Abstract
Insulin pens are developed to address specific needs of diabetes patients for their pens, such as ease of use, portability, and discreetness. Like many consumer-based products, the development of insulin pens can pose significant challenges to the development team in that they must balance substantial accuracy requirements with aesthetic desires. The HumaPen® Memoir™ team learned valuable lessons throughout the development process that may be worth highlighting. A keen understanding of the unmet needs of the patient population and a skillfully planned product generation map are critical to successful device development. A development team must decide whether to use a Quality Functional Deployment or system engineering-based development plan and, additionally, recognize where proof of concept ends and product development begins to maintain a strict timeline for the project. A proficiency in understanding and managing technical risk is critical to ensure a timely and high-quality product launch to the marketplace.
Keywords: development, insulin pens, medical device, system engineering, type 1 diabetes, type 2 diabetes
Introduction
Rigorous control of blood glucose levels is essential for both type 1 and type 2 diabetes patients, and patient adherence to treatment regimens, including insulin administration, is key to preventing the chronic complications of diabetes. Insulin delivery pens have been developed as an alternative to vial and syringe to increase ease of administration and adherence to medication regimens. Studies have shown that insulin pens are preferred by patients over vial and syringe,1–3 are more accurate than vial and syringe,4 and increase medication adherence.5,6 When asked to explain their preference, patients note the increased ease of administration, flexibility, convenience, discreetness, and decreased pain of injection.2,7 Prior to development of the HumaPen® Memoir™ (Eli Lilly and Company, Indianapolis, IN), several reusable and prefilled insulin pens were available to insulin-requiring diabetes patients. However, it was noted that treatment success was affected by patient forgetfulness, which was the primary reason for nonadherence to an oral treatment regimen in a diabetes prevention trial.8 Using this knowledge, along with guidance from Lilly's medical device advisory board, it was determined that an insulin pen with a memory feature and a large, easy-to-read display would fulfill an important unmet patient need, and the HumaPen Memoir was therefore developed.
The HumaPen Memoir is a reusable electromechanical injection device that uses Lilly 3-ml insulin cartridges. The pen has a metal exterior and a liquid crystal display that allows the user to see the date, time, and number of insulin units to be injected and has the memory capability to recall the last 16 doses, including priming doses. During development of the HumaPen Memoir, many important lessons were learned. While this article is not a step-by-step guide to medical device development nor a detailed description of well-documented techniques, it briefly discusses several key elements that all device development project managers may wish to consider.
Device Concept and Product Generation Maps
During device development, a clear vision of the key product requirements and the product life cycle plan are essential elements that allow designers and project managers to navigate the development process.
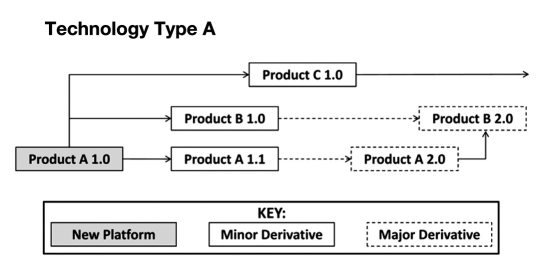
The HumaPen Memoir was initially identified as a product platform from which multiple new technologies could emerge simultaneously as part of a product family, including the insulin pen and a next-generation pen for another self-administered therapeutic. Although any new technology can be a “platform” from which other products emerge, such an approach can greatly complicate coordination of the requirements. This coordination of effort may have been more successful with a less innovative platform, but the emerging technology was at the forefront of development. Without a clear product leader and product follower, the team labored to make design trade-offs for the two different products simultaneously. These trade-offs included basic design challenges, such as material of construction, length, and weight, as well as more challenging elements, such as the electronic circuit, microchip, and software design. Ultimately, the team was able to focus its development primarily on the HumaPen Memoir requirements, with future products delegated to separate development teams. This allowed the team the ability to concentrate on a single device and deliver a high-quality product. This example highlights the importance of creating a product generation map (Figure 1) prior to setting product requirements.9 A product generation map is a systematic depiction of how a core platform technology or product family is expected to evolve over the life cycle of a product. It makes clear which developments are expected at different time points and allows for shared learning.
Figure 1.
A product generation map is a product family tree showing how a single product can, through a planned series of iterations, create second-generation or hybrid products as other technologies are merged. This sample of a product generation map would be widely applicable to a variety of development programs. Format developed from Wheelwright and Clark.10
In addition to a product generation map, an appropriate set of product requirements is also essential. The HumaPen Memoir was developed successfully using the Quality Functional Deployment (QFD) process.11 The QFD is a customer-oriented approach to development, and the team found it to be an effective methodology for requirement setting that enabled primary consideration for the needs of diabetes patients who inject insulin. This process allowed for the capture of fundamental patient needs, incorporated knowledge of the competitive environment, provided for ranking of design specifications, and, by comparing the voice of the customer to the voice of the business, enabled the team to effectively make design trade-offs. The “voice of the customer” is a process used to capture the requirements/feedback from external stakeholders (primarily health care professionals in the case of the HumaPen Memoir) to provide best-in-class product quality. The “voice of the business” is a term used to describe the stated and unstated needs or requirements of the business (marketing, manufacturing, regulatory, etc.). The QFD process worked well for the HumaPen Memoir project because it was the first time a Lilly device project used a structured approach to requirement setting. Additionally, because the team was constrained to using the existing insulin cartridge and needle, QFD could focus only on the device and not integrate the design of a more complex system.
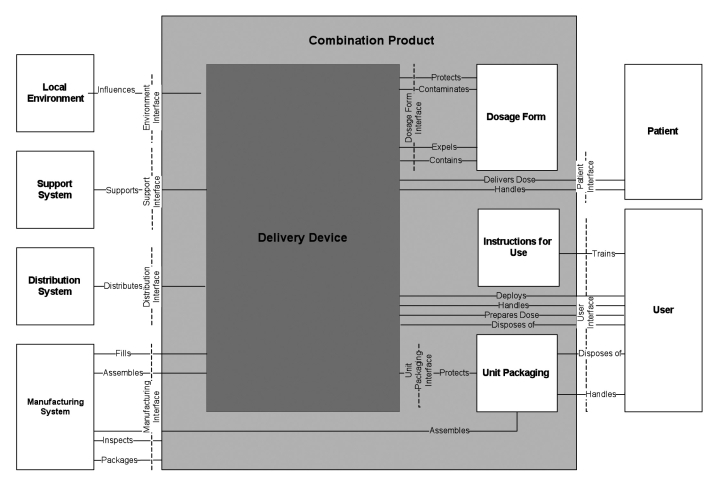
Increasingly, medical devices incorporate multiple new technologies that must function together in any operational state a user might encounter. To address the resultant challenges, system engineering is now being applied as an alternative to QFD earlier in development projects.12 While QFD is adequate for many development projects, system engineering is a more comprehensive and sophisticated approach to complex product development projects. In order to understand all of the requirements for a project, the requisites of each stakeholder must be defined and translated into product system and subsystem requirements. These stakeholders should be a broad mix of organizations and people, both internal and external to the development organization. It is critical to understand the needs of all relevant stakeholders of the targeted population. Stakeholders can be defined as groups who may be affected directly by product development. In the case of insulin pen development, patients, caregivers, prescribers, and payers are all key external stakeholders. In addition, the needs of manufacturing, quality, product complaint and investigation staff, product safety, distribution, and sales and marketing must be considered carefully to ensure delivery of a product to the marketplace that truly meets customer needs. While stakeholders are similar in QFD and system engineering, the two concepts diverge as system engineering creates system domain diagrams (Figure 2) and logical architectural diagrams (LAD)(Figure 3) to guide more complex and interdependent development projects.
Figure 2.
A system domain diagram and a logical architecture diagram of a combination product illustrate the relationship between the external environment and the device, which helps a development team determine the system requirements and the interaction of each input and output.
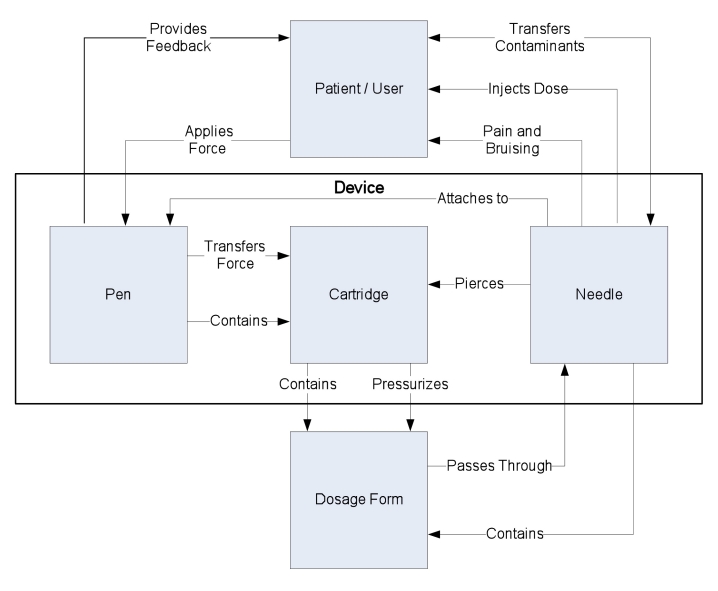
Figure 3.
A general device logical architecture diagram models the interaction between each component within a subsystem (here a delivery device). Additionally, it demonstrates how a subtle change in one component could impact other components within the device.
The system domain diagram (Figure 2) demonstrates critical requirements and their relationships between the delivery device and key aspects of the external environment, such as distribution and manufacturing. These requirements and relationships are then translated into inputs and outputs. If the system requirements are not well characterized (e.g., weight, length, color, storage requirements), the system domain diagram provides a starting point for discussion. Even if the requirements are well characterized [e.g., International Organization for Standardization (ISO); American Society for Testing and Materials; Food and Drug Administration], the diagram provides a graphical check for completeness of the requirements and a method of recording what has been considered and included in the design intent.
Inclusion of the design intent is important in that it provides essential context when feature trade-off decisions must be made. To do this effectively, each subsystem for a product must be examined using a logical architecture diagram (Figure 3).
An LAD places the emphasis on how the various components of the device interact. In the case of HumaPen Memoir pen, there were specifications for the overall length of the device; however, during detailed design, it became necessary to increase the length to accommodate internal components. Without a clear understanding and documentation of the intent of the length specification and its impact on other components, it was challenging to evaluate the consequences of the change.
From Research to Development
The development team may encounter many potential challenges while bringing a new device product concept to market. Balancing patient-centered technology challenges requiring scientific discovery versus business challenges, such as development time and cost, was a dilemma facing the HumaPen Memoir project team. At its core, good project management balances the inherent conflict between technology discovery needed to deliver on the unmet needs of patients and the time and cost needs of the business.
To accomplish this balance, project management typically splits activities into two distinct domains: research and development. In research, the team strives to identify or develop technologies needed to address pivotal issues and establish a projected overall development plan. In development, the team strives to execute the plan and deliver the new medical device. The critical optimization decision becomes determining how far to take research prior to entering development. Remaining in research too long (i.e., trying to address every issue prior to development) or not long enough (i.e., entering development with too many unanswered questions) results in overall development that is more costly and/or longer than necessary.
In the case of the HumaPen Memoir, the team moved into the development phase relatively quickly. Using paper-based analysis, they believed that the key technical challenges could be overcome and promptly proceeded to establish an overall development and launch schedule. During the development program, the team produced working models of the product concept and found that an internal subassembly was not performing optimally. Analysis of the deficiencies of the concept led the team to conclude that there was not a clear solution that fit within the overall constraints of the development and launch plan. As a result, the team was forced to reenter research and create a new concept for the internal sub-assembly. While this enabled resolution of the technical challenges, it slowed overall development and negatively impacted the timeline for the anticipated rollout of the new device.
It is typical to vacillate between research and development during device optimization, but two key lessons learned through the HumaPen Memoir development experience may help future teams minimize the need for additional research once development has begun. First, “key performance indicators” (KPIs) should be established for the research phase of a project, and KPIs should be met prior to moving into the development phase. By establishing these requirements a priori, the team is obligated to make the decision to move into full-scale development a deliberate one. (Note: “Key performance indicators” should be differentiated from “product requirements” in that product requirements are the essential items that the product must meet prior to market introduction, whereas KPIs are items that the team feels are essential to meet at a given phase, prior to making any additional investment in development.) The second key lesson learned is the importance of using physical models versus theoretical integration of existing technologies to demonstrate and communicate key challenges. Establishing firm demonstration requirements for key technical challenges arising during the KPI process may have eliminated the need for the HumaPen Memoir team to reenter research.
Through these improvements—KPI and the use of physical models—it is much less likely that a team will unintentionally be driven back into research after the initial transition to development. Further, the clearer understanding made possible by these improvements better enables the team to consciously choose not to discharge all technical risks by carrying some forward as part of the risk management plan. This likewise allows the team to avoid a protracted development cycle by remaining in research trying to discharge all technical risk.
A core concept for development of an effective risk management plan is a solid understanding of those aspects of the design approach that are “bleeding edge” versus “leading edge.” Although leading edge refers to novel developments, bleeding edge refers to designs that are at the forefront of development and therefore have the potential to incur substantial technical risk. Those that are bleeding edge warrant risk reduction throughout the development program, and if these are not clearly understood, the project will likely fail.
For HumaPen Memoir development, it was well recognized that the team was near the bleeding edge with certain aspects of the design, but the implications of this technology were not immediately apparent. As a result, significantly more resources were spent on manufacturing scale up at the project's end, and the transition to launch may have been easier with greater risk reduction earlier in the program. One of the best risk reduction tools to use is building and testing, building and testing, building and testing—subsystems, components, complete devices—wherever the risk lies. When eliminating a technical risk, there is no substitute for understanding the failure of a design approach or making clear the optimal design solution. Albeit at significant expense, the HumaPen Memoir team built and tested thousands of preproduction subassemblies and pens to verify reliability. Standard ISO and highly accelerated life cycle tests were conducted as components of design verification. User simulation studies were conducted during key stages of product development to test crucial features and functionality of the device to ensure that it functioned as it was intended in the target patient population.
Clinical Testing of Devices
In addition to benchtop and simulation testing, Lilly conducted a randomized, controlled clinical trial in insulin-injecting diabetes patients to further evaluate the functionality of the HumaPen Memoir in the target patient population, understand its complaint profile, and demonstrate its safety prior to launch in the marketplace. In this clinical trial, 300 patients used the HumaPen Memoir to inject either their prandial or basal insulin dose during the 6- to 10-week trial.13 The primary objective of this study was to determine if patients using the HumaPen Memoir injection device reported any device functionality issues deemed to be unacceptable. Questionnaires that captured satisfaction, preference, and confidence in both the prestudy insulin device and the HumaPen Memoir were administered to assess secondary objectives of the study. Because patients and health care providers are both stakeholders in the development of a new insulin delivery device, both groups were assessed. In addition to providing valuable insight into patient and provider preference for the pen, the trial offered a valuable opportunity to investigate and analyze device failures and complaints prior to launch.
Complaints were collected and categorized into functional (interfering with the delivery of the insulin dose), nonfunctional (not directly relating to the device functionality), or user-manual related. Of primary interest to the development team were the reported functional complaints. Complaint pens were returned to Lilly for investigation and analysis to validate that no new failure modes were identified and to assign complaint analysis categories. Of 24 total functional complaints (7.6% of all pens), 8 (2.5%) were considered device failures with no novel failure modes identified, with the remaining user related. When patients were asked at their final visit whether they preferred their prestudy insulin pen or the HumaPen Memoir, 81.4% of patients preferred the HumaPen Memoir. Patients and health care professionals alike rated the pen highly based on its ease of use and its memory feature.13 Study results demonstrated a favorable benefit–risk profile for the HumaPen Memoir. Following the clinical trial, corrective action projects were initiated based on complaint data to continually improve the robustness of the device. Through the use of benchtop testing, simulated user testing, and a clinical trial, the HumaPen Memoir design was optimized prior to full launch, enabling the development team to deliver the highest quality product to the marketplace.
Finalizing Development
Once a product such as the HumaPen Memoir has been optimized, there can still be a tendency for a project team to want to continue to evolve a product through another “better idea.” The potential merits of an idea need to be weighed carefully against the impact on development time and cost and the potential value to the patient to determine if continued development is prudent or if the idea should be captured as a revision to the product generation map. With this concept in mind, the motto of the HumaPen Memoir development team became: There comes a time when you must “shoot the engineers” and let the manufacturing crew push the product over the finish line and into the hands of the waiting patient.
Conclusions
The HumaPen Memoir project, despite its challenges, allowed both Eli Lilly and Company and Battelle Memorial Institute, codevelopers of the pen, to become better product development organizations that bring innovative devices to patients that better meet their unmet needs. Product generation maps are now being used to guide product strategies and focus engineering efforts. Based on knowledge gained during the design process for the HumaPen Memoir, system engineering is now being employed earlier to crystallize product requirements and their rationale. Early integration of system engineering enables developers to utilize metrics to determine when sufficient technical risk has been mitigated and when it is prudent to exit the research phase and enter the development phase of a project.
Managing the development plan for a novel insulin device is no minor achievement, and every program is ultimately an opportunity for continuous improvement and process refinement. The two organizations discovered that although product development process improvement was a substantial undertaking, a logical and traceable set of detailed requirements may enable future teams to plan and manage their programs more effectively. Ultimately, this innovative device was launched successfully and continues to address a formerly unmet need of diabetes patients. Through ongoing improvements to the product development process, insulin delivery devices will surely continue to advance in the future.
Acknowledgment
Assistance in manuscript preparation was provided by Tina Rees, Ph.D., of Eli Lilly and Company.
Abbreviations
- ISO
International Organization for Standardization
- KPI
key performance indicator
- LAD
logical architecture diagram
- QFD
quality functional deployment
References
- 1.Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Korytkowski M, Bell D, Jacobsen C. Suwannasari R; FlexPen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 3.Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27(10):2495–2497. doi: 10.2337/diacare.27.10.2495. [DOI] [PubMed] [Google Scholar]
- 4.Lteif AN, Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22(1):137–140. doi: 10.2337/diacare.22.1.137. [DOI] [PubMed] [Google Scholar]
- 5.Wilk T, Mora PF, Chaney S, Shaw K. Use of an insulin pen by homeless patients with diabetes mellitus. J Am Acad Nurse Pract. 2002;14(8):372–379. doi: 10.1111/j.1745-7599.2002.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712. doi: 10.1016/j.clinthera.2006.10.004. discussion 1710-1. [DOI] [PubMed] [Google Scholar]
- 7.Kadiri A, Chraibi A, Marouan F, Ababou MR, el Guermai N, Wadjinny A, Kerfati A, Douiri M, Bensouda JD, Belkhadir J, Arvanitis Y. Comparison of NovoPen 3 and syringes/vials in the acceptance of insulin therapy in NIDDM patients with secondary failure to oral hypoglycaemic agents. Diabetes Res Clin Pract. 1998;41(1):15–23. doi: 10.1016/s0168-8227(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 8.Walker EA, Molitch M, Kramer MK, Kahn S, Ma Y, Edelstein S, Smith K, Johnson MK, Kitabchi A, Crandall J. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006;29(9):1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes RH, Wheelwright SC, Clark KB. Dynamic manufacturing—creating the learning organization. 4th ed. New York: Free Press; 1988. [Google Scholar]
- 10.Wheelwright SC, Clark KB. Revolutionizing product development: quantum leaps in speed, efficiency, and quality. 1st ed. New York: Free Press; 1992. [Google Scholar]
- 11.Hauser JR, Clausing D. The house of quality. Harv Bus Rev. 1988;66:63–73. [Google Scholar]
- 12.Martin JN. Systems engineering guidebook: a process for developing systems and products. 1st ed. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 13.Venekamp WJ, Kerr L, Dowsett SA, Johnson PA, Wimberley D, McKenzie C, Malone J, Milicevic Z. Functionality and acceptability of a new electronic insulin injection pen with a memory feature. Curr Med Res Opin. 2006;22(2):315–325. doi: 10.1185/030079906X80477. [DOI] [PubMed] [Google Scholar]