Abstract
The oligodendrocyte myelin glycoprotein is a glycosylphosphatidylinositol-anchored protein expressed by neurons and oligodendrocytes in the central nervous system. Attempts have been made to identify the functions of the myelin-associated inhibitory proteins (MAIPs) after axonal lesion or in neurodegeneration. However, the developmental roles of some of these proteins and their receptors remain elusive. Recent studies indicate that NgR1 and the recently discovered receptor PirB restrict cortical synaptic plasticity. However, the putative factors that trigger these effects are unknown. Because Nogo-A is mostly associated with the endoplasmic reticulum and myelin associated glycoprotein appears late during development, the putative participation of OMgp should be considered. Here, we examine the pattern of development of OMgp immunoreactive elements during mouse telencephalic development. OMgp immunoreactivity in the developing cortex follows the establishment of the thalamo-cortical barrel field. At the cellular level, we located OMgp neuronal membranes in dendrites and axons as well as in brain synaptosome fractions and axon varicosities. Lastly, the analysis of the barrel field in OMgp-deficient mice revealed that although thalamo-cortical connections were formed, their targeting in layer IV was altered, and numerous axons ectopically invaded layers II–III. Our data support the idea that early expressed MAIPs play an active role during development and point to OMgp participating in thalamo-cortical connections.
Keywords: axon plasticity, barrel-field specification, cortical lamination, myelin
Introduction
The oligodendrocyte myelin glycoprotein (OMgp) is a glycosylphosphatidylinositol-anchored protein expressed by neurons and oligodendrocytes in the central nervous system (CNS) (Habib et al. 1998; Wang et al. 2002). Pioneer genomic studies reported that the omgp gene is located within intron 27b of the mouse NF1 gene, which encodes to Neurofibromin, a RasGAP protein, which, when mutated leads to neurofibromatosis type 1 (NF1) disease (Mikol, Alexakos et al. 1990). NF1-deficient mice display deficits in cortical development (especially in the development of the neocortical barrel field) (Lush et al. 2008). However, although function in adult in normal and neural degeneration is revealed, OMgp functions during development remain to be established.
OMgp belongs to a group of molecules located in CNS myelin protein fractions, with axon outgrowth inhibitory activity (Kottis et al. 2002; Wang et al. 2002). This group also includes Nogo-A (GrandPre et al. 2000; Huber and Schwab 2000; Prinjha et al. 2000) and myelin associated glycoprotein (MAG) (McKerracher et al. 1994; Mukhopadhyay et al. 1994). All 3 proteins may act via the same receptor, the Nogo receptor (NgR1) (Fournier et al. 2001; Fujitani et al. 2005) or its paralogues (NgR2 and/or NgR3) or the recently identified PirB (paired immunoglobulin-like receptor B) (Barton et al. 2003; Lauren et al. 2003; Pignot et al. 2003; Venkatesh et al. 2005; Atwal et al. 2008). The participation and physiology of PirB is not fully known. However, NgR1 may form a complex with either p75NGFR (Domeniconi et al. 2002; Hu et al. 2002) or TROY (Domeniconi and Filbin 2005; Shao et al. 2005), which would transduce intracellular signals by activating RhoA (Yamashita and Tohyama 2003; Domeniconi and Filbin 2005; Shao et al. 2005). In addition, NgR1 may also interact with another coreceptor, Lingo-1 (Mi et al. 2004; Llorens et al. 2008), which mediates intracellular signaling through the serine–threonine kinase WNK1 (Zhang et al. 2009). Subsequent studies pointed out that ligands and their receptors may play crucial roles after lesion or in neurodegenerative diseases (e.g., Fournier et al. 2002; Karnezis et al. 2004; Teng and Tang 2005; Gil et al. 2006; Jokic et al. 2006; Park et al. 2006) or following alcohol abuse (Okamoto et al. 2006). However, although these myelin-associated inhibitory proteins (MAIPs) are widely expressed in the adult CNS, emerging data indicate that some of them may play additional roles at early stages of brain development, because they are expressed before NgR1 and long before the onset of brain myelination. A recent example has been reported for Nogo-A with high neuronal expression and different roles during neuronal migration, neurite formation, or oligodendrocyte maturation in the developing telencephalon (Mingorance-Le Meur et al. 2007; Zhao et al. 2007; Pernet et al. 2008). Another example is Lingo-1 (a coreceptor of NgR1, Carim-Todd et al. 2003; Mi et al. 2004), which can also bind to the postmitotic neuron-specific zinc finger protein Myt1l (Llorens et al. 2008). In the studies of Habib et al. and Vourc'h et al., omgp expression was analyzed during postnatal development, but earlier developmental stages were not studied.
Although oligodendrocyte expression of OMgp occurs at nodes of Ranvier with distinct roles in regulating nodal formation and function during CNS myelination (Apostolski et al. 1994; Huang et al. 2005; Nie et al. 2006), several studies suggest that OMgp is mainly a neuronal protein, which is also expressed in oligodendrocytes (Habib et al. 1998; Hunt, Coffin, and Anderson 2002; Koyama et al. 2008). However, the functions of neuronal OMgp during development have not been fully explored. Here, we examined the pattern of OMgp expression in the embryonic mouse forebrain using a well-characterized antibody, paying special attention to neurons. In addition, the cellular distribution and expression changes of neuronal OMgp protein were analyzed in vivo and in vitro. We report that neuronal OMgp is present at early stages of development (from E14), localized in the growing axons during axonal tract formation following the maturation of cortical connections (e.g., perforant pathway and thalamo-cortical projection). In addition, subsets of hippocampal interneurons express OMgp in the adult stages. At the cellular level, OMgp is present in the neuronal membrane, synaptosomal fractions, and axonal varicosities in primary hippocampal cultures. Lastly, the role of OMgp in the organization of thalamo-cortical connections was analyzed in omgp −/− mice. The barrel field of omgp −/− mice was altered, and ectopic thalamic axons were seen in layers II–III. Taken together, our data provide a detailed characterization of the OMgp protein expression in the embryonic mouse telencephalon and indicate that OMgp has a role in axonal target specification and synaptic plasticity.
Materials and Methods
Animals
All animal experiments were carried out in accordance with the guidelines of the European Union (2003/65/CE) and current Spanish regulations (BOE 252/34367-91, 2005) for the use of laboratory animals. All experimental protocols were also approved by the local Ethical Committee. A total of 30 pregnant OF1 mice (Iffra Credo) were used. The morning of plug detection was considered as embryonic day 0 (E0) and the day of birth as postnatal day 0 (P0). Animals were killed at the following stages: E14, E16, P0, P5, P7, P10, P15, P21, and adults. Six to 12 animals (from 2 or more different litters) were used for each stage. In addition, 5 omgp −/− mice (stage P7) from 2 different litters were also used. omgp −/− mice were generated in the laboratory of Binhai Zheng (University of California, San Diego, CA). A detailed description of gene targeting at OMgp has recently been published (Lee et al. 2009). Briefly, the second exon, which contains all the coding sequence of the OMgp, is deleted, resulting in a null allele. This deletion does not interfere with NF1 expression (see below).
Antibodies
The following primary antibodies were used: OMgp (goat polyclonal, AF1674, R&D Systems, MN, 1:3000 for immunohistochemistry (IHC), 1:200 for immunofluorescence (IF), and 1:1000 for Western blot), Nogo-A (rabbit polyclonal, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA), myelin basic protein (MBP, mouse monoclonal, 1:500, Chemicon, Temecula, CA), neuron-specific β-III Tubulin (mouse monoclonal, 1:2000, Sigma, St Louis, MO), Calbindin-28 kDa (CALB, rabbit polyclonal, 1:5000, Swant, Bellinzona, Switzerland), Calretinin (rabbit polyclonal, 1:500, Swant), CCK (rabbit polyclonal, 1:100, CRB, Cleveland, United Kingdom), Parvalbumin (rabbit polyclonal,1:250, Swant), Somatostatin (SOM, rabbit polyclonal, 1:5000, Swant), SNAP-25 (SMI81, mouse monoclonal, 1:5000, Covance, Princeton, NJ), Syntaxin 1 (mouse monoclonal, clone HPC-1, 1:5000, Sigma), Synaptophysin (mouse monoclonal, 1:1000, Dako, Glostrup, Denmark), Synapsin (rabbit polyclonal, 1:1000, Synaptics System, Goettingen, Germany), MAP2 (mouse monoclonal, 1:200, Sigma), Actin (mouse monoclonal, 1:1000, Chemicon), serotonin (5-HT) transporter (602–622) (5-HTT, rabbit polyclonal, 1:1000, Calbiochem, Gibbstown, NJ), HNK-1(412) (rat monoclonal, 1:500, kindly provided by Prof Melitta Schachner), HNK-1 (clone VC1.1, mouse monoclonal, 1:2000, Sigma), and Neurofibromin (NF1) (rabbit polyclonal, SC-67, 1:1000, Santa Cruz Biotechnology).
Preparation of Adult Brain Myelin
CNS myelin was isolated following the procedure described by Norton and Poduslo (1973). Briefly, adult Sprague–Dawley rat brains were homogenized in 0.32 M sucrose at 4 °C in a Dounce homogenizer. This homogenate was layered over 0.85 M sucrose solution and centrifuged at 25 000 rpm for 30 min. The CNS myelin at the interface of the 2 sucrose layers was collected in water and centrifuged at 25 000 rpm for 15 min. The resultant pellet was obtained, collected in water, and centrifuged at 10 000 rpm for 10 min twice. The white pellet was then suspended in 0.32 M sucrose, and the initial gradient was replicated as described previously. Finally, the myelin was removed from the interface and washed in water and spun at 25 000 rpm for 10 min to remove sucrose. The final pellet was freeze dried overnight, and protein content was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Cell Transfection and OMgp Detection
EBNA-293T cells were cultured with Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, glutamine, and antibiotics (all purchased from GIBCO Life Technologies, Paisley, United Kingdom). Cells were grown in 35-mm ø 6-well multiplates (Nunc, Roskilde, Denmark) containing 10-mm ø glass coverslips to 60–70% confluence and transfected with pCMV-SPORT6-OMgp (full-length cDNA clone IRAVp968C0766D purchased from RZPD, Germany) using Lipofectamine-Plus reagents according to the manufacturer's instructions (GIBCO Life Technologies). Seventy-two hours later, cells were scraped and harvested in Laemmli sample buffer. Cell extracts were separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), electrotransferred to nitrocellulose membranes, and immunoblotted with OMgp antibody. In parallel, protein samples of total adult brain and myelin extract were also included in the experiment as controls.
Immunohistochemical Methods
For IHC, fetuses were removed by caesarean section after deep anesthesia of the mother with chloral hydrate (3.5 mg/kg i.p. injection) and transcardially perfused with 4% paraformaldehyde dissolved in 0.1M phosphate buffered saline (PBS). Postnatal mice were anesthetized with chloral hydrate and perfused. After perfusion, brains were removed and postfixed in the same solution for 12 h, cryoprotected in 30% sucrose, and sectioned on a freezing microtome (Leica, Wetzlar, Germany) (50 μm thick for E16 and 30 μm for P0 adult). They were then processed for the immunocytochemical detection of OMgp following an immunoperoxidase protocol. Briefly, free-floating sections from different developmental stages were processed in parallel. Free-floating sections were rinsed in 0.1 M PBS and endogenous peroxidase activity was blocked by incubation in 3% H2O2 and 10% methanol dissolved in 0.1 M PBS. After extensive rinsing, sections were incubated in 0.1 M PBS containing 0.2% gelatin, 10% normal goat serum, 0.2% glycine, and 0.2% Triton X-100 for 1 h at room temperature. Afterward, sections were incubated for 36 h at 4 °C with the primary antibody. Thereafter, sections were incubated with secondary biotinylated antibodies (2 h, 1:200 diluted) and Streptavidin–Horseradish peroxidase complex (2 h, 1:400 diluted). Peroxidase activity was revealed with 0.025% diaminobenzidine (DAB) and 0.003% hydrogen peroxide. After rinsing, sections were mounted onto slides, dehydrated, and coverslipped with Eukitt (Merck, Darmstadt, Germany). In embryonic stages, the peroxidase activity was developed following the intensification method of Hancock (1986), using DAB–nickel ammonium sulfate as chromogen. Immunocytochemical controls, including omission of the primary antibody or its replacement by normal serum, prevented immunostaining.
To characterize OMgp expression in adult hippocampal interneurons, additional sections from p21-adult brains were processed for double IF detection of OMgp and several markers of local circuit neurons, such as Calbindin, Parvalbumin, and Calretinin, or neuropeptides (CCK and SOM) by using Alexa Fluor 488 and Alexa Fluor 568–tagged secondary antibodies (Molecular Probes, Eugene, OR). Sections were mounted on Fluoromount (Vector Labs, Burlingame, CA) and analyzed on an Olympus Fluoview SV 500 confocal microscope. All images were obtained in sequential-scanning laser mode to avoid fluorochrome crossexcitation.
To determine differences between omgp −/− and omgp +/+ cortical barrel fields, coronal sections of P7 pups were processed in parallel in blind experiments. After genotype identification by Western blotting, the parietal cortex was photodocumented using an Olympus BX61 microscope equipped with a cooled digital DP72L camera. Pictures were densitometrically analyzed using the Image-J software (NIH, United States). Brightness and contrast were calibrated in each picture using a pseudocolor lookup table (Rainbow RGB LUT) settled between 108 (background) and 248 (maximum) gray scale values.
Western Blotting Techniques
Mice were anesthetized, their brains were dissected out, and the telencephalic portion was homogenized on ice in homogenization buffer containing 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 10% glycerol, 1% Triton X-100, and 1× protease inhibitor cocktail. The homogenate was clarified by centrifugation at 13 000 × g for 15 min, and the protein content of soluble fractions was determined using the Bio-Rad detergent-compatible assay. Tissue extracts (30 μg) were boiled in Laemmli sample buffer at 100 °C for 10 min, followed by 8% SDS-PAGE, and electrotransferred to nitrocellulose membranes (Amersham Biosciences, England, United Kingdom). Following transfer, membranes were incubated overnight at 4 °C with α-OMgp antibody, α-Nogo-A or α-MBP, and α-Tubulin to ensure equal amounts of protein in all samples. Membranes were subsequently incubated with peroxidase-tagged secondary antibodies (α-IgG raised in goat, rabbit, or mouse, respectively, Dako), and peroxidase activity was visualized using the ECL-plus kit (Amersham Biosciences). Cell extracts from OMgp-transfected EBNA-293T cells were used as an internal control.
Primary Neuronal Cultures and Immunocytochemical Methods
E16 mouse brains were dissected in PBS containing 0.6% glucose, and the hippocampus was dissected out. After gentle trypsinization, tissue pieces were dissociated by gentle sweeping. Cells were then counted and seeded onto poly-D-lysine-coated coverslips in Neurobasal medium containing B27 supplement (GIBCO Life Technologies). Cells were cultured for 7 days. Coverslips were fixed in 2% buffered paraformaldehyde, permeabilized with Triton X-100 in 0.1 M PBS, and blocked with 10% normal serum in 0.1 M PBS. Cells were sequentially incubated overnight with primary antibodies at 4 °C and then with Alexa Fluor–tagged secondary antibodies for 2 h. After rinsing in PBS, cells were stained with Bisbenzimide (Hoescht 32444, 1 μM in 0.1 M PBS, for 10 min), rinsed, mounted on Fluoromount (Vector Labs), and analyzed with a confocal microscope (TCS SPII, Leica). To determine whether hippocampal neurons express OMgp, we incubated the cultures with α-OMgp and the neuronal marker α-MAP2 antibodies. To study the colocalization of OMgp with presynaptic markers, we labeled the cultures with α-OMgp and α-Synapsin antibodies.
Synaptosome Subfractionation
Adult-mouse forebrains were homogenized in 30 mL of Sol. A buffer (320 mM sucrose, 5 mM Na-4-(2-fydroxyethil)-1-piperazineethanesulfonic acid (HEPES)/HCl, pH 7.4) with 10 strokes at 600 rpm in a glass–Teflon homogenizer. The homogenate was centrifuged (5000 rpm, SS34 rotor, for 5 min at 4 °C). The resulting postnuclear supernatant was centrifuged twice at 11 000 rpm (SS34, for 12 min at 4 °C), and the crude synaptosomal fraction was resuspended in 4–8 mL of Sol. A buffer. This sample was layered on top of a discontinuous Ficoll gradient of 12%–9%–5%. After centrifugation for 35 min at 22 500 rpm in an SW28 rotor (Beckman Coulter Inc., Fullerton, CA), the synaptosomes were collected at the 5–9% and 9–12% interphases and resuspended in 15 mL of Sol. B buffer (10 mM glucose, 5 mM KCl, 140 mM NaCl, 5 mM NaHCO3, 1 mM MgCl2, 1.2 mM Na2HPO4, and 20 mM HEPES/NaOH, pH 7.4). After centrifugation for 12 min at 11 000 rpm, the pellet was resuspended in 300 μL of Sol. B buffer and 2.7 mL of H2O. This sample was layered on top of a discontinuous sucrose gradient (0.4 M, 0.6 M, 0.8 M, 1.0 M, 1.2 M, 1.4 M, 1.6 M, and 1.8 M). Gradients were centrifuged for 3 h at 33,000 rpm in an SW41 rotor (Beckman) and were collected as 0.5-mL fractions. The purity of the fractions was assessed with membrane markers by immunoblotting with α-Syntaxin 1, α-Synaptophysin, and α-SNAP-25 antibodies.
Results
Characterization of α-OMgp Antibody
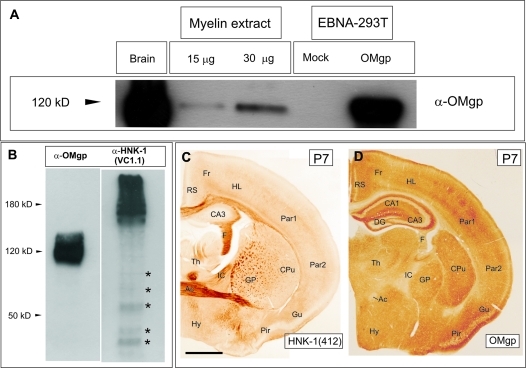
In this study, we used a commercial α-OMgp antibody from R&D Systems. This antibody was produced in goat immunized with mouse OMgp. However, to further characterize the specificity of the α-OMgp antibody, we transfected EBNA-293T cells with plasmid-encoding mouse OMgp. Immunoblot analysis using the α-OMgp antibody (Fig. 1A) exclusively detected a band of approximately 120–130 kDa in mouse-brain extracts, myelin extracts (see Material and Methods for details) and lysates of OMgp-transfected EBNA-293T cells. Labeling was absent in mock-transfected cells. It has been described that OMgp carries the HNK-1 epitope that is also present in other proteins such us NCAM or MAG (Mikol, Gulcher et al. 1990). To further corroborate that the OMgp antibody used in the present study does not recognize the HNK-1 carbohydrate, adult-brain extracts were immunobloted using the OMgp antibody and the HNK1 antibody (clone VC1.1, Sigma) (Fig. 1B). The HNK-1(VC1.1) recognizes the HNK-1 epitope in several brain proteins such us NCAM, MAG, and some chondroitin sulfate proteoglycans of different molecular weights. HNK-1(VC1.1) immunoblots render a strong smear labeling at >180–200 kDa and an additional labeling of several bands of less than 150 kDa that were not recognized by the OMgp antibody (Fig. 1B). In addition, to further corroborate these data in tissue sections, coronal brain sections from the same animal were immunostained using the HNK-1(412) and OMgp antibodies (Fig. 1C,D). The pattern of staining was completely different. Specific areas of the telencephalon (e.g., hippocampal fimbria, the anterior commissure, or globus pallidus) were HNK-1(412) positive. In contrast, although cortical layer IV was labeled with OMgp, HNK1(412) labeling was not observed (Fig. 1C,D). Lastly, the OMgp antibody did not label any band in omgp −/− derived protein extracts (see below) nor in omgp −/− brain sections (data not shown). We conclude that the goat α-OMgp used in the present study only recognized OMgp.
Figure 1.
Characterization of α-OMgp antibody. (A) Immunoblot of OMgp using the goat α-OMgp antibody in adult-brain protein extracts, purified myelin and protein extracts of OMgp-transfected, and Mock-transfected cells. For SDS-PAGE and Western blotting, 40 μg of adult- and cell-protein extracts, and 15 and 30 μg of myelin extract were used. (B) Immunoblot in adult-brain protein extracts using the goat α-OMgp antibody and the HNK-1(VC1.1). Blots with HNK-1 showed a pattern of staining (asterisks) different from those seen in parallel OMgp blots. (C,D) Low-power photomicrographs illustrating HNK-1 (C) and parallel OMgp-staining (C). Notice the different pattern of staining. Scale bar in (C) = 500 μm also pertains to (D) Abbreviations: Fr, frontal cortex; RS, retrosplenial cortex; CA1–CA3 “cornus ammonis” 1–3; DG, dentate gyrus; F, fimbria; Par1-2, parietal cortex 1 and 2; CPu, caudate putamen; GP, globus palidus; Th, thalamus; IC, internal capsule; AC, anterior commissure; Hy, hypothalamus; Pir, pyriform cortex; and Gu, gustatory cortex.
Developmental Expression of OMgp during Brain Development
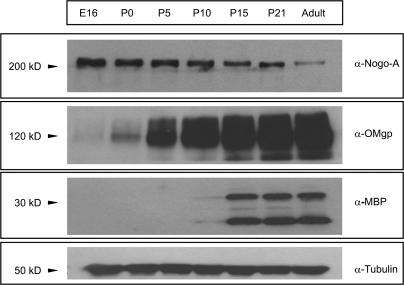
To determine the expression levels of OMgp during development, we first performed a Western blot analysis of protein extracts from developing telecephalon (Fig. 2). The results were compared with the developmental expression of Nogo-A and the MBP in parallel immunoblots. Immunoblot analysis using the rabbit α-Nogo-A antibody detected a band of approximately 200–210 kDa that decreases from E16 to adult stages (Fig. 2). In parallel immunoblots, a pale band of OMgp was first seen at E16. OMgp levels increased from P0 onward reaching maximum levels in the adult. Similar postnatal results were reported by Vourc'h et al. (2003) using semiquantitative real time-polimerase chain reaction. In contrast, MBP, a marker of the myelination, was detected only from P10 onward in brain extracts. In a recent study (Mingorance et al. 2005), we described that the first relevant expression of the MAG, a marker of myelinating oligodendrocytes, appeared at P8–P10 in the cortical white matter, which correlates with MBP expression revealed by immunoblotting. Taken together, our data indicate that OMgp is expressed at embryonic stages long before the onset of the brain myelination, which suggests that OMgp may play additional roles during perinatal development as reported for other MAIPs (e.g., NogoA, Mingorance, Soriano-Garcia, and del Rio 2004; Mingorance-Le Meur et al. 2007; Montani, Gerrits, Gehrig, Dimou et al. 2009).
Figure 2.
Developmental expression of Nogo-A, OMgp, and MBP in Western Blotting. α-OMgp antibody detected a band in brain samples with an apparent molecular weight of 120–130 kDa. Membranes were reprobed with α-Tubulin antibody for protein standardization. Notice that Nogo-A expression started at E16 and continued until adult stages. A faint OMgp band can be seen at E16 long before the onset of myelination as marked by the first appearance of MBP labeling at P10.
Next, we aimed to corroborate these data by analyzing the protein-expression pattern of OMgp during telencephalic development in brain sections, from E14 until adult stages (Fig. 3). Coronal or longitudinal sections from developmental series were immunohistochemically processed. The antibody mainly labeled neurons, although strong staining was also observed at late postnatal stages in white matter tracks, and labeled oligodendrocytes were identified in longitudinal spinal-cord sections with similar morphologies and localization to those reported in oligodendroglial-like cells in paranodal sections (Huang et al. 2005) (Fig. 3M, see also Fig. 2 of Huang et al., for details).
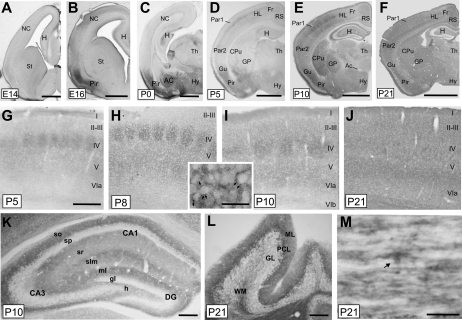
Figure 3.
Pattern of OMgp–protein expression during telencephalic development. (A–F) General views of mouse brains at different stages during development: embryonic stage 14 (E14), E16, P0, P5, P10, and P21. (G–J) High magnifications of the primary somatosensory cortex of mice aged from P5 to P21. Notice the relevant staining of the barrel field at layer IV between P5 and P10. A high magnification of an OMgp immunopositive barrel at P7 is shown in the insert. (K) High-power photomicrograph illustrating OMgp staining in P10 hippocampus. (L) OMgp staining in the cerebellum at P21. Purkinje cells are strongly labeled. (M) Transversal section of adult spinal cord labeled with anti-OMgp. An oligodendrocyte-like cell is OMgp positive (arrow) as well as other longitudinal thin processes. Abbreviations as in Figure 1 including AC, amygdaloid complex; HL, hindlimb; H, hippocampus; NC, neocortex and I–VIb, cortical layers; gl, granular cell layer; h, hilus; ml, molecular layer; slm, stratum lacunosum-moleculare; so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum; GL, granular layer; ML, molecular layer; PCL, Purkinje cell layer; and WM, white matter. Scale bars: (A–F) 500 μm; (G–K) 200 μm; (L) 100 μm, and (M) 10 μm.
At E14, OMgp immunoreactivity was almost absent from the mouse telencephalon (Fig. 3A) except for mammillotegmental and mammillothalamic tracts and raphe dorsalis and dorsal thalamus nuclei (data not shown). At E16, OMgp labeling was prominent in the pyriform/entorhinal region (Fig. 3B), and after nickel-intensified DAB intensification, projecting neurons in layers II–III were seen over the intense neuropil, which expands to layer I and lower layers of the pyriform/entorhinal cortex. In addition, pale neuropil staining was seen in the “stratum lacunosum-moleculare” of the developing hippocampus at these stages. At P0, strong immunoreactivity was also observed in the amygdaloid complex and several hypothalamic nuclei (Fig. 3C,D). At P5, puncta-like immunocytochemical staining was observed in all neocortical areas with higher levels in lateral than medial cortical regions. Particularly, pale OMgp staining was observed in all cortical layers in contrast to layer IV where, in the parietal cortex, immunostaining elements were grouped in clusters corresponding to barrels while septa were clearly defined (Fig. 3D,G). In addition, Purkinje cells and axonal tracts of the cerebellum were also stained at P5 (data not shown). At P8–P10, the barrel field was clearly identifiable, and increasing OMgp labeling was observed in subgranular cortical layers in the parietal cortex as well as in the formerly less immunoreactive regions of the cortex, striatum, and dorsal thalamic nuclei (Fig. 3E,H-I). From P15 onward, the labeling of the cortical barrel field was diluted with the intense OMgp immunoreactivity in the telencephalon (Fig. 3F,J). Particularly in the hippocampus, at P5 but especially at P8–P10, the stratum lacunosum-moleculare and the molecular layer of the dentate gyrus and the “stratum oriens” displayed strong OMgp immunoreactivity (Fig. 3E,K). From P15 to P21, the staining of the stratum lacunosum-moleculare gradually decreased to an intensity that was similar to that in the “stratum radiatum” but lower than in the stratum oriens. In the cerebellum, as indicated above, Purkinje cells were stained from P5 onward (Fig. 3L).
OMgp Expression by Adult Hippocampal Interneurons of the CA1 Region
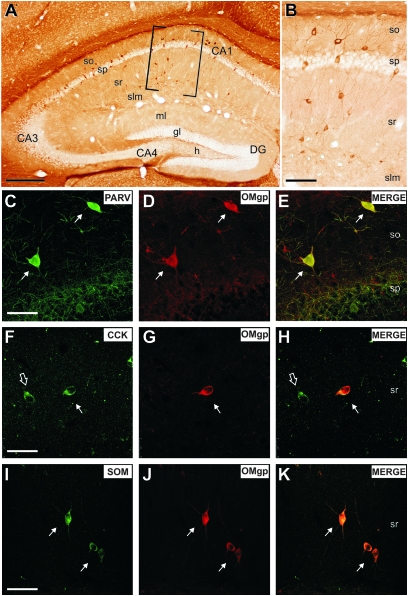
Habib et al. (1998) reported OMgp in local and projection neurons. In addition, in the Allen Brain Atlas, OMgp mRNA is expressed in neurons throughout the CNS. These data were also confirmed by Hunt, Coffin, and Anderson (2002) and recently by Koyama et al. (2008). In previous studies, we analyzed the developmental expression of myelin-associated proteins and receptors in the entorhino-hippocampal system in mouse, rat, and human (Mingorance, Fontana et al. 2004; Mingorance et al. 2005; Gil et al. 2006; Llorens et al. 2008). Thus, to analyze the pattern of labeling of OMgp in the adult hippocampus, we processed horizontal and coronal sections of the adult hippocampal formation (Fig. 4). We found that OMgp was expressed by numerous interneurons mainly in the CA1 region (Fig. 4A). OMgp staining in hippocampal neurons delineated the complete neuron including dendrite and axon. Cell labeling was intense, and several neuronal morphologies, ranking from multipolar to bipolar shapes, were observed scattered in plexiform layers (Fig. 4A,B). Double immunohistochemical labeling of OMgp and markers of local circuit neurons illustrated OMgp immunoreactivity in nonpyramidal cells expressing calcium-binding proteins (Parvalbumin, Calretinin, and Calbindin positive) (see Fig. 4C–E for examples of double-labeled Parvalbumin–OMgp interneurons) as well as some neuropeptides (CCK or SOM) (Fig. 4F–K). Although we were unable to establish a clear and specific colocalization of OMgp with particular subsets of hippocampal interneurons, the first appearance of OMgp staining in hippocampal interneurons coincided with the first appearance of inhibitory potentials in the hippocampus (see Discussion).
Figure 4.
OMgp expression in the adult hippocampus. (A): Low-power photomicrograph of OMgp labeling in the hippocampus. (B): High magnification of the boxed area in (A), OMgp-positive cells in the CA1 region corresponded to hippocampal local circuit neurons. (C–H) Confocal microphotographs illustrating double-labeled (PARV/OMgp) interneurons in the stratum oriens (arrows in C–E); (CCK/OMgp) interneurons in the stratum radiatum (arrows in F–H). Note that some CCK-positive cells are not labeled with OMgp antibody (open arrow in F,H). (I–K) Confocal microphotographs illustrating double-labeled (SOM/OMgp) interneurons in the stratum radiatum (arrows in I–K). Abbreviations as in Figure 3. Scale bars: (A) 200 μm, (B) 100 μm, and (C–K) 25 μm.
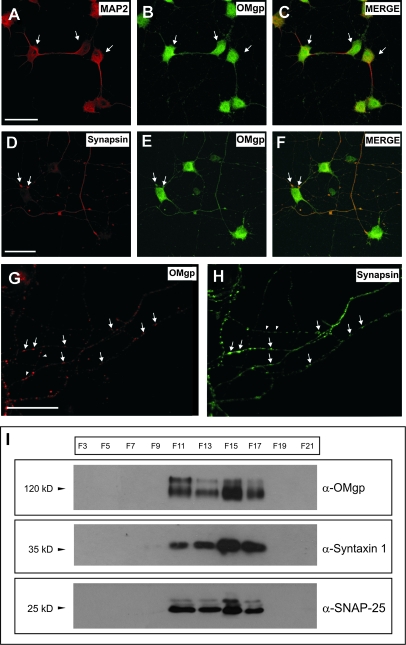
OMgp Colocalizes with Presynaptic Axonal Markers in vitro and with Presynaptic Proteins in Synaptosomal Fractions In Vivo
As indicated, OMgp is present during perinatal development in neocortical layer IV in puncta-like staining as well as in developmental axonal tracts. Thus, we next examined the putative presence of OMgp at the synapse (Fig. 5). First, OMgp localization was studied in primary hippocampal cultures after 7 days in vitro (Fig. 5A–H). In cultured neurons, OMgp completely labeled MAP2-positive hippocampal neurons (Fig. 5A–C) but was also present in axonal-like varicosities close to neurites or the perikaryon of other cultured neurons. Further, double immunohistochemical studies showed that OMgp colocalized in axonal varicosities with presynaptic proteins such as Synapsin (Fig. 5D–H). To further confirm that OMgp was present in the presynaptic terminals, we analyzed the distribution of well-known presynaptic markers (Syntaxin 1 and SNAP-25) in adult sucrose-fractioned brain synaptosomes, and we compared their distribution with OMgp by Western blotting. Fractionation of synaptosomal preparation showed that OMgp was present in the membrane and vesicular fractions (F11–F17), sharing distribution with synaptic markers but not with the cytosolic or mitochondrial fractions (F3–F9 and F19) (Fig. 5I). Taken together, the data suggest that OMgp is localized in axons and synaptosomes in developing and adult neurons.
Figure 5.
OMgp expression in cultured MAP2-positive hippocampal neurons and Synapsin-labeled presynaptic terminals in vitro. (A–C) Confocal microphotographs illustrating hippocampal cultures (7DIV) incubated with antibodies α-OMgp and α-MAP2 to demonstrate the expression of OMgp in MAP2-positive neurons (arrows). (D–F) Parallel cultures were incubated with antibodies α-OMgp and α-Synapsin to demonstrate the presence of OMgp in axon terminals close to neuronal perikaryons (Synapsin positive, arrows). (G,H) OMgp expression in Sinapsin-positive (arrows) axonal varicosities in cultured hippocampal neurons. Note that few OMgp-positive varicosities (arrowheads) are Synapsin negative. (I) OMgp expression in adult forebrain fractionated synaptosomes. Note that OMgp is detected in the same fractions (membrane/vesicles) that are immunoreactive for Syntaxin 1 and SNAP-25. Scale bars: (A,D,G) 25 μm pertains to (B,E), (C,F), and (H), respectively.
Altered Thalamo-Cortical Targeting and Barrel-Field Development in omgp −/− mice
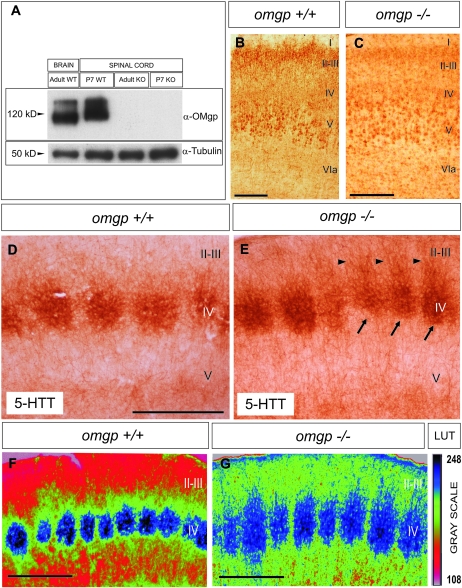
As indicated, OMgp is present in developing axonal tracts. To further determine the role of OMgp in the development of the cortical barrel field, we analyzed the distribution of 5-HTT (serotonin transporter) immunoreactivity in coronal brain sections from 5 omgp −/− and 6 wild-type mice at P7 (Fig. 6). The experiments were conducted blind, with no knowledge of the genotype of the brain being processed, and all free-floating sections were bulk processed during the immunolabeling. After the experiment and data acquisition, the genotype of each mouse was determined by immunoblotting (Fig. 6A). First, as indicated, the omgp gene is located within intron 27b of the mouse NF1 gene, which encodes to Neurofibromin (Mikol, Alexakos et al. 1990). NF1-deficient mice display deficits in development of the somatosensory barrel field (Lush et al. 2008). Thus, we aimed to determine whether the pattern of NF1 expression is altered in omgp −/− mice compared with wild-type mice at the postnatal stages of barrel-field formation. After immunostaining, omgp −/− and wild-type mice showed similar patterns of immunostaining in the neocortex (Fig. 6B,C) and hippocampus (Supplementary Fig. 1). In addition, cortical layering was maintained in adult omgp −/− mice compared with wild type (Supplementary Fig. 2). Next, we determined that the thalamo-cortical connection is formed in omgp −/− mice. However, our results revealed that the distribution of the 5-HTT immunostaining in the barrel field showed clear differences in omgp −/− compared with controls in the neocortex. In omgp −/− mice, barrels were less defined in the first parietal cortex with numerous 5-HTT-positive axons invading ectopically layers II–III (Fig. 6E). All the processed mutant mice showed these alterations. In Figure 6, we show the densitometric analysis in one of the analyzed mice and its parallel control littermate (Fig. 6F,G). In conclusion, omgp −/− mice showed altered distribution of thalamo-cortical axons in cortical layer IV, which indicates that OMgp is required to restrict correct thalamo-cortical axon targeting in the developing cortical barrel field.
Figure 6.
OMgp immunostaining in the primary somatosensory cortex in omgp −/− mice. (A) Western blot corroboration of the presence of the OMgp protein in omgp −/− mice and wild-type controls. (B,C) Low-power photomicrographs illustrating representative sections of the parietal cortex of a wild-type (B) and omgp −/− (C) mouse, immunostained using the α-NF1 antibody. (D–G) Low-power photomicrographs illustrating representative sections of the somatosensory barrel field in control (D,F) and omgp −/− (E,G) mice. Barrels in mutant mice (arrows in E) appeared less defined than in controls and numerous 5-HTT-positive axons were seen ectopically in layers II–III (arrowheads). After application of the pseudocolor correlation, the disorganization of the terminal thalamo-cortical field in the somatosensory cortex is better demonstrated. In the right, the LUT pseudocolor scale (Rainbow RGB) from the Image-J program indicating the gray scale value is shown. Abbreviations as in Figure 3. Scale bars: (B,D), 100 μm pertains to (C,E), respectively. (F,G) 100 μm.
Discussion
Neuronal OMgp Expression during Telencephalic Development
To date, most studies have analyzed the pattern of OMgp expression during postnatal development (Habib et al. 1998; Vourc'h et al. 2003) or in adult stages (Hunt, Coffin and Anderson 2002; Funahashi et al. 2008; Lee et al. 2009). Some studies reported that OMgp is expressed by oligodendrocytes (Funahashi et al. 2008), whereas others indicate a neuronal expression (Habib et al. 1998; Hunt, Coffin, and Anderson 2002; Koyama et al. 2008; Lee et al. 2009). These discrepancies in OMgp expression are very similar to those observed few years ago with the oligodendroglial and neuronal Nogo-A expression (see Mingorance, Fontana et al. 2004 for details). From a technical point of view, most authors used OMgp immunostaining because mRNA localization in oligodendrocytes is difficult and tissue treatments may underestimate the amount of mRNA in neurons (Schwab M, personal communication; see also Huber et al. 2002 for details). However, the available evidence indicates that OMgp is a neuronal protein that is also expressed by oligodendrocytes in healthy (Hunt, Coffin, and Anderson 2002) or damaged CNS (Guo et al. 2007), as well as in cultured oligodendrocytes (Habib et al. 1998). Interestingly, OMgp was found in the nodes of Ranvier, a nonmyelinated axon region (Apostolski et al. 1994; Huang et al. 2005; Nie et al. 2006). Huang et al. (2005) reported that OMgp was not localized in compact myelin, but in oligodendroglial-like cells, whose processes converge to form a ring that completely encircles the nodes.
Our results indicate that the goat α-OMgp antibody recognized endogenous and recombinant OMgp protein specifically. OMgp is present along nonmyelinated axonal tracts during telencephalic development and expressed in cultured MAP2-positive hippocampal neurons and adult hippocampal interneurons in vivo. Taken together, our results reinforce the notion that OMgp is expressed in neurons and oligodendrocytes. However, we did not observe OMgp-positive oligodendrocytes in the telencephalic regions due to the relevant neuropil staining of the sections from the second postnatal week onward. However, in transversal sections of the spinal cord, a similar staining to those presented by Huang and coworkers was observed.
Early Expression of OMgp during Cortical Development. A Role in Axon Target Specification?
We have determined that OMgp expression begins early in embryonic development long before the onset of brain myelination. This suggests that OMgp has additional roles other than the formation of the myelin sheath (Nie et al. 2006) or preventing axon regrowth after injury (Ji et al. 2008). OMgp immunostaining in the developing neocortex follows the targeting of the thalamo-cortical projection in layer IV in mice (Rice and Van der Loos 1977; Rebsam et al. 2002, 2005). As described, the early cortical barrel in mice appears as a patch around P4 and septa become noticeable at P6 (Rice and Van der Loos 1977). Intrinsic cortical connections in the developing somatosensory barrel field are detected from the first postnatal week after barrel formation (P8–P10) coinciding with the first appearance of spontaneous inhibitory potentials in middle cortical layers (Luhmann and Prince 1991). In our study, OMgp labeling in layer IV appeared during the first stage of barrel development (P4–P5). This suggests that OMgp plays an early role in the fine tuning of the thalamo-cortical axons in the developing cortex. This was corroborated by analyzing the parietal barrel field in omgp −/− mice, which displays ectopic 5-HTT labeling in layers II–III. A disrupted barrel-field pattern was also reported in nf1 −/− mice (Lush et al. 2008) as well in trkb −/− mice or MAOA-trkB double knockout (Vitalis et al. 2002). The omgp −/− mice used in the present study showed a normal pattern of NF1 protein compared with wild-type mice. Thus, it is unlikely that NF1 is involved in producing the present results. However, the phenotypes of the NF1-deficient mice and the OMgp knockout are different. As indicated by Lush et al. (2008), NF1 knockout mice showed profound differences in cortical layer IV because patterning of cortical cells into barrels was strongly reduced compared with wild-type mice. In contrast, the OMgp-deficient mice showed no apparent differences in the barrel formation and cortical layering (see Supplementary Fig. 2). The deficits observed in the NF1-deficient mice in the thalamo-cortical connection are stronger than those observed in the omgp −/− mice. Due to the particular location of the OMgp gene into the NF1 locus (see above), we cannot rule out an additional effect of the OMgp absence in the NF1 phenotype. However, OMgp expression was not determined in NF1 mice (Prof. Parada L, personal communication).
On the other hand, it has recently been reported that brain-derived neurotrophic factor (BDNF), the high-affinity ligand of TrkB receptor, which plays key roles during cortical development (see, e.g., Alcantara et al. 2006), stimulates the phosphorylation of NgR1 by Casein kinase II, suppressing Nogo-dependent inhibition of neurite outgrowth in neuroblastoma-derived neural cells (Takei 2009). Thus, the absence of TrkB may have a direct effect on NgR1-mediated axon inhibition and plasticity. It is not clear, whether OMgp expression is modulated by BDNF through TrkB receptor.
OMgp is located at the neuronal membrane (Habib et al. 1998) and carries the HNK-1 epitope (Mikol, Alexakos, et al. 1990), which is also present in well-characterized neural adhesion molecules such as NCAM, L1 or Tenascin R (see Schachner et al. 1995; Yamamoto et al. 2002; or Vourc'h and Andres 2004; for a review). Although our OMgp antibody does not recognized HNK-1, it has been reported that similar CA1 adult hippocampal interneurons labeled with OMgp are HNK-1-positive. The HNK-1 epitope is involved in synaptic plasticity and neuronal physiology both during development and in adulthood (Schachner et al. 1995; Yamamoto et al. 2002). Thus, a putative function of OMgp in neuronal physiology cannot be ruled out. On the other hand, a putative role of OMgp as an adhesion molecule during axonal development cannot be also discarded out either, even if we take into account the modifications of the distribution of 5-HTT axons in the omgp −/− mice. Furthermore, the absence of other neuronal MAIPs during development in vitro leads to increased neurite length and growth cone motility (Mingorance-Le Meur et al. 2007; Montani, Gerrits, Gehrig, Dimou et al. 2009). Although not considered in the present study, we cannot discard a putative function of OMgp modulating cytoskeleton dynamics and neurite length.
To our knowledge, this is the first description of a putative function of MAIPs in barrel-field formation and together with other studies (Martin et al. 2009) the first step toward understanding the role of OMgp during cortical development. Although Nogo-A has been associated with neurite extension (Mingorance-Le Meur et al. 2007; Montani, Gerrits, Gehrig, Dimou et al. 2009), its putative role in the development of the somatosensory barrel field is unlikely, because cortical layering develops normally in Nogo mutant mice (McGee et al. 2005; Mingorance-Le Meur et al. 2007). In addition, Nogo-A expression levels do not change during the critical period, at least in the mouse visual cortex (P20–P26) (McGee et al. 2005), and other MAIPs, such as MAG, appear in the white matter of the somatosensory cortex at P5 (Mingorance et al. 2005). Indeed, numerous studies indicate that nonmyelin-related mechanisms may limit somatosensory barrel-field plasticity because the relevant critical period ends earlier in development (P1–P4), before cortical myelination matures (McGee et al. 2005). Thalamo-cortical axon targeting involves the participation of multiple lamina-specific molecules but relevantly its fine tuning via neural activity (see Yamamoto et al. 2007 for a review). Thalamic axons grow and reach the cortex in the absence of OMgp and in the absence of other MAIPs. Moreover, myelination is absent during early barrel-field formation as indicated above, but a role of OMgp via NgR1 (expressed in layer IV neurons at these stages; Mingorance, Fontana et al. 2004) or other receptors (see below) could take place. NgR1 has recently been implicated in activity-dependent synaptic strength (Lee et al. 2008). In addition, NgR1-mediated signaling from myelin-derived proteins consolidates the neural circuitry established during experience-dependent plasticity (McGee et al. 2005). Furthermore, a recent study described a new MAIPs receptor: PirB (Atwal et al. 2008; Filbin 2008), which has been implicated in restricting cortical plasticity in the visual cortex (Syken et al. 2006). In this scenario, we cannot rule out the participation of OMgp together with other factors in restricting cortical plasticity.
Does OMgp Play a Role at the Synapse?
As indicated, emerging descriptions indicate several roles for myelin protein ligands and receptors in functions very different from those reported above (e.g., see Wang et al. 2006; Mingorance-Le Meur et al. 2007; Pernet et al. 2008; or Montani, Gerrits, Gehrig, Kempf et al. 2009). Nogo-A has been located at the neuronal synapse at the ultrastructural level in the postsynaptic active zone (Liu et al. 2003) as well as in developing axonal tracts (Tozaki et al. 2002; Mingorance-Le Meur et al. 2007). Here, we demonstrated, using biochemical and immunocytochemical methods, that OMgp is located in axonal tracts as well as in synaptosomal fractions and in axonal varicosities. Taken together, these data open up the field for a putative role of OMgp at the synapse. Whether these functions are structural or associated with neurotransmission warrants further study. Unfortunately, our antibody does not react with OMgp in postembedding protocols, so we cannot clearly define its location at the synaptic contact, as reported for Nogo-A (Liu et al. 2003). However, its location in puncta-like structures or synaptosomal fractions points to putative neuronal roles at the synapse, which would increase the new unexpected OMgp functions. For example, recent studies reported new functions for OMgp in controlling stem-cell physiology (Martin et al. 2009). Whether NgR1 or the recently discovered MAIPs receptor PirB or other unknown receptors mediate or participate in these new functions, including the targeting of thalamo-cortical axons, needs to be determined. In this respect, Lee et al. (2008) indicates that NgR1 modulates synaptic transmission by regulating fibroblat growth factor-fibroblast growth factor receptor-mediated signaling. It would be of interest to study whether OMgp–NgR1 or PirB interactions regulate FGF2 roles in the developing telencephalon. In addition, several HNK-1-binding molecules located in perineural nets have been described, such as laminin, selectins, brevican, or aggrecan, which also contribute to corticogenesis (Hall et al. 1993; Needham and Schnaar 1993; Miura et al. 2001; Domowicz et al. 2003). Interestingly, one of the most relevant compounds of the perineural nets is aggrecan, which also showed profound alterations in expression and distributions after sensory deprivation (McRae et al. 2007). Further studies will help to answer these challenging questions.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
MICINN, Instituto Carlos III, and EU-FP7 PRIORITY (to J.A.D.R. and SAF2005-00171 to E.S); Generalitat of Catalunya (grants SGR2009-366 to J.A.D.R. and SGR2009-1017 to E.S.); MICINN (MP4-CT-2006-031971 [EU] and TEC2007-60436 to E.C.T.); United States NIH/NINDS (Grant R0INS054734 to B.Z.); NRSA postdoctoral fellowship (to J.K.L.); Juan de la Cierva Program of the MICINN (to F.Ll.); ISCIII (to A.B.); MEC (to V.G. and O.S.); and Generalitat of Catalunya (to R.M.).
Supplementary Material
Acknowledgments
The authors thank R. Rycroft for linguistic advice and I. Jiménez for technical assistance. The authors also thank Prof Christian Andres and Prof Patrick Vourc‘h (Université François-Rabelais, France), Prof Melitta Schachner (University of Hamburg, Germany), and Javier Saez-Valero (Instituto de Neurociencias de Alicante, Spain) for reagents. Conflict of Interest: None declared.
References
- Alcantara S, Pozas E, Ibanez CF, Soriano E. BDNF-modulated spatial organization of Cajal-Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cereb Cortex. 2006;16:487–499. doi: 10.1093/cercor/bhi128. [DOI] [PubMed] [Google Scholar]
- Apostolski S, Sadiq SA, Hays A, Corbo M, Suturkova-Milosevic L, Chaliff P, Stefansson K, LeBaron RG, Ruoslahti E, Hays AP, et al. Identification of Gal(beta 1-3)GalNAc bearing glycoproteins at the nodes of Ranvier in peripheral nerve. J Neurosci Res. 1994;38:134–141. doi: 10.1002/jnr.490380203. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science (New York, NY) 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carim-Todd L, Escarceller M, Estivill X, Sumoy L. LRRN6A/LERN1 (leucine-rich repeat neuronal protein 1), a novel gene with enriched expression in limbic system and neocortex. Eur J Neurosci. 2003;18:3167–3182. doi: 10.1111/j.1460-9568.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Domowicz MS, Mueller MM, Novak TE, Schwartz LE, Schwartz NB. Developmental expression of the HNK-1 carbohydrate epitope on aggrecan during chondrogenesis. Dev Dyn. 2003;226:42–50. doi: 10.1002/dvdy.10214. [DOI] [PubMed] [Google Scholar]
- Filbin MT. PirB, a second receptor for the myelin inhibitors of axonal regeneration Nogo66, MAG, and OMgp: implications for regeneration in vivo. Neuron. 2008;60:740–742. doi: 10.1016/j.neuron.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fujitani M, Kawai H, Proia RL, Kashiwagi A, Yasuda H, Yamashita T. Binding of soluble myelin-associated glycoprotein to specific gangliosides induces the association of p75NTR to lipid rafts and signal transduction. J Neurochem. 2005;94:15–21. doi: 10.1111/j.1471-4159.2005.03121.x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Hasegawa T, Nagano A, Sato K. Differential expression patterns of messenger RNAs encoding Nogo receptors and their ligands in the rat central nervous system. J Comp Neurol. 2008;506:141–160. doi: 10.1002/cne.21541. [DOI] [PubMed] [Google Scholar]
- Gil V, Nicolas O, Mingorance A, Urena JM, Tang BL, Hirata T, Saez-Valero J, Ferrer I, Soriano E, del Rio JA. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:433–444. doi: 10.1097/01.jnen.0000222894.59293.98. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Guo Q, Li S, Su B. Expression of oligodendrocyte myelin glycoprotein and its receptor NgR after the injury of rat central nervous system. Neurosci Lett. 2007;422:103–108. doi: 10.1016/j.neulet.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Habib AA, Marton LS, Allwardt B, Gulcher JR, Mikol DD, Hognason T, Chattopadhyay N, Stefansson K. Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J Neurochem. 1998;70:1704–1711. doi: 10.1046/j.1471-4159.1998.70041704.x. [DOI] [PubMed] [Google Scholar]
- Hall H, Liu L, Schachner M, Schmitz B. The L2/HNK-1 carbohydrate mediates adhesion of neural cells to laminin. Eur J Neurosci. 1993;5:34–42. doi: 10.1111/j.1460-9568.1993.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;175:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Hu WH, Hausmann ON, Yan MS, Walters WM, Wong PK, Bethea JR. Identification and characterization of a novel Nogo-interacting mitochondrial protein (NIMP) J Neurochem. 2002;81:36–45. doi: 10.1046/j.1471-4159.2002.00788.x. [DOI] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, 3rd, Colman DR, et al. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science (New York) 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Huber AB, Schwab ME. Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biol Chem. 2000;381:407–419. doi: 10.1515/BC.2000.053. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Anderson PN. The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review. J Neurocytol. 2002;31:93–120. doi: 10.1023/a:1023941421781. [DOI] [PubMed] [Google Scholar]
- Ji B, Case LC, Liu K, Shao Z, Lee X, Yang Z, Wang J, Tian T, Shulga-Morskaya S, et al. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokic N, Gonzalez de Aguilar JL, Dimou L, Lin S, Fergani A, Ruegg MA, Schwab ME, Dupuis L, Loeffler JP. The neurite outgrowth inhibitor Nogo-A promotes denervation in an amyotrophic lateral sclerosis model. EMBO Rep. 2006;7:1162–1167. doi: 10.1038/sj.embor.7400826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Lavigne M, Bernard CC. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, Braun PE. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Fujiwara T, Kubo T, Tomita K, Yano K, Hosokawa K, Tohyama M. Reduction of oligodendrocyte myelin glycoprotein expression following facial nerve transection. J Chem Neuroanat. 2008;36:209–215. doi: 10.1016/j.jchemneu.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci. 2003;24:581–594. doi: 10.1016/s1044-7431(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Case LC, Chan AF, Zhu Y, Tessier-Lavigne M, Zheng B. Generation of an OMgp allelic series in mice. Genesis. 2009;00:1–6. doi: 10.1002/dvg.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Jin WL, Liu HL, Ju G. Electron microscopic localization of Nogo-A at the postsynaptic active zone of the rat. Neurosci Lett. 2003;346:153–156. doi: 10.1016/s0304-3940(03)00508-1. [DOI] [PubMed] [Google Scholar]
- Llorens F, Gil V, Iraola S, Carim-Todd L, Marti E, Estivill X, Soriano E, Del Rio JA, Sumoy L. Developmental analysis of Lingo-1/Lern1 protein expression in the mouse brain: interaction of its intracellular domain with Myt1l. Dev Neurobiol. 2008;68:521–541. doi: 10.1002/dneu.20607. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Lush ME, Li Y, Kwon CH, Chen J, Parada LF. Neurofibromin is required for barrel formation in the mouse somatosensory cortex. J Neurosci. 2008;28:1580–1587. doi: 10.1523/JNEUROSCI.5236-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Andres CR, Vedrine S, Tabagh R, Michelle C, Jourdan ML, Heuze-Vourc'h N, Corcia P, Duittoz A, Vourc'h P. Effect of the oligodendrocyte myelin glycoprotein (OMgp) on the expansion and neuronal differentiation of rat neural stem cells. Brain Res. 2009;11:22–30. doi: 10.1016/j.brainres.2009.05.070. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science (New York, NY) 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Mikol DD, Alexakos MJ, Bayley CA, Lemons RS, Le Beau MM, Stefansson K. Structure and chromosomal localization of the gene for the oligodendrocyte-myelin glycoprotein. J Cell Biol. 1990;111:2673–2679. doi: 10.1083/jcb.111.6.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol DD, Gulcher JR, Stefansson K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J Cell Biol. 1990;110:471–479. doi: 10.1083/jcb.110.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance A, Fontana X, Sole M, Burgaya F, Urena JM, Teng FY, Tang BL, Hunt D, Anderson PN, Bethea JR, et al. Regulation of Nogo and Nogo receptor during the development of the entorhino-hippocampal pathway and after adult hippocampal lesions. Mol Cell Neurosci. 2004;26:34–49. doi: 10.1016/j.mcn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mingorance A, Fontana X, Soriano E, Del Rio JA. Overexpression of myelin-associated glycoprotein after axotomy of the perforant pathway. Mol Cell Neurosci. 2005;29:471–483. doi: 10.1016/j.mcn.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Mingorance A, Soriano-Garcia E, del Rio JA. [Nogo-A functions during the development of the central nervous system and in the adult] Rev Neurol. 2004;39:440–446. [PubMed] [Google Scholar]
- Mingorance-Le Meur A, Zheng B, Soriano E, del Rio JA. Involvement of the myelin-associated inhibitor Nogo-A in early cortical development and neuronal maturation. Cereb Cortex. 2007;17:2375–2386. doi: 10.1093/cercor/bhl146. [DOI] [PubMed] [Google Scholar]
- Miura R, Ethell IM, Yamaguchi Y. Carbohydrate–protein interactions between HNK-1-reactive sulfoglucuronyl glycolipids and the proteoglycan lectin domain mediate neuronal cell adhesion and neurite outgrowth. J Neurochem. 2001;76:413–424. doi: 10.1046/j.1471-4159.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Montani L, Gerrits B, Gehrig P, Kempf A, Dimou L, Wollscheid B, Schwab ME. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/cofilin in the unlesioned adult nervous system. J Biol Chem. 2009;284:10793–10807. doi: 10.1074/jbc.M808297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Needham LK, Schnaar RL. The HNK-1 reactive sulfoglucuronyl glycolipids are ligands for L-selectin and P-selectin but not E-selectin. Proc Natl Acad Sci U S A. 1993;90:1359–1363. doi: 10.1073/pnas.90.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie DY, Ma QH, Law JW, Chia CP, Dhingra NK, Shimoda Y, Yang WL, Gong N, Chen QW, Xu G, et al. Oligodendrocytes regulate formation of nodes of Ranvier via the recognition molecule OMgp. Neuron Glia Biol. 2006;2:151–164. doi: 10.1017/S1740925X06000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Miki T, Lee KY, Yokoyama T, Kuma H, Wang ZY, Gu H, Li HP, Matsumoto Y, Irawan S, et al. Oligodendrocyte myelin glycoprotein (OMgp) in rat hippocampus is depleted by chronic ethanol consumption. Neurosci Lett. 2006;406:76–80. doi: 10.1016/j.neulet.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-a and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci. 2008;28:7435–7444. doi: 10.1523/JNEUROSCI.0727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignot V, Hein AE, Barske C, Wiessner C, Walmsley AR, Kaupmann K, Mayeur H, Sommer B, Mir AK, Frentzel S. Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J Neurochem. 2003;85:717–728. doi: 10.1046/j.1471-4159.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Dissociating barrel development and lesion-induced plasticity in the mouse somatosensory cortex. J Neurosci. 2005;25:706–710. doi: 10.1523/JNEUROSCI.4191-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Van der Loos H. Development of the barrels and barrel field in the somatosensory cortex of the mouse. J Comp Neurol. 1977;171:545–560. doi: 10.1002/cne.901710408. [DOI] [PubMed] [Google Scholar]
- Schachner M, Martini R, Hall H, Orberger G. Functions of the L2/HNK-1 carbohydrate in the nervous system. Prog Brain Res. 1995;105:183–188. doi: 10.1016/s0079-6123(08)63294-x. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science (New York, NY) 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Takei Y. Phosphorylation of Nogo receptors suppresses Nogo signaling, allowing neurite regeneration. Sci Signal. 2009;2:ra14. doi: 10.1126/scisignal.2000062. [DOI] [PubMed] [Google Scholar]
- Teng FY, Tang BL. Why do Nogo/Nogo-66 receptor gene knockouts result in inferior regeneration compared to treatment with neutralizing agents? J Neurochem. 2005;94:865–874. doi: 10.1111/j.1471-4159.2005.03238.x. [DOI] [PubMed] [Google Scholar]
- Tozaki H, Kawasaki T, Takagi Y, Hirata T. Expression of Nogo protein by growing axons in the developing nervous system. Brain Res Mol Brain Res. 2002;104:111–119. doi: 10.1016/s0169-328x(02)00172-9. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Gillies K, Hanoun N, Hamon M, Seif I, Gaspar P, Kind P, Price DJ. Interactions between TrkB signaling and serotonin excess in the developing murine somatosensory cortex: a role in tangential and radial organization of thalamocortical axons. J Neurosci. 2002;22:4987–5000. doi: 10.1523/JNEUROSCI.22-12-04987.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vourc'h P, Andres C. Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res Brain Res Rev. 2004;45:115–124. doi: 10.1016/j.brainresrev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Vourc'h P, Dessay S, Mbarek O, Marouillat Vedrine S, Muh JP, Andres C. The oligodendrocyte-myelin glycoprotein gene is highly expressed during the late stages of myelination in the rat central nervous system. Brain Res. 2003;144:159–168. doi: 10.1016/s0165-3806(03)00167-6. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Liu YY, Liu JP, You SW, Ju G. Nogo-66 receptor at the gap junctions between pituicytes of the rat. Neuroreport. 2006;17:605–609. doi: 10.1097/00001756-200604240-00010. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Maruyama T, Uesaka N, Hayano Y, Takemoto M, Yamada A. Molecular mechanisms of thalamocortical axon targeting. Novartis Foundation symposium. 2007;288:199–208. doi: 10.1002/9780470994030.ch14. discussion 208-111, 276–181. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Oka S, Inoue M, Shimuta M, Manabe T, Takahashi H, Miyamoto M, Asano M, Sakagami J, Sudo K, et al. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J Biol Chem. 2002;277:27227–27231. doi: 10.1074/jbc.C200296200. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu X, Zhang Y, Zhou J, Yu Z, He C. LINGO-1 interacts with WNK1 to regulate nogo-induced inhibition of neurite extension. J Biol Chem. 2009;284:15717–15728. doi: 10.1074/jbc.M808751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Jin WL, Ju G. An in vitro study on the involvement of LINGO-1 and Rho GTPases in Nogo-A regulated differentiation of oligodendrocyte precursor cells. Mol Cell Neurosci. 2007;36:260–269. doi: 10.1016/j.mcn.2007.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.