, relatively mild hypokalemia from gastrointestinal losses was sufficient in this case to cause presumed TdP resulting in syncope.
Quinidine is associated with a high incidence of gastrointestinal adverse effects, with loose bowel movements and diarrhea being among the most common.
CASE 4
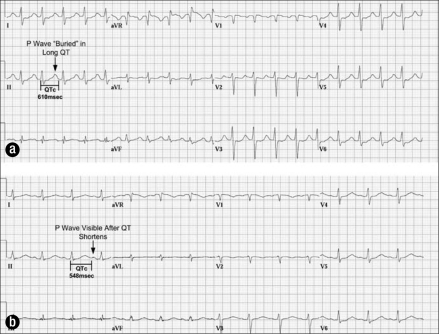
A 42-year-old woman was transferred to our hospital with severe hyperthermia, delirium, and respiratory failure; she had been documented as having a “wide complex tachycardia” that required direct current cardioversion. She had a history of psychiatric disease, and her medications included amitriptyline and haloperidol. The initial ECG on arrival (Figure 4a) showed a QRS duration of 118 msec and a QTc interval of 610 msec. Her serum potassium level was 2.7 mEq/L, and her magnesium level was 1.6 mg/dL. She was managed supportively with external cooling measures for hyperthermia due to suspected tricyclic overdose, and her QRS and QTc intervals normalized (Figure 4b).
Figure 4.
Electrocardiograms for case 4. (a) Marked QTc prolongation (610 msec) that obscures the subsequent P wave. QRS widening is noted consistent with tricyclic overdose. (b) Improvement in QTc 20 hours later.
Important point:
Amitriptyline and haloperidol both resulted in drug-induced LQTS. Hypokalemia and hypomagnesemia were contributing factors.
DISCUSSION
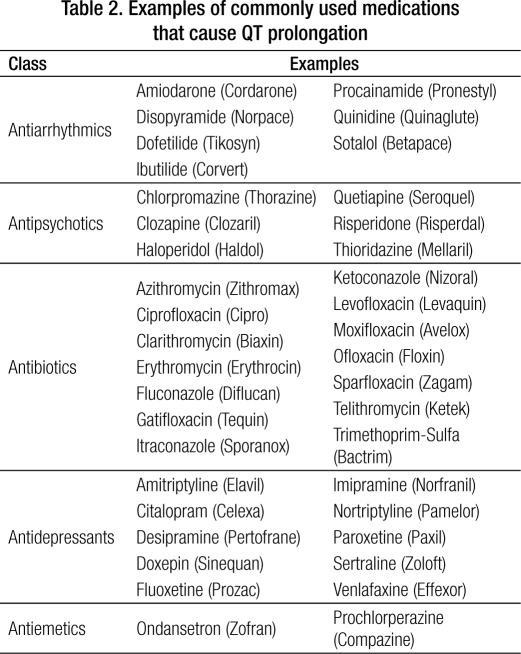
The acquired form of LQTS is a potentially fatal medical condition, which can be exacerbated by a wide range of both cardiac and noncardiac medications. As such, physicians in all specialties should be aware of LQTS and have a familiarity with those medications that exacerbate it. More than 50 medications approved by the Food and Drug Administration (FDA) can affect the QT interval. The most potent QT-prolonging medications are antiarrhythmic agents, particularly amiodarone, dofetilide, quinidine, and sotalol, with quinidine possibly having the most torsadogenic potential. Examples of noncardiac medications (Table 2) include antibiotics (macrolides and quinolones), antidepressants (tricyclics and selective serotonin reuptake inhibitors), antipsychotics (haloperidol and phenothiazines), and antiemetics such as ondansetron. The Arizona Center for Research and Education on Therapeutics maintains a current online reference for medications that can prolong the QT interval (4). Drugs are included on the list based on information from the medical literature, the FDA-approved drug labeling, and reports submitted to the FDA Adverse Events Reporting System database.
Table 2.
Examples of commonly used medications that cause QT prolongation
The mechanism for most potential QT-prolonging medications is inhibition of the KCNH2-encoded HERG (human ether-à-go-go related gene) potassium channel (5). The HERG channel mediates IKr (rapid component of the delayed rectifier potassium current) that is important for phase 3 of cardiac action potential repolarization (Figure 5). Inhibition of this current results in prolongation of the action potential duration and a prolonged QT interval.
Figure 5.
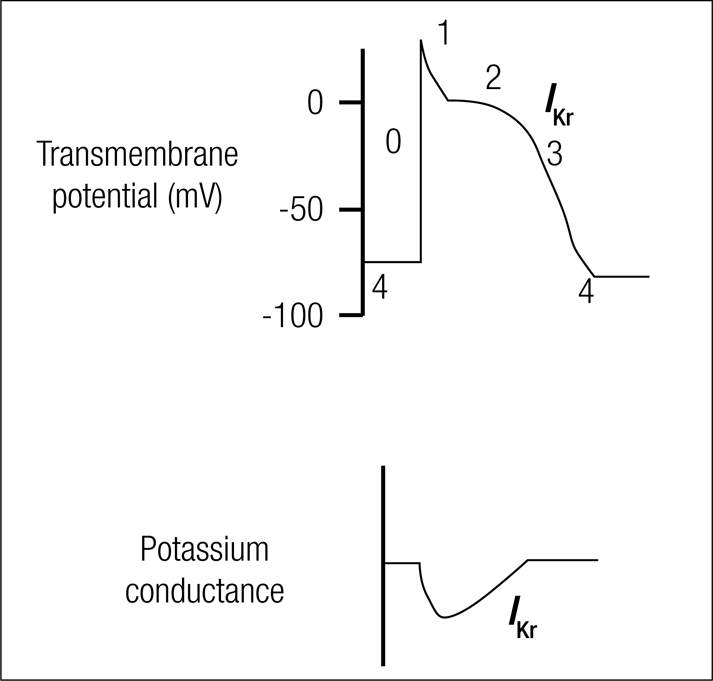
Cardiac action potential. Phase 3 depolarization is mediated by IKr, the delayed rectifier potassium current. Almost all of the drugs that cause LQTS block this current.
Interestingly, mutations in the KCNH2-encoded IKr channel are also responsible for congenital type 2 LQTS (LQT2). Suppressing IKr function, due to either genetic defects or adverse drug effects, can lead to LQTS. Thus, drug-induced LQTS and LQT2 are partially phenocopies stemming from either pharmacologically or genetically mediated perturbations in the IKr potassium channel. In fact, an estimated 10% of patients with drug-induced LQTS actually possess quiescent LQTS-susceptibility mutations, and an adverse drug reaction could be the sentinel event disclosing the presence of underlying congenital LQT2 (6). “Repolarization reserve” describes the redundancy of repolarizing currents that allow a LQTS mutation to remain clinically silent, only to produce clinical manifestations when another insult such as a drug or electrolyte derangement is coincident (6). One of our patients had a persistence of QTc prolongation despite avoiding offending medications and correcting electrolytes, which may be attributable to latent congenital LQT2.
All four patients had at least one risk factor for LQTS and TdP in addition to the offending drug. Most patients with drug-induced TdP have one or more risk factors such as advanced age (>65 years), bradycardia, hypokalemia, hypomagnesemia, occult or latent congenital LQTS, and female gender (7). Drugs are more appropriately viewed as contributors to the overall risk rather than causes of an idiosyncratic event. Although cases of drug-induced QT prolongation and TdP have been reported in the absence of predisposing factors, practitioners should be especially cautious and have a heightened awareness in the presence of such risk factors.
Three of our patients were women. Female gender is the most frequently associated risk factor for TdP (8). Compared to men, women have a longer QTc and greater response to drugs that block IKr (9). One possible explanation for this observation is that sex hormones can modify ion channel expression (9).
Hypokalemia or hypomagnesemia was seen in three of our patients. Both electrolyte disorders are associated with an increased risk of LQTS and TdP. Hypokalemia and hypomagnesemia are commonly seen in patients on antiarrhythmics. Often, this is secondary to concurrent diuretic therapy for associated cardiac conditions or is a consequence of vomiting and diarrhea from an unrelated illness. It is postulated that increased IKr blockade may be driven by hypokalemia (10).
The mechanism by which hypomagnesemia promotes TdP is not as well understood. The effect of hypomagnesemia is supported by the benefit of infusing intravenous magnesium in the treatment of TdP even despite normal magnesium levels and without shortening the QT interval (11). Low magnesium levels may potentiate and high magnesium levels may block the phasic movement of calcium that is responsible for delayed afterdepolarization, which is a form of triggered activity that can initiate VT (12). Hence, the beneficial effect of intravenous magnesium therapy in TdP may be suppression of transient currents that cause afterdepolarization (13).
Both of the patients with bradycardia in the setting of LQTS had TdP. One patient required a permanent pacemaker for sinus node dysfunction and a persistently prolonged QTc. It is paramount to recognize bradycardia in a patient with LQTS for two reasons.
First, an episode of TdP is often preceded by bradycardia or a pause, known as pause-dependent polymorphic VT. Discontinuing medications that can cause bradycardia, such as beta-blockers and calcium channel blockers, is paramount. However, in cases of sick sinus syndrome or AV conduction system disease, isoproterenol or pacing (temporary and sometimes permanent) is often necessary to prevent recurrent TdP. In contrast, it should be noted that beta-blockers are indicated in some of the congenital, but not acquired, forms of LQTS. TdP in these cases of congenital LQTS is often precipitated by a sudden increase in adrenergic tone, which can be mitigated by administration of beta-blockers.
Second, drugs that can induce TdP often have a quality known as reverse-use dependence (14). Drugs that are reverse-use dependent predominantly bind during the rested state of the channel and hence show greatest effect at slower heart rates. Hence, as heart rate slows, the QT interval lengthens. As heart rate increases, the QT interval shortens. A lower heart rate also worsens drug-induced IKr inhibition through decreasing the extracellular potassium concentration. The degree of blockade of IKr is inversely related to the extracellular potassium concentration (10). Lower heart rates result in less repolarizations, thereby reducing potassium moving out of the cell and, consequently, decreased extracellular potassium concentration and worsened IKr inhibition.
TdP (polymorphic VT in the setting of prolonged QTc) occurred in three of our patients, and, while not recorded, likely caused syncope in the fourth. There is a gradual increase in the risk for TdP as the QTc increases. Each 10-msec increase in QTc attributes approximately a 5% to 7% exponential increase in risk for TdP (15). There is no threshold of QTc prolongation at which TdP is certain to occur. However, case reports and small series of patients with drug-induced TdP show increased risk when the threshold of QTc >500 msec is exceeded (15). The shortest QTc in our 4 patients was 550 msec.
One of our patients had TdP that was incorrectly treated initially with amiodarone. It is common practice to administer amiodarone for VT, typically after successful defibrillation. While suitable for treating monomorphic VT, amiodarone is a class III antiarrhythmic that prolongs the QTc and is contraindicated in cases of LQTS and TdP. Prior to administering any antiarrhythmic, the clinician should take special care in examining available ECG tracings to distinguish monomorphic from polymorphic VT and exclude significant QTc prolongation. In addition, the presence of multiple risk factors for TdP should heighten the clinician's suspicion of TdP rather than monomorphic VT.
References
- 1.Wang T, Bergstrand RH, Thompson KA, Siddoway LA, Duff HJ, Woosley RL, Roden DM. Concentration-dependent pharmacologic properties of sotalol. Am J Cardiol. 1986;57(13):1160–1165. doi: 10.1016/0002-9149(86)90692-2. [DOI] [PubMed] [Google Scholar]
- 2.DiSegni E, Klein HO, David D, Libhaber C, Kaplinsky E. Overdrive pacing in quinidine syncope and other long QT-interval syndromes. Arch Intern Med. 1980;140(8):1036–1040. [PubMed] [Google Scholar]
- 3.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. American Association for Thoracic Surgery. Society of Thoracic Surgeons ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation. 2008;117(21):e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 4.Woosley RL. QT Drug Lists by Risk Groups Tucson, AZ: Arizona Center for Research and Education on Therapeutics, 2009. Available at http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm; accessed January 18, 2010.
- 5.Katchman AN, Koerner J, Tosaka T, Woosley RL, Ebert SN. Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther. 2006;316(3):1098–1106. doi: 10.1124/jpet.105.093393. [DOI] [PubMed] [Google Scholar]
- 6.Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21(5):1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 7.Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82(4):282–290. doi: 10.1097/01.md.0000085057.63483.9b. [DOI] [PubMed] [Google Scholar]
- 8.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270(21):2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 9.Drici MD, Clément N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf. 2001;24(8):575–585. doi: 10.2165/00002018-200124080-00002. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93(3):407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- 11.Tzivoni D, Banai S, Schuger C, Benhorin J, Keren A, Gottlieb S, Stern S. Treatment of torsade de pointes with magnesium sulfate. Circulation. 1988;77(2):392–397. doi: 10.1161/01.cir.77.2.392. [DOI] [PubMed] [Google Scholar]
- 12.Schechter E, Freeman CC, Lazzara R. Afterdepolarizations as a mechanism for the long QT syndrome: electrophysiologic studies of a case. J Am Coll Cardiol. 1984;3(6):1556–1561. doi: 10.1016/s0735-1097(84)80296-x. [DOI] [PubMed] [Google Scholar]
- 13.Iseri LT, French JH. Magnesium: nature's physiologic calcium blocker. Am Heart J. 1984;108(1):188–193. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 14.Hondeghem LM, Snyders DJ. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81(2):686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- 15.Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W, American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation Prevention of torsade de pointes in hospital settings. J Am Coll Cardiol. 2010;55:934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]