Abstract
The resting brain is associated with significant intrinsic activity fluctuations, such as the correlated low-frequency (LF) blood oxygen level–dependent (BOLD) fluctuations measured by functional magnetic resonance imaging. Despite a recent expansion of studies investigating resting-state LF-BOLD correlations, their nature and function are poorly understood. A major constraint on LF-BOLD correlations appears to be stable properties of anatomic connectivity. There is also evidence that coupling can be modulated by recent or ongoing task performance, suggesting that certain components of correlated dynamics are malleable on short timescales. Here, we compared activity during extended periods of rest following performance of 2 distinct cognitive tasks using different categories of visual stimuli—faces and complex scenes. Prolonged exposure to these distinct categories of visual information caused frontal networks to couple differentially with posterior category-preferential visual regions during subsequent periods of rest. In addition, we report preliminary evidence suggesting that conditions exist in which the degree of modulation of LF-BOLD correlations predicts subsequent memory. The finding that resting-state LF-BOLD correlations are modulated by recent experience in functionally specific brain regions engaged during prior task performance clarifies their role as a dynamic phenomenon which may be involved in mnemonic processes.
Keywords: fMRI, functional connectivity, intrinsic activity, network, resting-state
Introduction
Spontaneous intrinsic neural activity represents a significant part of the brain's activity dynamics (Fox and Raichle 2007) and has been studied extensively in both humans and animals across a broad range of temporal and spatial domains using various neuroimaging modalities (for review, see Buzsaki and Draguhn 2004). A particularly robust example of spontaneous activity involves the low-frequency (LF: <0.1 Hz) fluctuations that can be measured indirectly using blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI). Initially observed during passive “rest states” (Biswal et al. 1995), these LF-BOLD fluctuations are correlated among functionally related brain regions.
Increasingly over the last decade, there has been extensive study of spontaneous LF-BOLD correlations, including hundreds of articles in the past 5 years (Bandettini 2009), primarily using functional connectivity (FC) analysis, which refers to any analysis of interregional correlations of neural activity (Friston et al. 1993; McIntosh 1999, 2004; Horwitz 2003). FC analysis of LF-BOLD correlations is a useful technique for characterization of age-related and disease-related pathology (for review, see Greicius 2008; Bandettini 2009) and cross-species comparisons (Vincent et al. 2007; Shmuel and Leopold 2008; Margulies et al. 2009). A number of studies of LF-BOLD correlations have now identified a multitude of functionally dissociable “resting-state networks” (e.g., Damoiseaux et al. 2006; De Luca et al. 2006; Fox et al. 2006; Vincent et al. 2008).
However, despite the recent expansion of research on the topic, the nature and function of LF-BOLD correlations remains unclear (Fox and Raichle 2007; Bandettini 2009; Van Dijk et al. 2009). They persist during a variety of rest states (Fox et al. 2005, 2006; Fair et al. 2007), task states (Arfanakis et al. 2000; Greicius et al. 2004; Fransson 2006; Buckner et al. 2009), sleep (Fukunaga et al. 2006; Dang-Vu et al. 2008; Horovitz et al. 2008), under anesthesia (Kiviniemi et al. 2005; Peltier et al. 2005; Greicius et al. 2008), and during various other altered states of consciousness (Boly et al. 2008). The persistence of these correlation patterns across various states suggests that they reflect, in part, intrinsic properties of neuroanatomical networks. Furthermore, recent analysis of connectivity measured using diffusion-based magnetic resonance imaging (MRI) techniques suggests that LF-BOLD correlations are constrained by anatomic connectivity but are more pervasive, reflecting polysynaptic projections and common driving inputs (Greicius et al. 2009; Honey et al. 2009; for review, see Van Dijk et al. 2009).
However, LF-BOLD fluctuations also have dynamic components that modulate on timescales too brief to reflect anatomical modifications. These patterns of correlated activity likely reflect a combination of 1) intrinsic activity among anatomically connected brain systems and 2) experience-dependent fluctuations. In support of the latter notion, there is recent evidence that patterns of correlated activity are modulated by ongoing tasks (Fransson 2006; Fair et al. 2007; Buckner et al. 2009; Hasson et al. 2009; Wang et al. 2009) and recent experience (Waites et al. 2005; Albert et al. 2009; Hasson et al. 2009; Lewis et al. 2009). These results have raised the interesting possibility that correlated LF-BOLD fluctuations “support the off-line processing of past events for memory consolidation” (Miall and Robertson 2006). More generally, proposed functional roles include consolidation, maintenance of neuroanatomical networks, and preparation for future behavior (Miall and Robertson 2006; Buckner and Vincent 2007; Raichle and Snyder 2007).
Two lines of evidence would support the hypothesis that LF-BOLD correlations during rest are involved in learning and/or memory consolidation. First, performance of a given task should modulate the pattern of LF-BOLD correlations during subsequent rest periods, specifically within brain regions or networks that are functionally relevant to the task recently performed. Second, the magnitude of observed changes of the FC of these regions could predict subsequent behavior, such as performance on the same or a related task (e.g., improved motor performance, improved subsequent memory, etc.).
An early study that compared LF-BOLD correlations during prolonged (>5 min) periods of rest before and after a simple language task (orthographic lexical retrieval) showed differences in 6 individual subjects but very modest consistent effects at the group level, providing tentative evidence of increased FC within a purported language network following a language task (Waites et al. 2005). However, a more recent study argued that task induced changes in LF-BOLD correlations during subsequent rest occur exclusively following learning (Albert et al. 2009). Using probabilistic independent components analysis, Albert et al. (2009) identified 2 resting-state networks—a frontoparietal network and a cerebellar network, thought to be involved in motor performance—which showed increased component strength following a novel motor learning task but not following a simple motor performance task. The authors stressed that because changes in resting-state activity occurred only following the more difficult motor learning task, this modulation was attributable to learning per se. However, the possibility cannot be ruled out that the observed differences in resting-state activity could be attributed to the increased difficulty or attentional demand of the novel motor learning task relative to the simple motor performance task. Further, despite evident learning demonstrated by the participants during the motor learning task, analyses of potential brain-behavior correlations failed to show any relationship between LF-BOLD activity at rest and previous or subsequent task performance across a number of performance measures.
While the latter study provided evidence to support the hypothesis that LF-BOLD correlations may reflect learning, by satisfying the first of 2 lines of evidence outlined above, a direct link with measures of actual performance was not observed. Indeed, while recent evidence suggests that FC during task performance is related to individual differences in subsequent memory (Hasson et al. 2009) and intrinsic BOLD fluctuations interact with ongoing task performance (Fox et al. 2007), to date there is no direct evidence that modulation of LF-BOLD fluctuations during sustained periods of “rest” is predictive of subsequent memory or cognition (but see Lewis et al. 2009 for effects on perceptual learning). Further, the task that presumably modulated subsequent LF-BOLD correlations in the latter study (Hasson et al. 2009) was a passive listening task without any active learning component. Thus, it seems plausible that modulation of LF-BOLD correlations during rest may occur automatically within networks previously engaged during prior task performance, akin to a passive “echo” or “ripple effect” within recently coactivated brain regions. Given these competing hypotheses, questions that remain are 1) whether resting-state LF-BOLD correlations among brain regions engaged by a particular task can be modulated by simple prior task performance per se (Waites et al. 2005; Hasson et al. 2009), or whether these modulations are directly related to a prior learning episode (Albert et al. 2009; Lewis et al. 2009); and 2) to what extent modulations of LF-BOLD activity during rest might impact subsequent memory or cognition.
Here, we explored the possibility that LF-BOLD correlations during sustained periods of rest are modulated by recent experience in functionally relevant brain regions by varying the content of stimulus exposure and examining the dynamics of activity in subsequent rest states. We specifically probed the activity of functionally well-characterized brain regions known to be preferentially associated with processing of specific categories of visual stimuli (faces and complex scenes). We considered it to be an open question as to whether LF-BOLD correlations are involved in mnemonic processes, but we reasoned that if modulation of LF-BOLD correlations is directly related to learning, then this modulation should be related to subsequent performance or cognition in some way.
Exposure to particular categories of visual stimuli has long been known to differentially engage dissociable “category-preferential” regions within the human ventral visual cortex, for example, a scene-preferential (SP) region in bilateral parahippocampal cortex (PHC) for complex visual scenes (Epstein and Kanwisher 1998) and a face-preferential (FP) region in the right fusiform gyrus (FG) for faces (Kanwisher et al. 1997). Furthermore, processing these categories of visual stimuli involves functional interactions between nodes of distributed networks of brain regions, depending on the type of cognitive task being performed (Haxby et al. 2000; Epstein et al. 2007). Previous studies have shown that during a memory task involving faces and scenes, top-down modulation of category-preferential visual regions associated with memory performance involves not only the enhancement of “category-relevant” visual regions but also the simultaneous suppression of “category-irrelevant” visual regions (Gazzaley, Cooney, McEvoy, et al. 2005; Gazzaley, Cooney, Rissman, and D'Esposito 2005); this top-down modulation involves long-range functional interactions with prefrontal brain regions (Gazzaley et al. 2007). Interestingly, age-related memory deficits may be attributable to a failure to suppress activity in category-irrelevant visual regions, despite preserved enhancement of activity in category-relevant regions (Gazzaley, Cooney, Rissman, and D'Esposito 2005).
A significant body of research highlights the importance of long-range functional interactions between category-preferential visual regions and the right inferior frontal gyrus (rIFG) in particular. A recent study of patients suffering from congenital prosopagnosia showed that disruption of long-range structural connectivity between the rIFG and posterior FP visual cortex in the right FG underlies impaired face processing (Thomas et al. 2009). Indeed, evidence of an important role of the rIFG in processing of both faces and scenes across a range of cognitive tasks is abundant. For example, a number of fMRI studies have demonstrated that the rIFG is involved in both encoding and retrieval of nonverbal stimuli including faces and visuospatial scenes specifically (e.g., Wagner et al. 1998; Rajah et al. 1999; Golby et al. 2001; Wig et al. 2004). Thus, regions such as the rIFG that play an important role in both face and scene processing may be candidate regions likely to show modulation of LF-BOLD correlations with FP and SP visual regions during rest, depending on the stimulus category previously experienced.
We hypothesized that if intrinsic activity events captured by resting-state LF-BOLD fluctuations play a role in memory consolidation or preparation for future behavior, then recent stimulus exposure should modulate patterns of LF-BOLD correlations among functionally relevant brain regions during subsequent rest periods. Furthermore, if modulation of LF-BOLD correlations is related to learning per se, rather than being an automatic or passive consequence of previous task performance, then variation in these fluctuations during rest should predict subsequent memory performance. To test our hypotheses, we used FC analyses to compare fMRI LF-BOLD fluctuations (<0.08 Hz) of category-preferential visual brain regions (FP and SP regions) during sustained periods of rest following 2 distinct cognitive tasks using different categories of visual stimuli—faces and complex scenes. We then assessed the degree to which the magnitude of LF-BOLD modulation predicted subsequent memory for these visual images.
Materials and Methods
Participants
Participants were 34 healthy, right-handed, young adults (mean age ± standard deviation [SD] = 24.6 ± 3.9; range = 18–33; 16 females/18 males), with normal or corrected-to-normal visual acuity and no history of psychiatric, neurological, or other medical illness or history of drug or alcohol abuse that might compromise cognitive functions. All participants were paid for their participation and gave written informed consent prior to participation, in accordance with the guidelines of the institutional review board of Harvard University and the Human Subjects Research Committee at Massachusetts General Hospital. Of the 34 participants that underwent fMRI scanning in this study, 4 were excluded from the analyses presented here due to excessive movement during fMRI scanning. The data and analyses reported here include 30 participants (mean age ± SD = 24.3 ± 3.9; range = 18–33; 14 females/16 males).
Behavioral Tasks
The 2 distinct cognitive tasks that preceded the rest periods of interest involved semantic classification of faces and scenes in a repetition priming paradigm, the results of which are beyond the scope of the current paper and will be reported separately. A full description of the tasks and stimuli used is provided in the Supplementary methods and depicted in Supplementary Figures 1–3. Briefly, face stimuli were grayscale images of faces, half were female and half were male (Endl et al. 1998; Jaeger et al. 2005). Scene stimuli were grayscale images of outdoor scenes, half were “man-made” (i.e., scene contained at least one man-made object) and half were “nature” (i.e., scene contained no man-made objects). Each participant first performed either a face classification task (“male” vs. “female”) or a scene classification task (nature vs. man-made) for the duration of 4 consecutive event-related runs (total duration >15 min), followed by 2 consecutive rest runs (total duration >9 min). Immediately preceding each of the rest runs was a brief “primer” run (duration = 20 s), wherein participants were presented with an additional series of 10 novel faces or scenes (consistent with the current task stimulus category); the same semantic classification was performed. Due to the long combined duration of 2 consecutive rest runs (i.e., >9 min), the purpose of these short primer runs was to ensure that participants had been exposed to the relevant category of stimuli with a moderate degree of recency (i.e., within ∼4.7 min). The intent was to maximize the likelihood that LF-BOLD fluctuations could be modulated by the recent task (see Supplementary methods; Supplementary Fig. 3). During rest runs, participants were instructed to fixate on a centrally located crosshair but were not otherwise directed to engage in any particular cognitive activity. This sequence of task runs followed by rest runs was then repeated with the other task; order of task presentation was counterbalanced across participants. The rationale for the tasks that preceded the rest periods was to maximize the duration and number of repeated exposures to images of a single category of visual stimuli in an attempt to increase the likelihood of modulating subsequent resting-state LF-BOLD fluctuations.
At the end of the experiment, participants were scanned during 2 blocked runs of alternating face and scene blocks that served as an independent task-based functional localizer that allowed us to map individual FP and SP regions within the ventral visual cortex of each participant. During the face and scene trials of the localizer runs, participants performed an incidental recognition memory task that allowed us to assess subsequent memory performance for the stimuli previously viewed in the classification tasks. Due to the rapid presentation of trials inherent in the block design, neural responses to individual trials could not be estimated, and thus, analyses of BOLD activity during these runs were restricted to a block analysis of overall responses to the face and scene blocks for localization purposes. Task-based functional localizers typically involve performance of some type of cognitive task to ensure vigilance (Epstein and Kanwisher 1998; Kanwisher et al. 1998), but due to the statistical power and efficiency of block contrasts (Friston et al. 1999), this has negligible bearing on the main contrast of stimulus category, and thus, in no way does this compromise the independence of these runs as functional localizers (Friston et al. 2006). The localizer runs consisted of alternating “recognition blocks” of face and scene recognition tasks, interleaved with short “fixation blocks” (i.e., presentation of a centrally located crosshair for the duration of the 20-s block). Each recognition block contained 20 successive face or scene trials (2500 ms: 800-ms face or scene presentation; 1700-ms interstimulus fixation crosshair), consisting of 10 novel pictures and 10 repeated pictures from the previous face or scene tasks, presented in random order. Half of the blocks were “easy recognition” blocks, wherein repeated pictures had been viewed 4 times previously in the face and scene tasks; half of the blocks were “difficult recognition” blocks, wherein the repeated pictures had only been viewed once previously in the face and scene tasks. For each picture, participants were required to make an “old/new” judgment.
Stimuli were presented to participants using Presentation software (Neurobehavioral Systems Inc., Albany, CA) run on a Dell Latitude D820 laptop computer, projected onto a screen positioned at the head of the MRI scanner bore using JVC (model SX21/s) D-ILA projector, and viewed by participants via a mirror attached to the head coil.
fMRI Scanning
Participants were scanned at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital (Charlestown, MA) using a 3-T Siemens Magnetom TimTrio Scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a 12-channel phased-array whole-head coil. All participants were fitted with MRI-compatible corrective lenses (zero-correction control lenses were worn by participants with normal vision). Cushions and clamps were used to minimize head movement during scanning.
Anatomical images were acquired using a high-resolution 3D magnetization prepared rapid gradient echo sequence (MPRAGE: 128 sagittal slices; time repetition [TR] = 2530 ms; time echo [TE] = 3.45 ms; flip angle = 7°; voxel size = 1 × 1 × 1.33 mm). Functional images for the rest and the localizer/recognition runs were collected in 6 runs using T2* gradient echo, echo planar imaging sensitive BOLD contrast (TR = 2500 ms; TE = 30 ms; flip angle = 90°; voxel size = 4 × 4 × 4 mm). Sets of 112 (4 runs) and 138 (2 runs) volumes for each of the rest and localizer runs, respectively, were acquired axially, aligned parallel to the anterior commisure-posterior commisure plane in 36 slices, yielding whole-brain coverage.
Data Analysis
Recognition Performance
To assess memory for faces and scenes, recognition accuracy (proportion hits – proportion false alarms) was calculated separately for faces and scenes in easy recognition blocks, difficult recognition blocks, and all blocks collapsed across difficulty, for each participant. A 2 × 2 repeated-measures analysis of variance (ANOVA) was conducted with stimulus category (face vs. scene) and difficulty (easy vs. difficult) as within-subjects factors. Significant main effects were followed up with planned paired samples t-tests.
fMRI Preprocessing
All fMRI data were preprocessed using a combination of procedures using both FSL (FMRIB) and SPM2 (Friston et al. 1995; Wellcome Department of Imaging Neuroscience) tools. The first 4 volumes (10 s) in each run were excluded from analyses to allow for T1-equilibration effects. Data were corrected for slice-dependent time shifts for each whole-brain volume (SPM2) and for head motion within and across runs using a rigid body correction (Jenkinson et al. 2002; FMRIB). Motion parameters generated in the latter process were used later as nuisance regressors in the general linear model (GLM) and correlation analyses. Images were then normalized to a standard anatomical atlas space by first computing affine transforms connecting the first image volume of the first functional run with the T1-weighted structural images (Jenkinson and Smith 2001; FMRIB). Our atlas representative template includes MPRAGE data from 12 normal individuals and was made to conform to the Montreal Neurological Institute (MNI) template using previously described methods (Buckner et al. 2004). The final preprocessing step combined motion correction and atlas transformation in one step to yield a motion-corrected volumetric time series sampled at 2-mm cubic voxels, as previously described (Kahn et al. 2008).
Task-Based Functional Localizer
After preprocessing, fMRI images from the localizer runs were spatially smoothed with a Gaussian kernel with a full width at half maximum (FWHM) of 6 mm. The fMRI data were then analyzed using the GLM in SPM2. Variables of noninterest were entered as regressors into the GLM, including run means, linear trends to account for LF noise (e.g., scanner drift), and 6 movement parameters obtained from the previous motion correction procedure. The face and scene blocks were modeled separately with a box-car function convolved with the SPM2 canonical homodynamic response functions, with the onset and offset points coinciding with the beginning and end of each task block, and entered as regressors of interest into the GLM.
To identify a category-preferential brain region of interest (ROI) for both faces and scenes in ventral visual cortex, whole-brain voxel-wise statistical parametric maps were computed for each participant for the contrasts face > scene and scene > face for FP and SP regions, respectively. Definition of the FP- and SP-ROIs was restricted to the right hemisphere to control for potential differences in laterality and facilitate comparisons with the FP-ROI, which was most consistently right lateralized across participants. A FP-ROI was defined individually for each participant as all voxels for which activity exceeded a given threshold (t = 2.76, P < 0.005) within an approximate sphere with a radius of 8 mm centered on the peak activated voxel in the right FG in the contrast face > scene. A SP-ROI was defined for each participant in the same manner but centered on the peak activated voxel within the right PHC in the contrast scenes > faces. The relatively liberal threshold (i.e., t = 2.76, P < 0.005) was used in order to include as many face- and scene-responsive voxels in the FP- and SP-ROIs, respectively; when subsequently using these a priori ROIs as seed regions for FC analyses, this yields a more conservative approach than if a higher threshold were used to define the ROIs (e.g., using only the peak activated voxel).
To identify brain regions involved in face processing and scene processing per se across participants, statistical parametric maps for the contrasts faces > fixation and scenes > fixation were calculated, then used in conventional random-effects analyses to assess voxel-wise statistical significance at the group level.
FC Analyses
After preprocessing, the fMRI data from the rest runs were subjected to additional processing steps, as described previously (Fox et al. 2005; Vincent et al. 2006; Van Dijk et al. 2009), prior to FC analysis of LF-BOLD fluctuations. First, a temporal band-pass filter was applied to the atlas-aligned BOLD data, retaining signal within the frequency range of 0.009–0.08 Hz. Data were then spatially smoothed with a Gaussian kernel (FWHM = 6 mm). Then, sources of variance of noninterest were removed from the data by regression of nuisance variables (in addition to first temporal derivatives of each), including: the 6 motion parameters obtained during the motion correction procedure; the mean whole-brain signal; the mean signal from the lateral ventricles; and the mean signal from a region within the deep cerebral white matter.
FC analysis typically involves creation of a ROI centered on a given coordinate that is used as a common seed region across multiple participants. Here, in order to account for individual variability and increase specificity, we used the individually defined FP-ROI and SP-ROI from the independent localizer as seed regions for the FC analyses in each participant. To identify patterns of LF-BOLD fluctuations correlated with the FP- and SP-ROIs, the BOLD data were collapsed across all rest runs. For each participant, the mean BOLD signal time course was extracted from the FP- and SP-ROIs, and the correlation coefficient for each of these time courses with the time course for every voxel in the brain was computed separately using Pearson's product-moment formula. These values were then converted to z values using Fisher's r-to-z transformation (Zar 1996). Whole-brain voxel-wise z-maps were then subjected to random-effects analyses to asses statistical significance across participants at the group level using t-tests performed in SPM2.
To identify correlated activity specific to the FP-ROI, a FP-map was defined for each participant as the t-map contrast FP > SP; likewise, to identify correlated activity specific to the SP-ROI, a SP-map was defined as the opposite contrast SP > FP. With participant as a random factor, group t-maps (threshold: t > 3.4, P < 0.001) were created to define the group FP-map and SP-map.
To determine whether correlated activity with the FP- and SP-ROIs was modulated by recent cognitive experience (i.e., category of stimuli in the preceding task), “face–rest” (i.e., rest runs following the face task; 2 concatenated runs) and “scene–rest” (i.e., rest runs following the scene task; 2 concatenated runs) were analyzed separately. For each participant, the “difference-map” defined by the contrast FP > SP was calculated separately during face–rest and scene–rest. We predicted that there may be brain regions involved in both face and scene processing (e.g., rIFG) that would show modulation of LF-BOLD correlations with the FP- and SP-ROIs depending on the category of stimuli in the previous task. Therefore, to identify any region(s) in the brain showing such differential modulation, we calculated a whole-brain “difference of differences” map (i.e., task × ROI interaction map) defined by the contrast:
(FP − SP during face–rest) − (FP − SP during scene–rest).
This interaction contrast directly tested the prediction that there would be brain regions showing increased correlations with the FP visual region in the right FG following a prolonged period of face processing relative to scene processing; and conversely, increased correlations with the SP visual region in PHC following a prolonged period of scene processing relative to face processing.
To characterize the nature of the interaction identified by this contrast in the rIFG (task × ROI: t > 3.4, P < 0.001, cluster size = 113 contiguous voxels; MNI coordinates: x = 48, y = 36, z = 4. See Results), the mean correlation z value of this region was calculated for each of the 4 conditions (task: face–rest vs. scene–rest × ROI: FP vs. SP). Statistical significance of the simple main effect of task for both the FP-ROI and the SP-ROI was assessed using paired samples t-tests.
Brain–Behavior Correlations
Finally, to assess the degree to which modulation of LF-BOLD correlations by the preceding task might be correlated with subsequent memory performance, the following procedures were carried out. Because top-down modulation of category-preferential visual regions associated with memory performance involves the simultaneous enhancement of category-relevant visual regions and suppression of category-irrelevant visual regions (Gazzaley, Cooney, McEvoy, et al. 2005; Gazzaley, Cooney, Rissman, and D'Esposito 2005), we tested whether the strikingly similar pattern seen here during subsequent rest periods would predict subsequent memory. Thus, we correlated the magnitude of the task by ROI interaction in the rIFG with subsequent recognition performance across participants. Because definition of all ROIs in this study was completely independent of any measure of behavior (i.e., priming or subsequent memory performance), this correlation analysis did not in any way confound definition of the rIFG ROI (i.e., region showing a significant task [face–rest vs. scene–rest] by FC [FP-ROI vs. SP-ROI] interaction) with the variable it was correlated with (i.e., subsequent memory). Thus, the logical fallacy of “nonindependence,” which is unfortunately ubiquitous among fMRI studies reporting ROI/brain–behavior correlation analyses does not apply to the analyses reported here. To apply a more statistically conservative assessment of a potential correlation between the FC of the rIFG region and subsequent memory performance (see ROI definition above), an interaction ROI was defined as a larger cluster that included all voxels exceeding the statistical threshold of t > 2.76, P < 0.005 in the task × ROI interaction contrast described above. Then, the mean magnitude of the task × ROI interaction within this ROI was calculated for each participant individually. Finally, the magnitude of the interaction in this region was correlated with subsequent face and scene recognition accuracy (proportion hits − proportion false alarms) across participants.
For all figures, fMRI data are displayed projected onto the partially inflated cortical surface (population average landmark surface: PALS-B12) using Caret software (Van Essen 2005).
Results
Recognition Performance
To assess memory for faces and scenes, recognition accuracy (proportion hits − proportion false alarms) was calculated separately for faces and scenes in the easy and difficult recognition blocks (Supplementary Fig. 4A). A 2 × 2 repeated-measures ANOVA with stimulus category (face vs. scene) and difficulty (easy vs. difficult) as within-subjects factors revealed significant main effects of stimulus category (F = 40.4, P < 0.001), and as expected, difficulty (F = 213.3, P < 0.001), with higher recognition accuracy for scenes than for faces and for easy recognition blocks than for difficult recognition blocks. There was no significant stimulus category by difficulty interaction (F = 1.75, P = 0.2).
Localizer Analyses
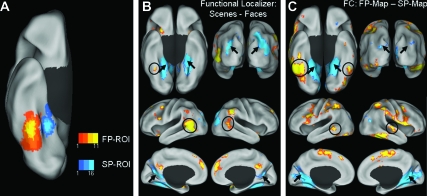
As expected, there was a considerable degree of variability in the spatial location and extent of the FP- and SP-ROIs across participants, but there was little or no overlap between FP- and SP-ROIs either within or across participants (Fig. 1A). The spatial variability of the FP- and SP-ROIs highlights the potential advantage of using individually defined ROIs as seed regions for the FC analyses. In addition to the anticipated FP and SP regions in the right FG and PHC, respectively, a number of other brain regions typically associated with face and scene processing were identified by the localizer contrasts as well (Fig. 1B).
Figure 1.
Comparison of task-based functional localizer activity and FC maps. (A) Overlap of the FP- and SP-ROIs for all participants (n = 30) overlaid on the inflated right ventral cortical surface. (B) Group difference-maps for FP (yellow/orange scale) and SP (blue scale) regions defined by the contrast faces – scenes in the task-based functional localizer, overlaid on the inflated bilateral ventral (top left), posterior (top right), lateral (middle), and medial (bottom) cortical surfaces. (C) Group difference-maps for LF-BOLD correlations collapsed across all rest runs, specific to the FP-ROI (yellow/orange scale) and SP-ROI (blue scale) defined by the contrast FP-map – SP-map, overlaid on inflated cortical surfaces as in panel B. The functional localizer maps and FC difference-maps show similarities between the pattern of activation and correlated activity, respectively. Arrows indicate regions associated with scene processing: bilateral PHC, transverse occipital sulcus, and retrosplenial cortex, on the ventral, posterior, and medial surfaces, respectively. Circles indicate regions associated with face processing: FG and lateral temporal cortex, on the ventral and lateral surfaces, respectively.
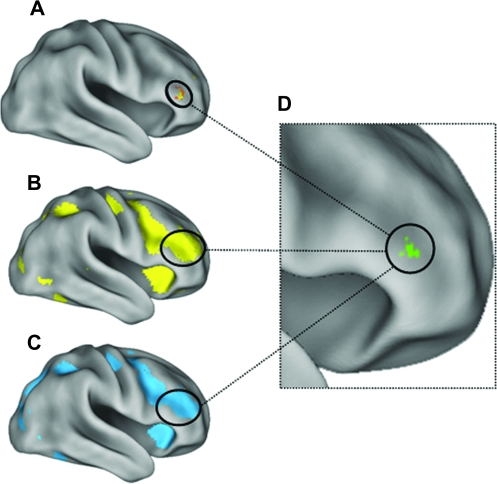
In addition to regions showing preferential activation for either faces or scenes, localizer task contrasts also allowed us to identify common brain regions involved in processing of both faces and scenes. Importantly, the main effect contrasts for face processing (face > fixation; Fig. 3B) and scene processing (scene > fixation; Fig. 3C) revealed a common region within the rIFG showing a strikingly similar pattern of activation, both in spatial location (coordinates of peak activation within 2 mm) and extent. Regions such as the rIFG, showing similar involvement in both face and scene processing, were potential candidate regions that may show modulation of LF-BOLD correlations with FP and SP visual regions by the task stimulus category manipulation. (N.B. we did not use these contrasts to define any ROIs.)
Figure 3.
Both face and scene processing engage the rIFG during both task performance and subsequent rest. Region showing the task by ROI interaction in rIFG (A) overlapped peak activation for the task-based localizer contrast for faces (B: faces > fixation) and for scenes (C: scenes > fixation). (D) Conjunction of activation for all 3 contrasts, each contrast thresholded at: t > 2.76, P < 0.005. All activation maps displayed at a threshold of: t > 2.76, P < 0.005.
FC Analyses
The “difference-maps,” showing correlated activity patterns specific to each of the category-preferential ROIs, were created for each participant by contrasting the correlation map for the FP- and SP-ROIs, collapsed across all rest runs (“FP-map” = FP > SP; “SP-map” = SP > FP). The group FP-map and SP-map were functionally specific, showing similarities to the networks of regions typically identified in task-based functional localizers for face and scene processing, respectively (Kanwisher et al. 1997; Epstein and Kanwisher 1998; Haxby et al. 2000; Epstein et al. 2007; Fig. 1C).
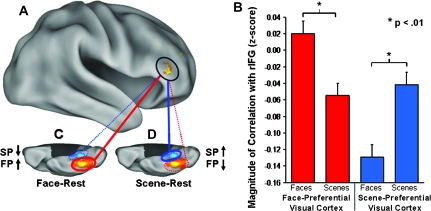
The interaction contrast ([FP − SP during face–rest] > [FP − SP during scene–rest]) was designed to identify any brain region(s) showing increased correlations with the FP visual region in the right FG following a prolonged period of face processing relative to scene processing; and conversely, increased correlations with the SP visual region in PHC following a prolonged period of scene processing relative to face processing. Consistent with our prediction, this contrast revealed a significant task by ROI interaction in a sizeable region within the rIFG (Fig. 2A: Brodmann area 45/47; t > 3.4, P < 0.001, cluster size k = 113 contiguous voxels; MNI coordinates of the peak activated voxel: x = 48, y = 36, z = 4), indicating that the correlation of activity in this region with the FP- and SP-ROIs during rest was differentially modulated depending on the preceding task performed. Importantly, the rIFG region showing the interaction between ROI and task (Fig. 3A) overlapped the region that showed common activation for both face (Fig. 3B) and scene (Fig. 3C) processing identified in the localizer contrasts, encompassing the peak voxels from both contrasts (Fig. 3D).
Figure 2.
LF-BOLD correlations during rest were modulated by previous task. (A) Region in the rIFG (circled) showing a task by ROI interaction for correlations with the FP- and SP-ROIs (t > 3.4, P < 0.001, 113 contiguous voxels; peak activation in MNI coordinates: x = 48, y = 36, z = 4; activation displayed at a threshold of: t > 2.76, P < 0.005). (B) Paired samples comparisons revealed a significant simple effect of task on correlations between rIFG and both the FP- and SP-ROIs (FP-ROI t = 3.0, P < 0.01, 2-tailed; SP-ROI t = −3.3, P < 0.01, 2-tailed). (C) Correlations with the FP-ROI increased during face–rest relative to scene–rest; (D) Correlations with the SP-ROI increased during scene-rest relative to face-rest. (C, D) The orange/yellow scale and blue-scale ROIs overlaid on the right ventral cortical surface images show the degree of overlap of all FP- and SP-ROIs, respectively, for all participants (n = 30); red and blue lines indicate correlation with FP-ROI and SP-ROI, respectively; solid lines and dotted lines indicate increased correlations and decreased correlations, respectively. Error bars represent standard error of the mean calculated for within-subjects design.
To determine the nature of this interaction in the rIFG, we extracted the mean correlation values from the region for paired samples comparisons, which revealed a double dissociation (cross-over interaction) between ROI and task (Fig. 2B). LF-BOLD correlation between the rIFG and the FP-ROI increased during face–rest relative to scene–rest (Fig. 2C: t = 3.0, P < 0.01, 2-tailed); and conversely, LF-BOLD correlation with the SP-ROI increased during scene–rest relative to face–rest (Fig. 2D: t = −3.3, P < 0.01, 2-tailed). It is important to note that regression of the global signal during FC analysis results in rescaling of the correlation values to a mean of approximately zero (Murphy et al. 2009). Thus, negative correlation values are not interpretable, and instead, the relative difference (increase vs. decrease) in correlation values between rest conditions (face–rest vs. scene–rest) for each ROI (FP vs. SP) is the relevant measure of interest.
Brain–Behavior Correlations
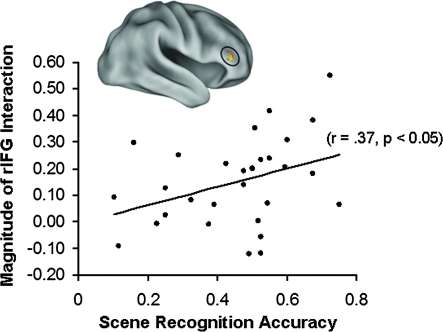
We tested the possibility that modulation of LF-BOLD correlations by previous task would predict subsequent memory. There was a significant correlation between the magnitude of the interaction in the rIFG and subsequent scene recognition (r = 0.37, P < 0.05, 2-tailed; Fig. 4). Further investigation revealed that this correlation was driven primarily by a stronger correlation with recognition performance during the easy recognition scene blocks (r = 0.43, P < 0.05, 2-tailed; Supplementary Fig. 4B); the correlation was not significant for the difficult recognition scene blocks or for face recognition.
Figure 4.
Coupling of LF-BOLD fluctuations in the rIFG with category-preferential visual regions during rest predicts subsequent memory. Across all participants (n = 30), the magnitude of the interaction in rIFG was significantly correlated with subsequent recognition accuracy for scenes (r = 0.37, P < 0.05, 2-tailed).
Discussion
The results of the current study support the hypothesis that certain components of correlated LF-BOLD fluctuations during rest play a role in learning. There was also evidence suggesting that this could reflect consolidation of recent cognitive experience. The first line of evidence outlined previously was satisfied by showing that LF-BOLD correlations during rest are modulated by previous cognitive experience in functionally specific brain regions. Furthermore, to the extent that modulation of LF-BOLD activity during rest was correlated with subsequent memory, our results provide some support for the second line of evidence that these modulations of resting-state activity could predict subsequent behavior and/or cognitive performance. However, these latter findings are preliminary. Taken together, our results indicate that intrinsic activity in the resting human brain has a dynamic component with possible relevance to mnemonic processes, thereby converging with recent findings that BOLD activity during rest is associated with motor and perceptual learning (Albert et al. 2009; Lewis et al. 2009), and individual differences in memory performance (Wig et al. 2008; Hasson et al. 2009).
It is important to reiterate that patterns of LF-BOLD fluctuations have stable components that persist across a broad range of task states and levels of consciousness (for discussion, see Fox and Raichle 2007; Van Dijk et al. 2009). Thus, the majority of variance in these patterns of correlated activity is unlikely to be a consequence of cognitive operations but rather reflects spontaneous intrinsic activity within neuroanatomical networks (for recent empirical support, see Greicius et al. 2009; Honey et al. 2009). However, studies showing modulation of LF-BOLD correlations related to recent or ongoing tasks (Waites et al. 2005; Fransson 2006; Fair et al. 2007; Albert et al. 2009; Buckner et al. 2009; Hasson et al. 2009; Lewis et al. 2009; Wang et al. 2009) demonstrate that some portion of this resting-state activity is malleable on a shorter timescale. The present results suggest that this latter portion of resting-state activity may contribute to learning or memory consolidation. Furthermore, Fox et al. (2007) provide evidence of a potential mechanism by which alterations in LF-BOLD correlations over time might also modulate ongoing task performance. In the foregoing study, spontaneous intrinsic BOLD fluctuations in motor regions accounted for a portion of intertrial variability in performance on a simple motor task. We suggest that such a mechanism might underlie an association between modulation of LF-BOLD fluctuations and learning or subsequent cognition as well.
We compared activity during sustained periods of rest that followed prolonged performance of 2 distinct cognitive tasks using different categories of visual stimuli—faces and complex scenes. LF-BOLD fluctuations in a frontal brain region (rIFG), shown to be involved during cognitive processing of both faces and scenes in an independent task, showed a double dissociation: this region transiently demonstrated increased coupling with a SP region in the right PHC following exposure to scenes; and conversely, increased coupling with a FP region in the right FG following exposure to faces. Furthermore, the degree to which rIFG coupled selectively to these posterior cortical systems during rest predicted subsequent memory performance, though not consistently across conditions. Contrasts from an independent task-based functional localizer revealed that this frontal region was also maximally activated for both face and scene processing per se during task performance (Fig. 3B,C).
Our finding of an important role of the rIFG in cognitive processing of both faces and scenes is consistent with a number of previous fMRI studies (e.g., Wagner et al. 1998; Rajah et al. 1999; Golby et al. 2001; Wig et al. 2004; Thomas et al. 2009) but provides novel evidence that its functional role is not limited to the temporal window of engagement in task performance. Instead, increased FC of this rIFG region with posterior visual brain regions persisted for at least several minutes after participants disengaged from the task, as measured by fMRI LF-BOLD activity.
While researchers have speculated for some time that LF-BOLD fluctuations during rest may play some functional role in learning, memory, or future behavior/cognition, only recently has it been demonstrated that resting-state activity is reliably affected by recent experience in a systematic way (Albert et al. 2009; Hasson et al. 2009; Lewis et al. 2009). These studies showed modulation of FC within large-scale brain networks following motor and perceptual learning tasks and a passive listening task. Our results bolster these recent observations and extend our understanding of the phenomenon by demonstrating robust modulation of LF-BOLD correlations of posterior visual brain regions that have been thoroughly studied, functionally well characterized, and are known to be preferentially recruited for processing different categories of visual stimuli.
While the results of these studies provide indirect support for the notion that LF-BOLD activity during rest may be associated with subsequent memory, a direct link remains elusive. Albert et al. (2009) showed that modulation of 2 motor learning-related networks occurred only following a motor learning task but not a trivial motor performance task, arguing that this established the link between modulation of LF-BOLD correlations and learning. Contrastingly though, Hasson et al. (2009) reported modulation of FC among default network regions during rest depending on a previous passive listening task, with no explicit task demands and no explicit learning component (see also Waites et al. 2005). However, it is important to note that “resting state” was defined in a very different way in the latter study—that is, short intermittent “rest trials” (duration = 16 s) that were interleaved with task trials were defined as rest. This paradigm may have involved different neural dynamics than studies examining sustained periods of rest, such as the present study (see also the Albert et al. 2009; Lewis et al. 2009). Nonetheless, the semantic classification task of the current study also contained no explicit learning component, as participants were only required to perform the trivial tasks of classifying faces by gender and scenes by content (i.e., containing man-made objects or not). Yet, we observed robust modulation of LF-BOLD coupling between a rIFG region and category-preferential posterior visual regions, mirroring the activity dynamics of brain regions known to be engaged during task performance.
The current results suggest that modulation of LF-BOLD correlations during rest is not exclusively a direct consequence of a prior explicit learning episode per se. Rather, it appears that even relatively passive cognitive engagement, or mere exposure to different categories of perceptual material, can modulate patterns of resting-state activity. However, this observation does not rule out the possibility that modulation of LF-BOLD activity is related to learning. On the contrary, our finding that LF-BOLD coupling between the rIFG and posterior visual regions predicted subsequent memory performance for scenes provides preliminary evidence of a potential role of resting-state activity in memory consolidation or preparation for future behavior. While we find the latter brain–behavior correlation to be intriguing, it should be interpreted with caution; future research is needed to demonstrate a definitive link between resting-state activity and subsequent memory. While there was a significant correlation between the magnitude of selective coupling of the rIFG with posterior category-preferential visual cortex and subsequent scene recognition accuracy across participants, this correlation was driven primarily by an even higher correlation with recognition accuracy in the easy recognition scene blocks. Conversely, the correlations between rIFG–visual cortex coupling and recognition accuracy in the difficult recognition scene blocks or for faces were not significant. This pattern could be explained by the fact that performance for face recognition was much lower than for scene recognition overall. Moreover, even in the easy recognition face blocks, recognition accuracy was not significantly different from performance during the difficult recognition scene blocks (t = 1.3, P = 0.2; Supplementary Fig. 3A). While it is tempting to conclude that the brain–behavior correlation was significant only for the easy recognition blocks due to increased learning in this condition, as indicated by higher subsequent recognition accuracy, further research will be required to substantiate this link.
In conclusion, a number of recent papers have now demonstrated that LF-BOLD fluctuations during rest are modulated by previous tasks within networks engaged during task performance (Waites et al. 2005; Albert et al. 2009; Hasson et al. 2009; Lewis et al. 2009); some providing support for the suggestion that resting-state activity may be involved in some forms of consolidation (e.g., Albert et al. 2009; Lewis et al. 2009). The current results extend these findings by demonstrating that LF-BOLD fluctuations during sustained periods of rest are modulated by recent cognitive experience with no explicit learning component, such as a simple classification task or mere exposure to different categories of visual stimuli. Furthermore, we showed that this modulation is specific to functionally relevant brain regions, in this case, regions known to be preferentially involved in processing of different categories of visual stimuli. Finally, we found preliminary evidence that the degree of this modulation predicts subsequent cognitive performance. Taken together, these findings extend our understanding of the dynamic nature of LF-BOLD fluctuations in the resting human brain and the role it plays in learning and subsequent cognition. Future studies that probe other cognitive domains and brain regions are needed to clarify the extent, temporal dynamics, and nature of interactions between correlated activity fluctuations in the resting brain and a range of cognitive functions.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (MH060941 to D.L.S.); Howard Hughes Medical Institute and a National Institutes of Health (AG021910 to R.L.B.).
Supplementary Material
Acknowledgments
We thank J.R. Andrews-Hanna, K.M. Visscher, and G.S. Wig for technical assistance and helpful discussion of analyses and results; I. Kahn and T. Talukdar for assistance with data analyses; and A. Gilmore for assistance in preparation of the manuscript. Conflict of Interest: None declared.
References
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18:921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- Bandettini PA. What's new in neuroimaging methods? Ann NY Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Peigneux P, Faymonville ME, Maquet P, et al. Consciousness and cerebral baseline activity fluctuations. Hum Brain Mapp. 2008;29:868–874. doi: 10.1002/hbm.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts ARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci USA. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Endl W, Walla P, Lindinger G, Lalouschek W, Barth FG, Deecke L, Lang W. Early cortical activation indicates preparation for retrieval of memory for faces: an event-related potential study. Neurosci Lett. 1998;240:58–60. doi: 10.1016/s0304-3940(97)00920-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM. Visual scene processing in familiar and unfamiliar environments. J Neurophysiol. 2007;97:3670–3683. doi: 10.1152/jn.00003.2007. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principle component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. Neuroimage. 2006;30:1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu R, Deckers RH, Leopold DA, Duyn JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney JW, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17:i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nat Neuroscience. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci USA. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Jaeger T, Seile KH, Mecklinger A. Picture database of morphed faces: technical report. Psydok online [Internet] 2005. Available at http://psydok.sulb.uni-saarland.de/volltexte/2005/505/. Accessed 9 December 2009. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Tong F, Nakayama K. The effect of face inversion on the human fusiform face area. Cognition. 1998;68:B1–B11. doi: 10.1016/s0010-0277(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpaa H, Kantola JH, Jauhiainen J, Vainionpaa V, Alahuhta S, Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23:531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri A, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0905314106. Advance Access published November 10, doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics. 2004;2:175–182. doi: 10.1385/NI:2:2:175. [DOI] [PubMed] [Google Scholar]
- Miall RC, Robertson EM. Functional imaging: is the resting brain resting? Curr Biol. 2006;16:R998–R1000. doi: 10.1016/j.cub.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR, Grady CL. Frontotemporal interactions in face encoding and recognition. Brain Res Cogn Brain Res. 1999;8:259–269. doi: 10.1016/s0926-6410(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung KJ, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2009 doi: 10.1152/jn.00783.2009. Advance Access published November 4, doi:10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge L, Desmond JE, Glover GH, Gabrieli JDE. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yu C, Xu L, Qin W, Li K, Jiang T. Offline memory reprocessing: involvement of the brain's default network in spontaneous thought processes. PLoS One. 2009;4:e4867. doi: 10.1371/journal.pone.0004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM. Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Natl Acad Sci USA. 2008;105:18555–18560. doi: 10.1073/pnas.0804546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Miller MB, Kingstone A, Kelley WM. Separable routes to human memory formation: dissociating task and material contributions in the prefrontal cortex. J Cogn Neurosci. 2004;16:139–148. doi: 10.1162/089892904322755629. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River (NJ): Prentice Hall; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.