Abstract
Background
Little is known of patient acceptance of an artificial pancreas (AP). The purpose of this study was to investigate future acceptance of an AP and its determinants.
Methods
Patients with type 1 diabetes treated with insulin pump therapy were interviewed using questions based on the technology acceptance model and completed the diabetes treatment and satisfaction questionnaire (DTSQ).
Results
Twenty-two adults with type 1 diabetes participated. Half of the patients were followed in a university hospital, and the others were under treatment in an affiliated teaching hospital. Half of the patients were male. The mean DTSQ score was 29 (range 23–33). The AP was perceived as likely to be useful. Perceived advantages were a stable glucose regulation, less need for self-monitoring of blood glucose, relief of daily concerns, and time saving. Participants were confident in their capability to use the system. Although many participants (58%) had been reluctant to start continuous subcutaneous insulin infusion, the majority (79%) felt they would have no barriers to start using the AP. Trust in the AP was related to the quality of glucose control it would provide. Almost everyone expressed the intention to use the new system when available, even if it would initially not cover 24/24 hours.
Conclusion
The overall attitude on the AP was positive. Intention to use was dependent on trust in the AP, which was related to the quality of glucose control provided by the AP.
Keywords: acceptance, artificial pancreas, perception, type 1 diabetes mellitus
Introduction
Treatment for type 1 diabetes is noncurative. The artificial pancreas (AP) will hopefully be a technological improvement in the treatment of diabetes.1 The AP will most likely consist of a subcutaneous glucose sensor, a continuous subcutaneous insulin pump, and a mathematical model that regulates the amount of insulin based on the glucose levels.
Today, patients with type 1 diabetes have to administer insulin subcutaneously to control their glucose regulation. Insulin can be administered via multiple daily injections (MDI) or via continuous subcutaneous insulin infusion (CSII). The latter method has resulted in better glycemic control in adults without a higher rate of hypoglycemia and added more flexibility.2,3 Furthermore, quality of life was better on CSII compared to MDI.4 On the other hand, using an insulin pump may raise concerns about perceived body image and social acceptance.5 Continuous glucose monitoring (CGM) systems are able to provide a glucose value every three to five minutes. Compared to self-monitoring of blood glucose (SMBG), CGM can detect more periods of hypoglycemia6 and more frequent nocturnal hypoglycemia.7 Their use in adults improves glycemic control.8 However, diminishing use over time as seen in adolescents and younger children is a concern. Reasons for not wearing the sensors include skin irritations, inaccurate readings, and excessive alarms.9
Due to these factors, concerns could be raised about acceptance of the AP, since the AP will contain both devices. To our knowledge, patient-related factors determining acceptance of the AP have not yet been investigated. The aim of this study is to investigate the future acceptance and use of the AP as well as its possible determinants. We used interviews based on the technology acceptance model (TAM). The original model studied acceptance of new computer systems in order to improve job performance.
Methods
Patients
All patients had type 1 diabetes and were treated with CSII for at least one year. Half of them were followed in the outpatient clinic of a university hospital in Amsterdam, the Netherlands, and the other half were under treatment in an affiliated teaching hospital in Amsterdam, the Netherlands. Eleven of the 22 patients had already worn a CGM for a couple of days. All invited patients gave written informed consent. The number of patients was increased until saturation was reached.10
Interview
The TAM provides a general explanation of the intention to use a specific system, originally the determinants of computer acceptance and computer usage behavior.11–14
The original TAM was based on two concepts: perceived usefulness and perceived ease of use. Perceived usefulness was defined as the degree to which a person believes that using the new system would enhance job performance. Perceived ease of use was defined as to which degree a person believes that using a particular system would be free of effort. To increase the predictive value of TAM, both variables were extended with determinants of perceived usefulness and ease of use.
The proposed determinants of perceived usefulness were social influences and cognitive instrumental processes. Social influences were divided into three parameters: subjective norm, voluntariness of using the system, and image. Subjective norm was defined as the perception of the opinion of significant others on the behavior in question. The response of a person to social normative influences to establish or maintain a favorable image within a reference group enclosed the definition of image. Cognitive instrumental processes could be subdivided into the degree to which the new system is applicable to the job (job relevance), how well the new system performs (output quality), and tangibility of the results of using the new system (result demonstrability).
Perceived ease of use was extended with the following determinants: self-efficacy to perform a task with the computer (perceptions of internal control); facilitating conditions, such as a specific training (perceptions of external control); computer playfulness or openness to the process of using systems (intrinsic motivation); and computer anxiety (emotion).
Besides perceived usefulness and perceived ease of use, trust in the new system was also integrated in the model by Pavlou.15
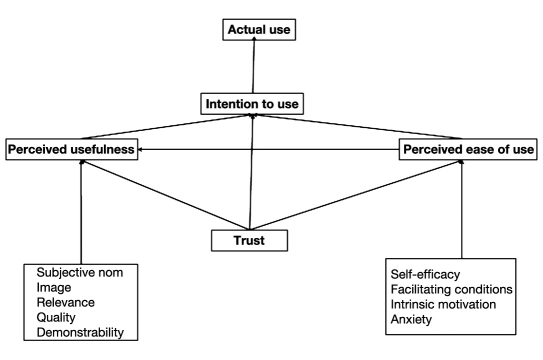
Figure 1 gives a visual representation of the model.
Figure 1.
The adapted technology acceptance model.
In the context of the AP, the intention to use the AP is the subjective probability that one will use the AP. Perceived usefulness is the degree to which the patient thinks the AP would facilitate glucose control. Its determinants are the degree of control of the glucose concentration (quality of the AP), results of the AP (health care cost and time saving, quality of life), relevance of using the AP, influence of relatives (subjective norm), and image in a peer group if using the AP.
Perceived ease of use is the degree to which the patient believes that using the AP would be free of effort. The determinants are self-efficacy to operate with the AP, training (external control), playfulness with the insulin pump (intrinsic motivation), and anxiety at the time of starting the new treatment. As a proxy for the latter, we inquired about anxiety at the time of starting insulin pump treatment. Trust in the AP manufacturer and trust in health care providers are additional factors.
Arianne C. van Bon conducted the in-depth interviews. At the start of the interview, a short introduction about the action of the AP was read, and a CGM (CGMS® System Gold™, Medtronic Minimed, Sylmar, CA) was shown to the persons who had not already worn a CGM. In the short introduction, the AP was described as a combination of CSII and CGM sensor, both connected to a mathematical model or controller what would be integrated in a device. The AP would control their basal insulin supplementation rate and would be able to give an insulin bolus in case of a snack or meal. A CGM would perhaps be inaccurate in the range of low and high glucose levels, so in case of an alarm, the patient would have to perform SMBG. Apart from checking the alarms, patients also would have to perform SBMG for calibration; this was set on twice a day. The sensor would need to be replaced every three to seven days. The interviews were recorded and transcribed literally.
Diabetes Treatment and Satisfaction Questionnaire
Before the start of the interview, the diabetes treatment and satisfaction questionnaire (DTSQ) was administered.16 This questionnaire contains eight items regarding satisfaction about the current treatment. Treatment satisfaction is the combined score of the first six items. The range of the score is 0 to 36, with higher scores indicating better satisfaction. The two remaining questions relate to perceived frequencies of hyperglycemia or hypoglycemia and are rated 0 to 6. Higher scores (e.g., 6 = most of the time) indicate respectively more hyperglycemia or hypo-glycemia.
Data Analysis
The data collection was an iterative process. Interviews underwent sequential analysis during data collection. All data relevant to each category were identified and examined using a process called constant comparison. The categories were mostly obtained deductively (Table 1). In order to analyze the interviews, they were imported in MAXQDA2007 (VERBI software, Marburg, Germany).
Table 1.
Overview of the Questions Based on the Technology Acceptance Model
| Overall attitude | Overall, do you like the AP? |
| If so, what do you like about it? | |
| Perceived usefulness | What possible advantages and disadvantages do you foresee? |
| Impact health care/relevance | Do you think the AP decrease the face-to-face visits of the doctor and the nurse? |
| Do you think that the use of the AP will improve the glucose control? | |
| Costs and time | Are you willing to pay a financial contribution? |
| Do you think the AP will save your time? | |
| Subjective norm | Do people who are important in your life influence the decision to use the AP? |
| Image | What kind of patients with diabetes will use the AP? |
| Which attitude (envy or serve as a guinea pig) will arise by other patients with diabetes if someone will use the AP? | |
| Perceived ease of use | Do you think the AP is easy to use? |
| Do you think other patients with diabetes would be able to use the AP? | |
| Perceived self-efficacy of using the AP | If the AP were available, are you confident that you could use the AP? |
| Perceptions of external control; training | What kind of training will suit you? |
| Intrinsic motivation; playfulness | Can you describe the daily use of the insulin pump? |
| Anxiety | Can you describe the period before the switch to CSII, especially your emotions? |
| Trust | Do you have confidence in the glucose regulation of the AP? |
| Are you willing to share the results of the AP with your doctor or nurse? | |
| Intention to use | If the AP were available, would you be interested in using it? |
| Conditions for use | If the AP is not able to perform 24 hours, are you willing to wear the AP? If so, which part of the day? |
| If you have to import the insulin bolus before meal, are you willing to wear the AP? | |
| What kind of alarm is necessary? | |
| What frequency of false alarms is acceptable? |
Results
Twenty-two patients were interviewed. Half of the 22 participants were male, and 16 of the 22 (72.7%) had high school education or held a university degree. Twelve persons had a paid job, four persons were retired, three were students, two were disabled, and one was a housewife.
The median age was 42 years (20–63 years). The median age at diagnosis was 15 years (5–40 years), and the median duration of pump use was 6 years (1–28 years).
Diabetes Treatment and Satisfaction Questionnaire
The mean treatment satisfaction score was 29 (range 23–33), indicating overall satisfaction with CSII. The scores for hyperglycemia and hypoglycemia were 4 and slightly above 3, respectively, comparable with regular occurrence of perceived hypoglycemia and hyperglycemia (Table 2).
Table 2.
Diabetes Treatment and Satisfaction Questionnairea
| Variable | Outcome |
|---|---|
| Treatment satisfaction | 29.0 (23–33) |
| Perceived frequency of hyperglycemia | 4.0 (2–6) |
| Perceived frequency of hypoglycemia | 3.1 (1–6) |
Values are in mean with the range of minimum and maximum.
Interview
Overall Attitude toward the Artificial Pancreas
The overall attitude about the AP was generally positive. Almost all patients labeled the AP as a positive or nice development. In their perceptions, the AP could realize a better glucose control. An overview is given in Table 3.
Table 3.
Overview of the Main Outcomes toward the Artificial Pancreasa
| Perceived usefulness | |
| Possible advantages | Stable glucose regulation (n = 17/22) |
| Diminish SMBG frequency (n = 10/22) | |
| Better quality of life (n = 20/20) | |
| Possible disadvantages | Second subcutaneous device (n = 20/22) |
| Technical accuracy (n = 8/22) | |
| Subjective norm (relatives' influences) | Yes (n = 3/20) |
| No (n = 17/20) | |
| Image (perceived persons to wear AP) | Insight in disease (n = 12/22) |
| Poorly regulated patients (n = 8/22) | |
| Adolescents (n = 5/22) | |
| Perceived ease of use | Easy to use in general (n = 15/22) |
| Easy to use for themselves (n = 22/22) | |
| Main barriers | Trust (n = 22) |
| Alarm frequency (n = 22) | |
| Intention to use | Yes (n = 19/22) |
Total number of patients is 22. The number of participants who gave an opinion on the issue mentioned is given per issue.
Perceived Usefulness
The most frequently stated perceived advantages of the AP were better and stable glucose control (77%) and lesser need for SMBG (46%). Furthermore, the presence of a display showing current glucose levels and alarms for too high or too low glucose levels was reassuring, and a display would give more insight in glucose regulation.
Positive psychological aspects were a better quality of life, mainly through better glucose control and less disease burden. But it was anticipated that the AP would not bring back normal life to most patients.
Two disadvantages were mentioned. The first was to wear another (second) subcutaneous device, but 75% of the patients thought they would overcome this problem because the advantages of the AP would outweigh this disadvantage. The second concern was technical accuracy of the AP, particularly during exercise, but again, the presence of a display showing the current glucose levels and facilitating trust in the AP would overcome this worry.
Costs and Savings
The frequency of visits to the doctor was estimated not to change, because screening for long-term complications and risk factors for cardiovascular disease would still be important. Besides, the visits to the diabetes nurse would temporarily increase when starting use of the AP. Also it would initially take more time to manage diabetes, mainly due to concerns about trust. Therefore, the overall frequency of SMBG would increase temporarily. But after some weeks, patients thought they would be less busy with their diabetes than before.
Almost half of the patients were not willing to (co)pay for using the AP. The reason was related to the fully reimbursed health care system in the Netherlands. The other half would be willing to pay up to 1–2% of their monthly salary.
Subjective Norm
The majority of the participants (17/20, 85%) would listen to arguments of their relatives but would make their own decision. The others would delay their decision if their partner was not convinced.
Image
The aspect of perception was assessed by two questions. The first question was “What kind of response of other patients with diabetes would you expect if you wear the AP? Would they see you as a guinea pig or would they be jealous?” Half of the patients answered a combination of jealousy and being seen as a guinea pig, and 36% of the participants expected a jealous response if they would wear the AP. Three participants assessed the response of others as being interested more than jealous or seeing them as a guinea pig.
The second question was “What kind of person do you think would use the AP?” Several answers per participant were given. Most patients (55%) described the type of person who would use the AP as a person who had a clear understanding of diabetes and was involved in its treatment. Also, patients with subjective poor glucose regulation (36%) were willing to use the AP. Adolescents were supposed to use the new system more readily than older adults.
Perceived Ease of Use
The overall opinion about the perceived ease of use of the AP was positive. The perceived capacity of others to use the AP varied from “everybody is able to use it” to “the use is dependent on having an open mind or being young.”
Self-Efficacy and Training
All participants expected themselves to be capable to use the AP, but they expected to need an introduction before the use of the AP. Everyone but two preferred to start with an individual training. Half of the patients wanted to join a group consultation after having used the AP for a couple of weeks.
Technical Skills and Anxiety
At the moment of the interview, half of the participants only used the basics of their insulin pump, such as basal rate of the insulin infusion and temporary reduction of the insulin rate. The other half used more possibilities of the insulin pump, such as long- or dual-wave insulin bolus.
Anxiety to use a new system was conceptualized with reference to the emotions at the time of starting the insulin pump. Almost half of the patients (42%) had not experienced any anxiety before starting the insulin pump. Others had been reluctant to start using the insulin pump. It had taken more than a year to change therapy from MDI to subcutaneous insulin therapy in six persons. The most frequently given reason for this reluctance had been the idea of having a device on the body, and for two persons, the needles in the abdomen had been a very unpleasant idea. One person was aware of a bad experience involving a close friend with fatal hypoglycemia when using the pump. This anxiety concerning the start with an AP was present in four participants and was thus related to trust issues.
Trust
Nobody would rely immediately on the action of the AP. Trust was related to the quality of glucose regulation provided by the AP. The participants would initially increase the frequency of SMBG to check the accuracy of the AP. Furthermore, a display that shows current glucose levels would augment trust. The average period of distrust was estimated to be two weeks.
All but two participants would prefer if the doctor or nurse were able to print out the glucose control. This printout would be an opportunity to discuss glucose regulation and to obtain more insight in one's glucose control.
Intention to Use
One person (4.5%) was not willing to use the AP. Two persons (9.1%) would prefer to test the AP, and if successful, they would continue using the AP. The others (86.4%) would certainly use the AP.
Minimal Conditions to Use the Artificial Pancreas
To find out the minimal conditions for use of the AP, four additional questions were asked. Even if it would be technically not feasible to use the AP for 24/24 hours, almost every participant would use the AP. If limited to either daytime or nighttime, daytime was mostly favored over nighttime, by 14 versus 6, respectively. Two persons did not have any preference. The participation would decrease if partial use of the AP would lead to extra needle insertions.
If the mealtime insulin bolus had to be given manually, all would still like to use the AP.
All participants would like to have alarms in the AP, mostly with both vibration and sound. The indications mentioned for the alarms to go off included the occurrence of hypo- and hyperglycemia—which would actually be failures of the AP—and low batteries. Some would like to see an alert if the basal insulin supplementation rate would change substantially.
Too many alarms, certainly any kind of false alarms, would impede the use of the AP. With regard to deviations between sensor glucose and SMBG values noticed after a confirmatory SMBG following an alarm, false alarms were divided into minor and major false alarms. A minor alarm was defined as less than a 1 mmol/liter glucose difference between the sensor glucose and the SMBG, and a major false alarm was defined as more than a 1.5–2 mmol/liter glucose difference. Minor false alarms were better accepted than major alarms. As to major alarms, they were mostly thought acceptable in a frequency of 1 out of 10 alarms. Minor alarms were mostly thought acceptable in a frequency of 2 out of 10.
Conclusions
The aim of the study was to investigate future acceptance of the AP and its possible determinants. To our knowledge, this study is the first analysis of patients' acceptance of the AP. Although most participants were relatively satisfied with their current treatment, almost all had the intention to use the AP. Strikingly, even if the system would not cover 24 hours or the mealtime bolus has to be given manually, they were still willing to use the AP. Furthermore, the use of a second subcutaneous device, CGM, was not a major obstacle for using the AP.
The major, logical concern that would influence the use of the AP was trust. At the start of using the AP, patients would increase their SMBG frequency to check the system. If the system would work well, defined as satisfactory glucose levels during, on average, the first two weeks, trust in the AP would augment and patients would diminish the frequency of SMBG. Remarkably, the device should have a display showing the current glucose levels, indicating that patients are willing to let the AP control the glucose regulation, but they still want to have the ability to check at any given time. Another important aspect of willingness to use the AP would be the occurrence of false alarms. The accepted frequency for minor false alarms was 2 out of 10 alarms and for major false alarms 1 out of 10. If false alarms would occur more frequently, trust in the AP would diminish and willingness to use the AP would decrease. The perceived usefulness was described as better well-being due to better glucose control and less impact of diabetes on daily life. The possibility to set alarms for too high or too low glucose levels was reassuring and was expected to augment the perceived usefulness. The perceived ease of use was expected to be large, and the participants expected themselves to be capable of using the AP. All patients agreed that training before using the AP would be useful.
This study has its limitations. First, all patients who were interviewed already wore an insulin pump, so generalizability of all these findings is limited to such patients. However, their previous experiences when starting pump treatment may result in a more realistic assessment of the expected problems at the start of the AP and the aspect of wearing two subcutaneous devices. If the perceived reluctance before using the AP can be deducted from the reluctance experienced before starting the insulin pump, half of the patients would have inhibitions to start using the AP. A second limitation could be that only half of the patients have worn a continuous glucose monitor, so not all patients have experienced wearing a second device. Another limitation is that the majority of the interviewees were highly educated. To extend the insight into the determinants of future acceptance and use of the AP, the next step of our research will be to develop a questionnaire based on the principles of the TAM and the outcome of these interviews. Such a questionnaire would enable investigations in a much larger number of patients.
To conclude, most participants had the intention to use the AP even though they were relatively satisfied with their current diabetes treatment. Trust in the new system was a concern, but most participants anticipated that they would trust the system after the initial period.
Acknowledgment
We thank C. B. Brouwer, M.D., Ph.D., physician of the patients interviewed from the affiliated hospital.
Abbreviations
- AP
artificial pancreas
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- DTSQ
diabetes treatment and satisfaction questionnaire
- MDI
multiple daily injections
- SMBG
self-monitoring of blood glucose
- TAM
technology acceptance model
References
- 1.Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcome. Diabetes Technol Ther. 2009;11(Suppl 1):S113–S119. doi: 10.1089/dia.2009.0031. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Hammond P. NICE guideline on continuous subcutaneaous insulin infusion 2008: review of the technology appraisal guidance. Diabet Med. 2009;26(1):1–4. doi: 10.1111/j.1464-5491.2008.02617.x. [DOI] [PubMed] [Google Scholar]
- 3.Retnakaran R, Hochman J, DeVries JH, Hanaire-Broutin H, Heine RJ, Melki V, Zinman B. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004;27(11):2590–2596. doi: 10.2337/diacare.27.11.2590. [DOI] [PubMed] [Google Scholar]
- 4.Hoogma RP, Hammond P, Gomis R, Kerr D, Bruttomesso D, Bouter KP, Wiefels KJ, de la Calle H, Schweitzer DH, Pfohl M, Torlone E, Krinelke LG, Bolli GB 5-Nations Study Group. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med. 2006;23(2):141–147. doi: 10.1111/j.1464-5491.2005.01738.x. [DOI] [PubMed] [Google Scholar]
- 5.Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care. 2007;30(3):549–554. doi: 10.2337/dc06-1755. [DOI] [PubMed] [Google Scholar]
- 6.Maia FF, Araújo LR. Efficacy of continuous glucose monitoring system (CGMS) to detect postprandial hyperglycemia and unrecognized hypoglycemia in type 1 diabetic patients. Diabetes Res Clin Pract. 2007;75(1):30–34. doi: 10.1016/j.diabres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Wentholt IM, Maran A, Masurel N, Heine RJ, Hoekstra JB, DeVries JH. Nocturnal hypoglycaemia in type 1 diabetic patients, assessed with continuous glucose monitoring: frequency, duration and associations. Diabet Med. 2007;24(5):527–532. doi: 10.1111/j.1464-5491.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 8.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Research in Children Network (DirecNet) Study Group. Youth and parent satisfaction with clinical use of the GlucoWatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care. 2005;28(8):1929–1935. doi: 10.2337/diacare.28.8.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowen GA. Naturalistic inquiry and the saturation concept: a research note. Qual Res. 2008;8(1):137–152. [Google Scholar]
- 11.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly. 1989;13(3):319–340. [Google Scholar]
- 12.Venkatesh V, Morris MG. Why don't men ever stop to ask for directions? Gender, social influence, and their role in technology acceptance and usage behavior. MIS Quarterly. 2000;24(1):115–139. [Google Scholar]
- 13.Venkatesh V. Determinants of perceived ease of use: integrating control, intrinsic motivation, and emotion into the technology acceptance model. Inform Sys Res. 2000;11(4):342–365. [Google Scholar]
- 14.Venkatesh V, Davis FD. A theoretical extension of the technology acceptance model: four longitudinal field studies. Manag Sci. 2000;46(2):186–204. [Google Scholar]
- 15.Pavlou PA. Consumer acceptance of electronic commerce: integrating trust and risk with the technology acceptance model. Int J Electron Commer. 2003;7(3):101–134. [Google Scholar]
- 16.Bradley C. Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Switzerland: Harwood Academic; 1994. pp. 111–132. [Google Scholar]