Abstract
Objective
The aim was to determine if frequently repeated glucose measurements mandated by an inpatient protocol led to falsely elevated reported rates of both hypo- and hyperglycemia.
Methods
In our academic medical center, a mandatory standardized subcutaneous insulin order form and protocol was implemented in May 2006. We analyzed point-of-care blood glucose (BG) measurements collected on all medical/surgical wards during the month of August in both 2005 and 2006 by all BGs measured, by patient admission, and by monitored patient-day. We then repeated all analyses using an algorithm that excluded BG values if another BG was measured less than 5 minutes later or 5-60 minutes earlier.
Results
In 2005 versus 2006, there were 7034 versus 8016 glucoses measured in 397 versus 389 patients over 1704 versus 1710 patient days, respectively. Analyses based on patient-day balanced differences in BG measurement frequency and patient length of stay. In both years, failure to exclude repeat values overestimated both the proportion of patient days with hypoglycemia (3.5% versus 1.8% in 2005, p = .003; 2.6% versus 1.3% in 2006, p = .007) and severe hyperglycemia (9.3% versus 7.4% in 2005, p = .09; 7.7% versus 5.9% in 2006, p = .08). Mean, median, and proportion of patient-day means within our target range (80-150 mg/dl) were not significantly different.
Conclusions
Glucometric reports should exclude repeated BG measurements from a single clinical episode of hypo- or hyperglycemia in order to accurately reflect inpatient glycemic control.
Keywords: glucometrics, glucose monitoring, hypoglycemia, inpatient diabetes
Introduction
Hypoglycemia is usually the limiting factor in implementation of tight inpatient glucose control and represents the most clinically significant potential adverse effect of aggressive antihyperglycemic therapy.1 As hospital glycemic control is now an important quality-of-care measure, standardized methods for quantifying the proportion of blood glucose (BG) within target ranges and the incidence of hypo- and hyperglycemia (glucometrics) are needed.2,3 Even though episodes of hypoglycemia and severe hyperglycemia usually prompt repeat BG measurements, previous reports of glucometrics include these BG measurements in analyses.4–10 Thus, themultiple frequent glucose measurements generally required for a single episode of hypoglycemia (to verify the low glucose and monitor until the hypoglycemia has resolved) may lead to a falsely increased reported rate of hypo-glycemia. In addition, frequent and confirmatory repeat measurements for a single hyperglycemic episode may lead to a falsely increased reported rate of hyperglycemia.
Algorithms for excluding repeat measurements must be developed and validated if hospital rates of hypo-glycemia are to be compared. Our inpatient diabetes quality improvement committee designed an algorithm for excluding glucose measurements that excluded BG measurements if another value was recorded either less than 5 min later or 5–60 min prior. We analyzed point-of-care BG measurements on all medical/surgical wards during one month and repeated these analyses excluding repeat values.
Research Design and Methods
In May 2006, a mandatory subcutaneous insulin order form and protocol was implemented at our academic medical center, including a standard hypoglycemia protocol (Figure 1). All point-of-care glucose measurements were analyzed on adult medical and surgical wards (non-intensive care unit) in August of 2006. De-identified glucose data were supplied from the clinical laboratory, and initial analysis of the data was performed using Microsoft Access programmed for the glucometrics algorithm as described here. Glucometric analyses are reported by two units of analysis: (1) without grouping and (2) grouped by monitored patient-day (which has been proposed to be a more accurate assessment of inpatient glycemic control).2 When analyzed “without grouping,” the mean glucose is calculated as the mean of all glucoses for all of the included hospital patients. When analyzed as “grouped by monitored patient-day,” the mean glucose is calculated from the mean of mean glucoses for each patient-day. Patients with less than 5 evaluable glucose measurements and days that patients received continuous insulin infusions (identified as greater than or equal to 10 glucose measurements per day) were excluded.
Figure 1.
Hypoglycemia protocol preprinted on the mandatory standardized subcutaneous insulin order form at the University of California San Francisco.
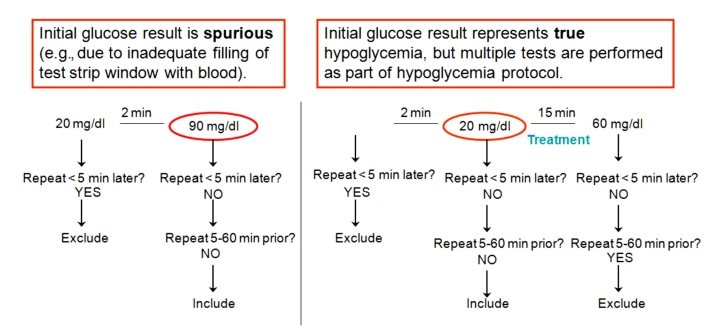
Our inpatient diabetes improvement committee (comprised of diabetologists, a hospitalist, a diabetes clinical nurse specialist, a nursing supervisor, pharmacists, and a quality improvement analyst) developed an algorithm for exclusion of repeat values: if another glucose measurement was performed less than 5 minutes later or 5–60 minutes earlier. This algorithm would exclude glucose values (1) when the initial glucose result is spurious (e.g., due to inadequate filling of test strip window with blood) and the immediately repeated value is accurate and should be included and (2) when the initial glucose result represents true hypoglycemia, but multiple tests are performed as part of hypoglycemia protocol (Figure 2). Initial statistical analysis was performed using Mann–Whitney tests and random effects logistic regression. These were complex analyses and are discussed in detail in Appendix 1.
Figure 2.
Algorithm developed by the Inpatient Diabetes Care Committee to exclude frequently repeated glucose values.
Results
Before repeat values were excluded, there were 6900 glucose measurements from 313 patients over 1570 patient days. Failure to exclude repeat values overestimated both hypoglycemia, defined as glucose less than 60 mg/dl (0.7% versus 0.3%, p ≤ .0001) and severe hyperglycemia, defined as glucose greater than 350 mg/dl (3.0% versus 2.3%, p ≤ .0001) (Table 1). Mean glucose ± standard deviation (175.1 ± 68.2 mg/dl versus 173.6 ± 68.2 mg/dl) and the proportion of glucose measurements outside our target range of 80–150 mg/dl (57.8% versus 57.2%, p < .0001) were lower after excluding repeat values, indicating that more high than low values were excluded with our algorithm. There was no significant difference in the median glucose, which is less sensitive to the inclusion of extremely high or low glucose measurements.
Table 1.
Failure to Exclude Frequently Repeat Glucose Values Overestimated Both Hypoglycemia and Hyperglycemiaa
| Repeat values | No grouping | Grouped by patient-day | ||||
|---|---|---|---|---|---|---|
| Includes | Excludes | p value | Includes | Excludes | p value | |
| Mean glucose (standard deviation) | 175.1 (73.5) | 173.6 (68.2) | <.0001 | 172.4 (55.9) | 171.7 (53.8) | .0002 |
| Median glucose | 158 | 158 | .08 | 159.7 | 160.4 | .02 |
| % hypoglycemia (<60) | 0.7 | 0.3 | <.0001 | 2.1 | 1.2 | –b |
| % hyperglycemia (>350) | 3.0 | 2.3 | <.0001 | 6.7 | 5.7 | –b |
| % outside range (<80 or >150) | 57.8 | 57.2 | <.0001 | 84.3 | 84.0 | –b |
All glucose values are expressed in mg/dl.
P values could not be calculated for comparisons involving measures based on at least one episode per day, since excluding repeat values could not increase these proportions.
The exclusion of repeat values also led to lower reported rates of hypoglycemia (2.1% versus 1.2%) and hyperglycemia (6.7% versus 5.7%) when analyzed grouped by patient-day. Both mean (172.4 ± 55.9 mg/dl versus 171.7 ± 53.8 mg/dl, p = .0002) and median (159.7 mg/dl versus 160.4 mg/dl, p = .02) glucose values were also significantly different.
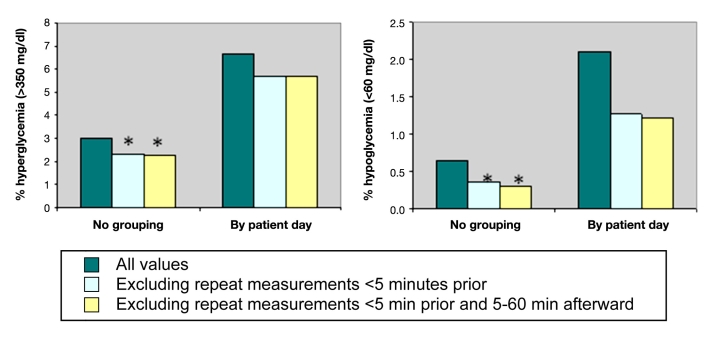
We then examined whether excluding repeat measurements made less than 5 minutes prior (spurious values) or those 5–60 minutes afterward (confirmation of adequate treatment) had a greater effect on reported rates of hypo- and hyperglycemia (Figure 3). The proportion of glucose measurements with hypoglycemia decreased from 0.7% to 0.4% after excluding repeat values less than 5 minutes prior and to 0.3% after further exclusion of repeat values 5–60 minutes afterward. Percent hyperglycemia decreased from 3.0% to 2.3% excluding spurious values and did not change further after excluding subsequent values recorded within 60 minutes.
Figure 3.
Rates of hyperglycemia and hypoglycemia analyzed without grouping and grouped by patient-day. Exclusion of repeat values within 5 min had a greater effect than exclusion of repeat values 5–60 min later. The asterisks represent p < .001 versus all values. P values could not be calculated for comparisons involving measures based on at least one episode per day (by patient-day), since excluding repeat values could not increase these proportions.
When analyzed grouped by patient-day, exclusion of repeat values within 5 minutes also had a greater effect than exclusion of repeat values 5–60 minutes later. Proportion of patient days with hypo- and hyperglycemia was reduced from 2.1% to 1.3% to 1.2% and from 6.7% to 5.7% to 5.7%, respectively, with the iterative exclusions of repeat measurements.
Discussion
Standardized protocols for glycemic management provide guidance to nursing and physician staff regarding the thresholds for treatment and prevent delays in treatment by providing anticipatory orders.11 Hypoglycemia protocols, in particular, may avoid overtreatment of hypoglycemia, which can result in hyperglycemia. Many hypoglycemia protocols, including the one used at our institution, mandate repeat measurements of BG until euglycemia is achieved. Reports of glucometrics that do not exclude repeat measurements may therefore “double-count” or “triple count” a single clinical episode. Indeed, hospitals that comply with such hypoglycemia protocols might appear to have higher rates of hypoglycemia.
In our academic medical center, excluding repeat values performed less than 5 minutes before a glucose measurement had a major impact on reported rates of hypo- and hyperglycemia. These values represent spurious values (e.g., if blood did not fill up the entire test strip window) and are disproportionately lower than other reported values, although they do not represent clinically significant events. Excluding repeat values performed 5–60 minutes after a glucose measurement, however, had minimal impact on reported rates of hypoglycemia, likely reflecting adequate treatment of hypoglycemia per protocol. When we performed analyses of glucometrics both including and excluding these repeat measurements, we found that inclusion significantly overestimated the incidence of hypo- and hyperglycemia.
Using the methods described in this study (glucose data entered into a database every month, grouped by patient-day and using the algorithm in Figure 2), our inpatient diabetes committee has continued to monitor glucose levels at our institution. As a result of the mandatory standardized insulin forms, education efforts, and glucose management feedback, the mean glucose levels on our medical–surgical units is now consistently 150–154 mg/dl with the rate of hypoglycemia <0.8%.
As the patient safety movement gains momentum12 with concrete financial consequences,13 there is a growing need for standardized methods of documentation of inpatient glycemic control. The Joint Commission';s Surgical Care Improvement Project includes the voluntary reporting of postoperative 6:00 AM glucoses in cardiac surgery patients,14 and the trend toward inclusion of glycemic control in quality-of-care measures is likely to increase. Several randomized trials of intensive glycemic control in hospitalized patients have been terminated early due to increased rates of severe hypoglycemia, highlighting the urgent need to track incidences of hypoglycemia reliably.15,16 Not only should standard glucometrics be used for comparisons among hospitals, but these reports should exclude repeat glucose measurements from a single clinical episode of either hypo- or hyperglycemia in order to accurately reflect inpatient glycemic control.
Acknowledgments
We gratefully acknowledge the University of California San Francisco Diabetes Improvement Committee, including Rosanne Rappazini, Mary Sullivan, Umesh Masharani, Lisa Kroon, Thomas Bookwalter, Heather Nye, Marlene Bedrich, and Paula Chin for input on the development of the algorithm to exclude repeat glucose measurements; Michael Kohn for assistance with data management; and Morris Schambelan, Ira Goldfine, Madhu Rao, and Elizabeth Murphy for their thoughtful comments.
Abbreviations
- BG
blood glucose
Appendix 1. Statistical Analysis
Crude summaries are not adjusted to account for clustering of multiple measurements or days within patients. The p values, however, do account for this clustering. P values for comparison of included versus excluded by-measurement data were calculated by mixed linear models with random person effects for means and by Mann–Whitney tests stratified by person for medians. Comparison of dichotomous outcomes was performed by random effects logistic regression, with exclusion as the predictor and a random person effect included in the model. For comparing by-patient-day continuous values, we first calculated the difference between each day';s mean using all measurements versus the mean, excluding either the spurious values or excluding both spurious and repeat values. For comparing means, we then modeled these differences in terms of an intercept term and a random person effect (to account for clustering of multiple days within person) and used the p value of the intercept term. The p values for medians were calculated by converting each day';s difference into a signed rank, similar to a Wilcoxon signed-rank test, and then fit a similar mixed-effects model. For the dichotomous by-patient-day outcomes, exclusion of some measurements cannot lack a net effect on the rate of the events. This is because events were defined as occurring if any one or more measurements were in the undesirable range, so exclusion of some measurements can only make events less likely. P values therefore cannot be calculated, because the null hypothesis of no net effect of excluding measurements is already known to be false.
References
- 1.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg PA, Bozzo JE, Thomas PG, Mesmer MM, Sakharova OV, Radford MJ, Inzucchi SE. “Glucometrics:” assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–569. doi: 10.1089/dia.2006.8.560. [DOI] [PubMed] [Google Scholar]
- 3.Garber AJ, Seidel J, Armbruster M. Current standards of care for inpatient glycemic management and metabolic control: is it time for definite standards and targets? Endocr Pract. 2004;10(Suppl 2):10–12. doi: 10.4158/EP.10.S2.10. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insula reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008–1011. doi: 10.2337/diacare.28.5.1008. [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30(9):2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 6.Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29–35. doi: 10.1370/afm.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545–552. [PubMed] [Google Scholar]
- 8.Alfonso A, Koops MK, Mong DP, Vigersky RA. Glycemic control with regular versus lispro insulin sliding scales in hospitalized type 2 diabetics. J Diabetes Complications. 2006;20(3):153–157. doi: 10.1016/j.jdiacomp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 10.Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G. Society of Hospital Medicine Glycemic Control Task Force. Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts. J Hosp Med. 2008;3(5 Suppl):66–75. doi: 10.1002/jhm.356. [DOI] [PubMed] [Google Scholar]
- 11.Braithwaite SS, Buie MM, Thompson CL, Baldwin DF, Oertel MD, Robertson BA, Mehrotra HP. Hospital hypoglycemia: not only treatment but also prevention. Endocr Pract. 2004;10(Suppl 2):89–99. doi: 10.4158/EP.10.S2.89. [DOI] [PubMed] [Google Scholar]
- 12.Leape LL, Berwick DM. Five years after To Err Is Human: what have we learned? JAMA. 2005;293(19):2384–2390. doi: 10.1001/jama.293.19.2384. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal MB. Nonpayment for performance? Medicare';s new reimbursement rule. N Engl J Med. 2007;357(16):1573–1575. doi: 10.1056/NEJMp078184. [DOI] [PubMed] [Google Scholar]
- 14.Joint Commission SCIP post-operative 6AM glucose in cardiac surgery patients. 2007. Available from: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/SCIP+Core+Measure+Set.htm.
- 15.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 16.Devos P, Preiser JC, Mélot C Glucontrol Steering Committee. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the Glucontrol study. Intensive Care Med. 2007;33:S189. [Google Scholar]