Abstract
Background
The need for lifelong, daily insulin injections can have a dramatic effect on patient compliance, can be painful, and runs the risk of local infections. Furthermore, needle-stick injuries are common, and the issue of needle disposal is troublesome. Injecting a long-acting insulin analog with needle-free administration would be a significant improvement for diabetic subjects, but is not currently feasible. To achieve a constant, reliable delivery of a novel, long-acting insulin analog, Lipoxen's SuliXen® (polysialylated insulin) in a solid dosage form capable of being delivered without a needle has been developed. The aim of this study was to evaluate the feasibility of Lipoxen's SuliXen delivery with the Glide solid dose injector, Glide SDI®.
Materials and Methods
A formulation containing 14 kDa polysialic acid (PSA)-recombinant human insulin conjugate was manufactured at Lipoxen PLC and transferred to Glide Pharma. The PSA–insulin conjugate solution was incorporated into different excipients at Glide Pharma (excipients 1 and 2), and formulations were manufactured containing implants with doses of 0.3 and 1.0 IU of insulin, respectively. Two different polymeric excipients were investigated for their suitable release profiles. The physicomechanical properties of the formulations were characterized in terms of solid dosage form strength (via three-point bend and compression) and disintegration time at 37°C. A preclinical efficacy study was performed in a nondiabetic rat model (Sprague-Dawley).
Results
The study demonstrated successful incorporation of PSA–insulin conjugate into formulations compatible for use with the solid dose injector. Physicochemical characterization indicated that each formulation produced was physically robust. For excipient 1, the compressive stress and three-point-bend-test values recorded for the 0.3 IU formulation were 106.99 ± 14.3 MPa and 30.6 ± 1.4 N (force in newtons), respectively. Corresponding values for the 1.0 IU dose were 53.10 ± 10.2 MPa and 16.66 ± 1.0 N. For excipient 2, the compressive stress and three-point-bend-test values recorded for the 0.3 IU dose were 53.10 ± 10.2 MPa and 7.64 ± 0.9 N, respectively, whereas the corresponding values recorded for the 1.0 IU dose were 41.61 ± 7.4 MPa and 13.18 ± 1.3 N. Each formulation successfully penetrated a laboratory substrate, achieving 100% penetration in each case. In vivo analysis demonstrated that PSA–insulin conjugate shows prolongation of activity (at least two-fold more compared to insulin) for more than 5 hours in the rat model.
Conclusion
Even though additional work may be required, for example, to develop several fixed dose formulations, the preliminary results show that solid dosage forms incorporating PSA–insulin conjugate maintained the prolongation of PSA–insulin conjugate activity in the rat model. Convenient and easy to use, the solid dose injector will not only ensure diabetic patient compliance and trust but also provide cost-effective solutions for safe, reliable, and controlled needle-free injection of PSA–insulin conjugate.
Keywords: colominic acid, diabetes, needle-free injection, polysialic acid, polysialic acid insulin, solid dose injector, SuliXen
Introduction
The effective use of peptide and protein drugs can be compromised by their enzymatic and hydrolytic instability in the body, rapid rates of clearance, premature uptake by tissues (for instance, the reticuloendothelial system), loss through the kidneys, and immunogenicity or antigenicity.1–4 To circumvent such problems, changes in the primary peptide structure, such as introduction of glycons into the structure or conjugation to polymers, have been investigated in an effort to render the active component less prone to degradation to improve its residence in the blood circulation and also to reduce immunogenicity.
By far the most successful approach to date is the conjugation of polyethylene glycol (PEG) with protein and peptide drugs.2–4 An increasing number of PEGylated drugs are now used clinically (e.g., asparaginase, interferon, tumor necrosis factor, and granulocyte-colony stimulating factor).1 However, PEG is not biodegradable and will be taken up by tissues participating in the uptake of the PEGylated constructs only to accumulate intralysosomally when such constructs are large enough to evade kidney clearance. Moreover, some PEGylated proteins have been found to generate anti-PEG antibodies that could influence the residence time of the conjugate in the circulating blood.4,5 Other PEG-associated drawbacks include high viscosity of the administered solutions and concerns regarding the toxicity of peroxides and byproducts following PEG oxidation in the body.6
It is now established that covalent coupling of the naturally occurring, highly hydrophilic, nonimmunogenic, linear polymer of N-acetylneuraminic acid (sialic acid) to drugs, including peptides and proteins, produces constructs exhibiting improved stability and prolonged survival in the blood circulation.7–16 Polysialic acids (PSAs; also referred to as colominic acids [CAs]) of varying chain lengths can be produced from bacteria, with long-chain PSAs attaining increased circulation times after intravenous injection (e.g., up to 40 h half-life in mice) than shorter ones.7 Polysialylation of protein therapeutics helps to reduce their immunogenicity and antigenicity and therefore is an effective form of protein drug delivery. Polysialic acid itself is a biodegradable, nonimmunogenic, nontoxic naturally occurring polymer. This is particularly important where a polymer is used to deliver therapeutics chronically or in large dosages.
It has been seen that covalent coupling of PSA to insulin improves the solubility (so as to prevent aggregation) and stability (unpublished data). It also extends its circulatory half-life, hence biological function, thereby reducing frequency of administration as well as reducing immunogenicity and antigenicity with overall improvement to the therapeutic index of the hormone.12 Results from a phase I trial of SuliXen® (14 kDa PSA–insulin) have shown the candidate to be safe and well-tolerated, with no adverse events attributed to the product being reported in the treated patients (unpublished data).
With regard to their administration into patients, most therapeutic proteins available cannot be given orally.17 Moreover, alternative options such as nasal and trans-dermal delivery routes are often not viable options due to impedance/clearance by mucus, while the large hydrophilic size of peptides can render them poor candidates for permeation across the stratum corneum. In such instances, parenteral administration is the only suitable delivery route. The majority of injection devices available (both needle-based and needle-free) deliver the formulation in a liquid form. Needles penetrate the skin, creating a hollow channel through which the liquid passes, while needle-free systems typically force a jet of liquid or powder through the skin. Owing to the universal dislike of needles, patient compliance is of particular concern where self-administration is necessary. As well as the cost of failing treatment, health care providers are becoming increasingly concerned about the cost of “wasted” drug therapy. Despite needle-free injection options being available, the traditional needle and syringe has been a hard habit to break, as it is dependable and cheap. The solid dose injector developed by Glide is a convenient, easy-to-use, inexpensive system that has the potential to increase patient compliance significantly. The system has been designed to address the issue of how to inject a solid dose accurately by using the dosage form itself as the delivery vehicle. Thus a solid dosage in the form of a tiny rod with a pointed end is pushed into the skin using a spring-powered, handheld actuator that resembles a pen. With minimal sensation, the solid dosage form pierces and penetrates the skin in a fraction of a second, and the simple click tells the patient that the drug has been delivered. The solid dosage form subsequently dissolves or degrades, releasing the drug at the desired rate.18
Here we report in vitro as well as in vivo testing results of solid dose formulations containing PSA–insulin conjugate suitable for delivery with the Glide injection system. The in vivo performance of the PSA–insulin conjugate in the formulations was evaluated in comparison with (a) subcutaneous injection of PSA–insulin conjugate (solution), (b) placebo (excipient used for the preparations of solid dosage forms), (c) Lantus® (solution), and (d) plain insulin (solution) into healthy rats. The present study aims to evaluate the feasibility of the development of the needle-free subcutaneous solid dosage formulations of PSA–insulin conjugate suitable for delivery using the Glide solid dose injector.
Materials and Methods
Preparation of Polysialic Acid–Insulin Conjugates
A conjugate of recombinant insulin (provided by the Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russian Federation, 117997) was prepared by the use of Lipoxen's PolyXen® drug delivery technology. A 14 kDa oxidized CA molecule (SII, India) was attached by reductive amination to the alpha-amino group of the B chain of the insulin molecule, as described previously. The conjugate was purified using reverse-phase chromatography (Tricon Resource 15 PRC column, 12.5 ml [GE Healthcare, United Kingdom]) to remove unreacted PSA and free recombinant human insulin, as well as conjugate species other than the monopolysialylated form. The final PSA–insulin conjugate formulation was composed of 15.4 mg/ml recombinant human insulin, 36.1 mg/ml PSA, 2.7 mg/ml metacresol, and 16 mg/ml ZnCl2 in pH 7.4 sodium phosphate dibasic buffer (10 mM).
Procedure Used for Production of Solid Dose Formulations Containing Polysialic Acid–Insulin Conjugate
Formulations were manufactured by incorporating the PSA–insulin conjugate solution as a granulation fluid into dry excipient powders, followed by use of a cold extrusion process. Overnight storage (2–8°C with desiccation) was sufficient for the drying of the formulations. Once dried, extrudates were cut, and analysis was conducted to characterize the prerequisite physicochemical properties of the formulations.
Rods of extrudate approximately 4 mm in length and with a point on one end were cut to a weight range of 2.18 mg ± 7%. Samples were subsequently placed into labeled foil laminate pouches containing desiccant, purged with nitrogen, and vacuum sealed. After storage at 2–8°C for 48 h, pouches were heat sealed to remove the desiccant. Formulations containing PSA–insulin conjugate were then chemically analyzed prior to the in vivo study. Table 1 gives a complete list of the formulations and their quantities, together with results from the physicomechanical testing and penetration.
Table 1.
Physicomechanical Characterization of Polysialic Acid–Insulin Containing Formulations
| Batch number | Formulation description | Physicomechanical Testing | ||||
|---|---|---|---|---|---|---|
| Compressive stress (MPa) | Three-point-bend (N) | Disintegration at 25 °C (min) | Disintegration at 37 °C (min) | Penetration (% success)a | ||
| 53/2-1 | Excipient 1 with 0.3 IU dose PSA–insulin | 106.99 ± 14.3 | 30.60 ± 1.4 | 20.6 ± 2.5 | 3.4 ± 0.2 | 100% |
| 53/2-2 | Excipient 2 with 0.3 IU dose PSA–insulin | 33.73 ± 7.8 | 7.64 ± 0.9 | 174.8 ± 16.7 | >2880 | 100% |
| 53/7-1 | Excipient 1 with 1.0 IU dose PSA–insulin | 53.10 ± 10.2 | 16.66 ± 1.0 | 20.1 ± 1.1 | 3.0 ± 0.2 | 100% |
| 53/7-2 | Excipient 2 with 1.0 IU dose PSA–insulin | 41.61 ± 7.4 | 13.18 ± 1.3 | 12.9 ± 0.4 | 2.8 ± 0.4 | 100% |
As a measure of robustness recorded while testing into an in vitro test bed.
In Vitro Characterization of Solid Dose Formulations Containing Polysialic Acid–Insulin Conjugate
The solid dose formulations containing PSA–insulin conjugate were suspended in 2 ml of phosphate-buffered saline buffer (pH 7.4) and incubated at 37°C until dissolved. The solution was then analyzed by size-exclusion high-performance liquid chromatography (SE-HPLC), bicinchoninic acid (BCA) protein assay, amino acid analysis, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (WB).
SDS-PAGE was performed using 18% Tris-glycine gels (Invitrogen). Samples were diluted with either reducing or nonreducing buffer, and 5.0 μg of protein was loaded into each well. The gels were run on a Tris-glycine buffer system as appropriate and stained with Instant Blue. Western blotting was performed using anti-PSA antibody (Millipore).
Protein Determination (Bicinchoninic acid)
Quantitative estimation of recombinant human insulin in the solid dose formulations was carried out by the BCA protein assay. The protein content of recombinant human insulin in solid dose formulations was also determined by amino acid analysis contracted out to Alta Biosciences, United Kingdom.
The endotoxin content of the solid dose formulations was determined using the portable test system Endosafe–PTS and Endosafe cartridges (Charles River Laboratories). A volume of 25 μl of sample was transferred into all four sample reservoirs. Determination was then carried out automatically. The results were displayed and consisted of sample endotoxin content in endotoxin unit (EU)/ml, sample percentage coefficient of variation (CV), spike EU/ml, spike percentage CV, and percentage spike recovery. Results were recorded accordingly.
In Vivo Characterization of Solid Dose Formulations Containing Polysialic Acid–Insulin Conjugate
The in vivo efficacy of the solid dose formulations was studied in rats (Sprague-Dawley) at the Royal Veterinary College of London, London, United Kingdom. A 3 IU insulin dose (on protein mass basis, not on activity) was injected into healthy rats subcutaneously. Animals were divided into groups of four or five and injected sub-cutaneously with PSA–insulin conjugate (solution), Glide formulations 26/185-1 (placebo excipient 1), Glide 53/7-1 (3 × 1.0 IU dose), Glide 53/2-2 (10 × 0.3 IU dose), Lantus (solution, 3.5–4 IU dose) and plain insulin (solution, 3 IU). Approximately 20 μl of blood was taken from each animal at predetermined time points, and blood glucose concentration (mM/liter) was measured by Accu-Chek Active (Roche Diagnostics).
Results and Discussion
Formulations produced for the feasibility study focused on the delivery of doses of 0.3 and 1.0 IU rod of PSA–insulin conjugate. Polysialic acid–insulin conjugate was incorporated into Glide Pharma excipients 1 and 2, which, as placebo implants, had the desired physico-mechanical properties and disintegration characteristics. Excipients 1 and 2 are both generally regarded as safe, parenterally approved polymers and are intended to dissolve slowly in the subcutaneous layer so as to exhibit a slow, prolonged release of PSA–insulin conjugate to the circulatory system of the animals. (Please note, the excipients used to formulate the solid dosage forms incorporating PSA–insulin referred to as “excipient 1” and “excipient 2” have not been disclosed, as the information has been regarded as proprietary at this early stage of the collaboration.)
The study demonstrated that solid dose formulations containing PSA–insulin conjugate can be successfully manufactured and characterized. Based on the results presented in Table 1, in the case of the two PSA–insulin conjugate doses investigated, both excipients used produced implants with desirable mechanical strength, as reflected in the high compressive stress (longitudinal strength) and three-point-bend-test (perpendicular strength) values and 100% penetration success into an in vitro test bed. Shergold and Fleck17 determined that the average penetration pressure required to penetrate human skin with a sharp tipped punch (similar in dimensions to the solid dosage forms manufactured for the study) is less than 10 mPa, and so the formulations were tested to determine if the maximum compressive stress they could withstand was above this value. With regard to excipient 1, an inverse relationship was observed between the concentration of PSA–insulin conjugate incorporated and the mechanical strength. The compressive stress and three-point-bend-test values recorded for the 0.3 IU formulation were 106.99 ± 14.3 MPa and 30.6 ± 1.4 N, respectively, whereas, for the 1.0 IU dose, values were 53.10 ± 10.2 MPa and 16.66 ± 1.0 N. It was also observed that the higher the dose of PSA–insulin conjugate, the lower the disintegration time. For the 0.3 IU dose, the average disintegration time at 25°C was 20.6 ± 2.5 min, and at 37°C, the average disintegration time was 3.4 ± 0.2 min. For the 1.0 IU dose, the average disintegration time at 25°C was 20.1 ± 1.1 min, and at 37°C, the average disintegration time was 3.0 ± 0.2 min.
By contrast, in the case of excipient 2, a slight reversal in the trend (compared to excipient 1) was observed in that the compressive stress and three-point-bend-test values recorded for the 0.3 IU dose were 53.10 ± 10.2 MPa and 7.64 ± 0.9 N, respectively, whereas the values recorded for the 1.0 IU dose were 41.61 ± 7.4 MPa and 13.18 ± 1.3 N. As excipient 2 is only sparingly soluble in water, it was incorporated to provide a slower releasing formulation than excipient 1. The physicomechanical behavior of the placebo implants (batch 47/63-1) used as the negative control was mirrored by batch 53/3-2 containing a 0.3 IU dose of PSA–insulin conjugate. At 37°C, the samples tested were still visible after 48 h. At 25°C, the average disintegration time was 174.8 ± 16.7 min. Conversely, increasing the dose of PSA–insulin conjugate to 1.0 IU had a marked effect on the disintegration characteristics. The disintegration time at 25°C was 12.9 ± 0.4 min and at 37°C was 2.8 ± 0.4 min. Trebling the dose of PSA–insulin conjugate with excipient 2 may have resulted in the generation of an unintentional disintegrant tipping point, causing a burst release effect.
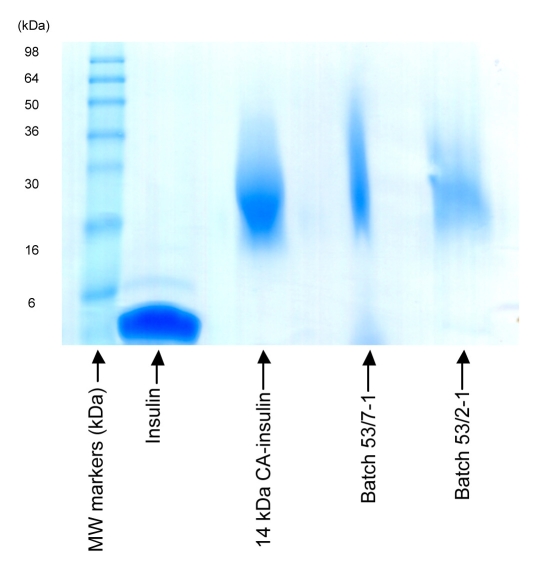
In vitro characterization of the solid dose formulations containing PSA–insulin conjugate was conducted to evaluate the concentration and integrity of PSA–insulin. SDS-PAGE revealed that PSA–insulin was intact during the formulation procedure as shown in Figure 1. As the PSA used in conjugation has a narrow distribution of molecular weight, less of a smear on the band can be observed compared with that of solid rod formulation, where the polymer excipients lag behind the migration band. Western blotting, however, failed to demonstrate the presence of PSA in the solid dose formulations. This could be due to interference of the excipient during the development of WB. A similar problem was encountered when SE-HPLC was used to analyze the solution extracted from solid dose formulations. In the future, attempts will be made to separate the excipient from formulations before performing WB and SE-HPLC.
Figure 1.
SDS-PAGE of PSA–insulin-containing formulations.
Bicinchoninic acid assay and amino acid analysis (Table 2) were used in order to determine the concentration of insulin in solid dose formulations. The concentration of insulin by BCA assay was found to be slightly lower than that of the theoretical insulin composition. However, the results of amino acid analysis were very close to the predicted values. Furthermore, the endotoxin content in the solid dose formulations containing PSA–insulin conjugate was acceptable for in vivo efficacy study.
Table 2.
Bicinchoninic Acid Assay and Amino Acid Analysis
| Parameters | Batch 53/2-1 0.3 IU (10.6 μg)/dose | Batch 53/7-1 1.0 IU (35.56 μg)/dose | Batch 53/2-2 0.3 IU (10.6 μg)/dose | Batch 53/7-2 1.0 IU (35.56 μg)/dose |
|---|---|---|---|---|
| BCA (per sample) | 9.5 μg | 37.0 μg | Not done | Not done |
| Amino acid analysis(per sample) | 13.3 μg | 41.9 μg | 9.8 μg | 39.9 μg |
| Endotoxin (rod dissolved in 100 μl water)a | Not done | 44.7 EU/ml | Not done | 53.1 EU/ml |
Batches not made under aseptic conditions.
Results of the in vivo efficacy in a rat model as displayed in Figure 2 suggest that the solid dose formulations containing PSA–insulin conjugate can induce prolongation of efficacy to at least 5 h. The data for injectable PSA–insulin conjugate revealed similar efficacy as Lantus in terms of hypoglycemic effect over time. This is apparent from areas over the curve. The starting (0 h) blood glucose levels for all these groups were different. Therefore, we used starting blood glucose as 100%, and percentage change in lowering blood glucose was recorded. The solid dose formulations containing PSA–insulin conjugate demonstrated a delayed response for the first hour after injection compared to PSA–insulin solution. In the case of Glide formulation 53/7-1, the profile was found to fluctuate at around 3 h. This indicates that the polymeric excipients used for the preparations of the solid dose formulations containing PSA–insulin conjugate need to be further optimized for the in vivo release of PSA-conjugate in vivo. Overall, t–test was conducted in order to determine the probability level of the difference between all study groups against native insulin group, p < .1, p < .05, and p < .01. This analysis showed that there is a significant difference between the PSA–insulin formulations as well as Lantus in comparison to native insulin toward later time periods. In addition to this, PSA–insulin formulations have up to 1.4-fold more area over the curve as compared to insulin.
Figure 2.
Blood glucose change versus time for Sprague-Dawley rats injected with insulin formulations. Blood glucose change ((Vt − V0) × 100% ÷ (V0)), where Vt is the value of blood glucose at interval period and is the value of blood glucose before subcutaneous injection) at interval period after subcutaneous injection of insulin formulations in Sprague-Dawley male rats at 5–6 weeks old (85 ± 12 g). Data are shown as means (n = 4–5) ± standard error of the mean.
The data generated in the in vivo study indicate that the injections of solid dose formulations produced using excipients 1 and 2 generated similar release profiles to the PSA–insulin conjugate solution injections. This indicates that PSA–insulin conjugate was delivered to the subcutaneous region of the animals at the same rate or faster than the biological effect recorded. To further lengthen the therapeutic window, the overall percentage of PSA–insulin conjugate incorporated into the solid dosage forms may be further decreased so as to minimize the changes observed in disintegration characteristics of the excipients as compared with placebos. In addition, PSA–insulin conjugate could be incorporated with polymers with increased molecular weight or similar classes of polymers so as to further increase the disintegration time of the solid dose formulations.
The Glide SDI® is a unique needle-free system that can be used to deliver small molecules, peptides, proteins, and vaccines in a solid dosage form, with polymeric excipients that can further stabilize such moieties.18 The facility to deliver molecules such as peptides by a low-sensation injection without a needle confers significant benefits for diabetic subjects. Furthermore, solid dosage forms are inherently more stable than liquid and thus have the potential to obviate the need for refrigeration, leading, in turn, to improved lifestyle choices for users, for example, being able to take a medicine on vacation without having to worry about cold storage. It is believed that a Glide insulin product, such as that combining the long-acting PSA–insulin conjugate, could offer a suitable alternative for insulin users, particularly for delivering fixed doses of insulin in type 2 diabetes subjects. One school of thought suggests that daily injections of fixed doses of insulin in newly diagnosed type 2 diabetes subjects can help maintain residual β-cell function and thus minimize future complications.19
In addition to insulin, the solid dose injection system has clear utility for other peptides (e.g., glucagon-like peptide-1 and glucagon) used in the treatment of diabetes and its consequences. For example, the currently available glucagon products require several preparatory steps, including having to reconstitute a powder formulation, prior to administration. Reports have shown that these preparation steps can take up to 12 minutes, drastically delaying the time to treatment and the speed with which the drug enters the bloodstream.20 Clearly, a ready-to-use, easy-to-administer alternative product would dramatically improve the ability to treat hypoglycemic attacks quickly and effectively and with minimum effort.
Conclusions
Solid dose formulations containing PSA–insulin conjugate for use with the Glide SDI were manufactured successfully, and the composition, amount, and integrity of the PSA–insulin conjugate were confirmed. An in vivo efficacy study in a rat model revealed that the solid dose formulations containing PSA–insulin conjugate showed prolongation of activity and a slow release profile. Overall, the model for such a study is established, but additional studies with a greater number of animals would be needed for further optimization.
Acknowledgment
We acknowledge the contributions to the collaboration of Dr. Shohre Nabahi, Dr. Chris White, and Dr. Simon Bennett at Glide Pharma and Dr. Brenda McCormack at Lipoxen PLC.
Abbreviations
- BCA
bicinchoninic acid
- CA
colominic acid
- CV
coefficient of variation
- EU
endotoxin unit
- N
Newton
- PEG
polyethylene glycol
- PSA
polysialic acid
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SE-HPLC
size-exclusion high-performance liquid chromatography
- WB
Western blotting
References
- 1.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 2.Vincent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: clinical applications and challenges for development. Adv Drug Deliv Rev. 2009;61(13):1117–1120. doi: 10.1016/j.addr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132(3):153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Mehvar R. Modulation of the pharmacokinetics and pharmacodynamics of proteins by polyethylene glycol conjugation. J Pharm Pharm Sci. 2000;3(1):125–136. [PubMed] [Google Scholar]
- 5.Caliceti P, Veronese FM. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 2003;55(10):1261–1277. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 6.Veronese FM. PEGylated proteins: an updated review. Presented at AAPS Meeting and Exposition; November 8–12; Los Angeles, CA. 2009. [Google Scholar]
- 7.Gregoriadis G, McCormack B, Wang Z, Lifely R. Polysialic acids: potential in drug delivery. FEBS Lett. 1993;315(3):271–276. doi: 10.1016/0014-5793(93)81177-2. [DOI] [PubMed] [Google Scholar]
- 8.Gregoriadis G, Fernandes A, Mital M, McCormack B. Polysialic acids: potential in improving the stability and pharmacokinetics of proteins and other therapeutics. Cell Mol Life Sci. 2000;57(13-14):1964–1969. doi: 10.1007/PL00000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes AI, Gregoriadis G. Polysialylated asparaginse: preparation, activity and pharmacokinetics. Biochim Biophys Acta. 1997;1341(1):26–34. doi: 10.1016/s0167-4838(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes AI, Gregoriadis G. Synthesis, characterization and properties of polysialylated catalase. Biochim Biophys Acta. 1996;1293(1):92–96. doi: 10.1016/0167-4838(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 11.Constantinou A, Epenetos AA, Hreczuk-Hirst D, Jain S, Deonarain MP. Modulation of antibody pharmacokinetics by chemical polysialylation. Bioconjug Chem. 2008;19(3):643–650. doi: 10.1021/bc700319r. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Hreczuk-Hirst D, McCormack B, Mital M, Epenetos A, Laing P, Gregoriadis G. Polysialylated insulsynthesis, characterization and biological activity in vivo. Biochim Biophys Acta. 2003;1622(1):42–49. doi: 10.1016/s0304-4165(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 13.Constantinou A, Epenetos AA, Hreczuk-Hirst D, Jain S, Wright M, Chester KA, Deonarain MP. Site-specific polysialylation of an antitumor single-chain Fv fragment. Bioconjug Chem. 2009;20(5):924–931. doi: 10.1021/bc8005122. [DOI] [PubMed] [Google Scholar]
- 14.Gregoriadis G, Jain S, Papaioannou I, Laing P. Improving the therapeutic efficacy of peptides and proteins: a role for polysialic acids. Int J Pharm. 2005;300(1-2):125–130. doi: 10.1016/j.ijpharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Jain S, Hreczuk-Hirst D, Laing P, Gregoriadis G. Polysialylation: the natural way to improve the stability and pharmacokinetics of protein and peptide drugs. Drug Deliv Sys Sci. 2004;4(1):3–9. [Google Scholar]
- 16.Florence AT, Hillery AM, Hussain N, Jani PU. Nanoparticles as carriers for oral peptide absorption: studies on particle uptake and fate. J Control Rel. 1995;36(1-2):39–46. [Google Scholar]
- 17.Shergold OA, Fleck NA. Experimental investigation into the deep penetration of soft solids by sharp and blunt punches, with application to the piercing of skin. J Biomech Eng. 2005;127(5):838–848. doi: 10.1115/1.1992528. [DOI] [PubMed] [Google Scholar]
- 18.Bennett S, Potter C. Delivering injectables: formulations, auto-injectors and needle-free. East Sussex: ONdrugDelivery; 2008. Solid dose injection of therapeutics and vaccines: effective, convenient and cost-effective alternative to needles; pp. 11–13. [Google Scholar]
- 19.Bolli GB. Initiation of insulin treatment in type 2 diabetes mellitus. Eur Endo Dis. 2007;1:40–41. [Google Scholar]
- 20.Harrism G, Diment A, Sulway M, Wilkinson M. Glucagon administration underevaluated and undertaught. Pract Diab Int. 2001;18(1):22–25. [Google Scholar]