Abstract
Introduction
While the endogenous first-phase insulin response has disappeared by the time of diagnosis of type 1 diabetes mellitus (T1DM), anecdotal evidence suggests that these patients can continue to have a second-phase insulin response during the first 12 months after diagnosis. We hypothesized that patients who are started on continuous subcutaneous insulin infusion (CSII) at the time of diagnosis of T1DM would have a lower basal insulin requirement than the 40-60% usually expected.
Methods
We analyzed 38 patients with T1DM, age 9.9 ± 6.4 years, 71% male, who were started on CSII within the first month of diagnosis.
Results
Average basal insulin requirements were 47–49% of total daily dose during the first 12 months after diagnosis and decreased from 0.30 U/kg/day at diagnosis to 0.20 U/kg/day by 12 months. Baseline percentage of basal insulin was significantly correlated with hemoglobin A1c at baseline and at six months. The percentage of basal insulin requirement at 12 months after diagnosis was significantly correlated with baseline body mass index (BMI) and current BMI. No other correlations between percentage of basal insulin requirements and any other factors were seen.
Conclusion
Our data suggest that, even though some endogenous insulin production remains during the first year after diagnosis of T1DM, the distribution of basal versus total daily insulin requirements remains the same as in the general population of people with diabetes. There may be benefits to starting patients on a higher basal rate at time of diagnosis for overall glycemic control during the first six months. Further research is needed to optimize starting insulin doses to maximize their potential in preserving beta-cell function.
Keywords: basal insulin production, basal insulin requirements, continuous subcutaneous insulin infusion, insulin pump, type 1 diabetes mellitus
Introduction
Insulin secretion in healthy individuals without diabetes is characterized by continuous basal secretion with peaks that occur soon after meals.1 Basal insulin secretion is the amount of insulin secreted in the fasting state in the absence of exogenous stimuli.2 It has been estimated that 50% of the total insulin secreted by a healthy pancreas is secreted under basal conditions.3 Stimulated insulin secretion, which comprises the remaining 50%, occurs in response to exogenous stimuli such as ingested meals. Glucose is the most potent stimulant of insulin release. When the glucose concentration in an individual is increased suddenly, an initial short-lived burst of insulin release occurs (first-phase insulin response); if the glucose concentration is held at this level, the insulin release gradually falls off and then begins to rise again to a steady level (second-phase insulin response).2
The amount of insulin produced by a lean, healthy individual is usually between 18 and 40 U/day or 0.2–0.5 U/kg/day. Because half of this amount is secreted in the basal state while the other in response to meals, the basal insulin secretion is about 0.5–1.0 U/h. After meal ingestion, insulin secretion rapidly increases to up to six times the baseline secretion in response to the oral glucose load and reaches a peak within 60 minutes.3,4
In type 1 diabetes mellitus (T1DM) there is a marked deficiency of beta-cell mass. Prior to the development of diabetes, there is a prolonged period when the auto-immune disease is thought to be active. During this latent period, a progressive decline in first-phase insulin secretion has been documented, as well as an impairment in insulin pulses. By the time of clinical presentation of T1DM, 90% of beta cells have been lost, although some capacity for endogenous insulin secretion remains. The endogenous insulin production rapidly declines over the next two years after diagnosis.5
However, clinical evidence has shown that individuals with T1DM can continue to have a second-phase insulin response during the first 12 months after diagnosis. In the same way, it has been repeatedly demonstrated that treatment with continuous subcutaneous insulin infusion (CSII), or insulin pumps, approximates normal insulin physiology better than multiple daily injections of short- and long-acting insulin and is the more logical mode of treatment for T1DM.6–9
In patients with established T1DM, the daily basal insulin requirement is generally expected to be in the range of 40–60% of the total daily insulin dose.10,11 However, in light of persisting second-phase insulin secretion early in the course of T1DM, individuals who are started on CSII at the time of diagnosis of T1DM should theoretically require less exogenous basal insulin than individuals with long-standing diabetes. Alternatively, these individuals may have lower bolus insulin requirements and therefore be on a higher percentage of basal insulin, because part of the endogenous bolus feature is preserved. This study was designed to test this hypothesis and to find what factors, if any, were associated with these individuals' exogenous basal insulin requirements.
Research Design and Methods
This study is part of a larger study that was designed to examine the feasibility and effects of starting children and young adults on insulin pumps from the time of diagnosis of T1DM.6 Subjects were recruited for this 12-month prospective study from two major medical centers in New York City. Patients and their families were approached during the initial hospitalization or an outpatient visit soon after being diagnosed with T1DM to inquire if they wanted to participate in a research study. The clinicians who approached these patients ensured that the families had time to absorb the diagnosis of T1DM before discussing the study with them. Both the patient and their family were educated about diabetes, and the physician explained the rationale of starting pump therapy early. Upon agreeing to participate in the study, subjects and their families were taught insulin pump mechanics and troubleshooting, and carbohydrate counting was discussed with a nutritionist. Inpatients (n = 12) were started on loaner insulin pumps with lispro insulin immediately upon completion of learning pump mechanics and troubleshooting, while outpatients (n = 26) were started on loaner insulin pumps filled with saline, which they wore for one week to become comfortable with CSII before filling the pump with lispro insulin.
Initial follow-up was done via daily phone calls to a designated member of the diabetes team for the first few days. Further follow-up included weekly phone calls to the diabetes provider for blood glucose (BG) management and troubleshooting lifestyle issues. The loaner pumps were replaced by subjects' own pumps upon receipt of the latter. Every subject was seen in the office for a follow-up visit one month after the initiation of CSII and once every three months thereafter, or more often if needed.
To measure endogenous insulin production, a 3 hour mixed meal tolerance test (MMTT) was performed in 28 subjects at baseline, 6 months, and 12 months after diagnosis. Subjects' pumps were disconnected at the 6- and 12-month MMTTs 4 h prior to the scheduled start of the MMTT to minimize the suppressive influence of exogenous insulin on endogenous insulin production. In case of extremely high blood sugars and moderate or large ketones, the MMTT could be stopped at the discretion of the researchers. No MMTT had to be stopped because of ketosis. Blood glucose excursions during the MMTT were calculated as BGpeak – BGt = 0. C-peptide values were analyzed looking at the area under the curve (AUC).
The data were analyzed using both Statistical Package for the Social Sciences and Microsoft Excel for calculating demographic data, mean and standard deviations, unpaired two-tailed t-tests, and correlations (Pearson correlation coefficients).
Results
Thirty-eight patients (27 males, 11 females) were started on CSII within one month of diagnosis of T1DM. The subjects ranged in age from 1 to 32 years old, with a mean age of 9.9 ± 6.4 years. Hemoglobin A1c (HbA1c) decreased to 6.6 ± 1.1% by three months after diagnosis and continued to stay at levels indicative of good glycemic control throughout the study. Demographic variables of the study population are presented in Table 1. The majority of patients (68%) were treated on an outpatient basis when diagnosed.
Table 1.
Demographic Data
| N | 38 |
| Male | 71% (n = 27) |
| Female | 29% (n = 11) |
| Age at diagnosis(range) | 9.9 ± 6.4 years |
| (1.3–32.3 years) | |
| Ethnicity | Caucasian: 25 (66%) |
| Hispanic: 8 (21%) | |
| Black: 1 (3%) | |
| Asian: 1 (3%) | |
| Middle Eastern: 1 (3%) | |
| Other: 2 (5%) |
Basal insulin requirement as a percentage of total daily dose (TDD) was found to stay relatively constant during the first 12 months after diagnosis and averaged 47–49% of the TDD (range = 16–100%, p = not significant between values at 0, 6, and 12 months). Total daily dose expressed in units per kilogram also did not decrease significantly during this time period. However, the absolute basal insulin requirements decreased from 0.30 U/kg/day at the time of diagnosis to 0.20 U/kg/day by 12 months (p = .05; p = not significant between other time points) (Table 2).
Table 2.
Hemoglobin A1c, Insulin Requirements, and Body Mass Index Over Time
| 0 months | 6 months | 12 months | |
|---|---|---|---|
| HbA1c (%) | 10.9 ± 2.3 | 6.6 ± 1.2 | 7.4 ± 1.8 |
| TDD U/kg/day | 0.55 ± 0.25 | 0.46 ± 0.22 | 0.50 ± 0.19 |
| Basal U/kg/day | 0.30 ± 0.16 | 0.24 ± 0.13 | 0.20 ± 0.11 |
| Basal as percentage of TDD | 48 ± 6% | 49 ± 20% | 47 ± 23% |
| BMI (kg/m2) | 20.0 ± 6.4 | 19.6 ± 4.7 | 22.0 ± 7.1 |
The percentage of basal insulin at baseline inversely correlated with HbA1c at baseline (r = -0.513, p = .01) as well as six months after diagnosis (r = -0.501, p = .03). A higher body mass index (BMI) at baseline and at 12 months was significantly correlated with higher basal insulin requirements at 12 months after diagnosis (r = 0.636, p < .05 and r = 0.651, p = .04, respectively). No significant correlations were noted between basal insulin requirements and age at diagnosis, HbA1c, weight, BMI, or total exogenous insulin requirements.
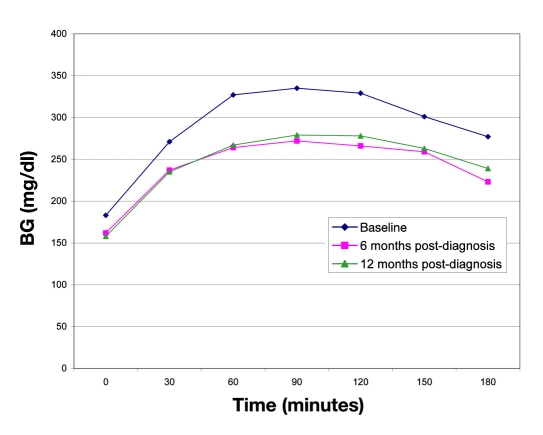
On the 3-hour MMTT, the BG measurements peaked at 90 minutes and then decreased. However, none of the differences observed after the 90-minute time point achieved statistical significance. The BG excursions at 0, 6, and 12 months were 176, 134, and 132 mg/dl, respectively (p = not significant). The BG measurements on MMTT are depicted in Figure 1. Additionally, C-peptide AUC values during the MMTTs did not significantly differ during the first 12 months after diagnosis (C-peptide AUCbaseline = 267 ± 363 ng/dl, C-peptide AUC6 months = 110 ± 94 ng/dl, C-peptide AUC12 months = 312 ± 364 ng/dl, p = not significant between all time points).6
Figure 1.
Average BG values during 3-hour MMTTs.
Discussion
The hypothesis that the presence of endogenous insulin secretion would cause a lower exogenous basal insulin requirement did not hold true in this study, nor was a decrease in the amount of bolus insulin as a percentage of TDD observed. Despite being in honeymoon and having evidence of endogenous insulin secretion during the first 12 months of follow-up that was not significantly different than the amount that existed at the time of diagnosis of T1DM,6 the subjects in this study required roughly the same percentage of exogenous basal insulin as do people with longstanding diabetes.10,11 It is presumed that the basal insulin requirements observed are actual basal requirements, as most subjects' BG levels were within normal limits and very rarely rose above 140 mg/dl (7.8 mmol/liter) during the first 12 months after diagnosis. Correction boluses had to be given so infrequently that subjects involved in this study often did not remember what their insulin sensitivity factor was. Other researchers have found basal insulin requirements to be as low as 20–30% of TDD in pediatric patients on CSII.11,12 However, their results are based on cross-sectional data from subjects of varying durations of T1DM. No other study that has been done to date specifically describes insulin requirements in children specifically with new-onset T1DM. It is unknown if our study population differs from the subjects in the aforementioned studies in regards to the postprandial glycemic rise or AUC, as this information is not available for comparison.
The decrease in exogenous basal insulin requirements that was observed during the course of this study may be due to reversal of the initial glucose toxicity that occurs when T1DM presents. However, since the basal insulin requirements were not significantly different from baseline until 12 months after diagnosis, this is likely not the case. Rather, the improvement may be indicative of a slow recovery of beta-cell function. A more detailed prospective study should be done to determine the physiological factors associated with our observations.
The results of the MMTT, specifically, automatically decreasing BG after the 90-minute time point in the 3-hour test, were also suggestive of a second-phase insulin response. However, given the results, it appears possible that the apparent glycemic improvement may have been caused by glucose decay rather than endogenous insulin secretion, or perhaps by a combination of the two.
Various large studies, including the Diabetes Control and Complications Trial, have demonstrated the efficacy of CSII in lowering HbA1c and improving metabolic control, including in the pediatric population, without increasing the risk for hypoglycemia.13–19 Additionally, the Epidemiology of Diabetes Interventions and Complications study showed that good control achieved early in the course of the disease has better long-term outcomes than achieving good control later on, even if this degree of glycemic control is not able to be maintained over time.18 The findings from our study support the same trend toward initiating CSII soon after diagnosis of diabetes.
Additionally, in this particular cohort of patients, a significant correlation was noted between a higher exogenous basal insulin dose and lower HbA1c at diagnosis and six months later. As has also been found by others in subjects with type 2 diabetes mellitus, this inverse correlation suggests that the use of a higher basal insulin infusion at diagnosis may be beneficial for achieving better glycemic control during the first six months after diagnosis.20,21 The beneficial effects of this initial intensive therapy have the potential to persist for several years afterward, even if glycemic control should worsen over time.18 However, these benefits do not last indefinitely.19 Further research needs to be done to determine the impact of good glycemic control right from the time of diagnosis on the incidence of diabetes complications in the future.
Conclusion
In patients with newly diagnosed T1DM, the distribution of basal and total daily insulin requirements is similar to the general population of people with established diabetes, even during the honeymoon phase. An observed decrease in BG after the 90-minute time point of a MMTT does not support the hypothesis that patients with newly diagnosed T1DM require less basal insulin because of endogenous basal insulin production. In fact, those who had a higher percentage of basal insulin through six months after diagnosis of T1DM had lower HbA1c levels, suggesting that there may be benefits to starting patients on a higher basal rate at the time of diagnosis for overall glycemic control during the first six months. Further research needs to be done so insulin doses can be optimized to maximize their potential in preserving beta-cell function.
Abbreviations
- AUC
area under the curve
- BG
blood glucose
- BMI
body mass index
- CSII
continuous subcutaneous insulin infusion
- HbA1c
hemoglobin A1c
- MMTT
mixed meal tolerance test
- T1DM
type 1 diabetes mellitus
- TDD
total daily dose
References
- 1.Ferrannini E, Pilo A. Pattern of insulin delivery after intravenous glucose injection in man and its relation to plasma glucose disappearance. J Clin Invest. 1979;64(1):243–254. doi: 10.1172/JCI109445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner DG, Shoback D. Greenspan's basic and clinical endocrinology. 8th. New York: McGraw Hill; 2007. [Google Scholar]
- 3.Kronenberg HM, Melmed S, Polonsky KS, Larsen PR. Williams textbook of endocrinology. 11th. St. Louis: Saunders; 2008. [Google Scholar]
- 4.Brunton L, Parker K, Blumenthal D, Buxton I. Goodman and Gilman's manual of pharmacology and therapeutics. New York: McGraw-Hill; 2008. [Google Scholar]
- 5.Degroot L, Jameson JL. Endocrinology: volume 1. 5th. Philadelphia: Saunders; 2005. [Google Scholar]
- 6.Ramchandani N, Ten S, Anhalt H, Sinha S, Ching J, Finkelstein A, Maclaren NK. Insulin pump therapy from the time of diagnosis of type 1 diabetes. Diabetes Technol Ther. 2006;8(6):663–670. doi: 10.1089/dia.2006.8.663. [DOI] [PubMed] [Google Scholar]
- 7.Bolli GB. Insulin treatment in type 1 diabetes. Endocr Pract. 2006;12(Suppl 1):105–109. doi: 10.4158/EP.12.S1.105. [DOI] [PubMed] [Google Scholar]
- 8.Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27(7):1554–1558. doi: 10.2337/diacare.27.7.1554. [DOI] [PubMed] [Google Scholar]
- 9.Renard E. Intensive insulin therapy today: ‘basal-bolus’ using multiple daily injections or CSII? Diabetes Metab. 2005;31(4 Pt 2):4S40–4S44. doi: 10.1016/s1262-3636(05)88266-7. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract. 2006;73(1):35–40. doi: 10.1016/j.diabres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Shalitin S, Phillip M. The use of insulin pump therapy in the pediatric age group. Horm Res. 2008;70(1):14–21. doi: 10.1159/000129673. [DOI] [PubMed] [Google Scholar]
- 12.Pańkowska E, Szypowska A, Lipka M. Basal insulin and total daily insulin dose in children with type 1 diabetes using insulin pumps. Pediatr Diabetes. 2008;9(3 Pt 1):208–213. doi: 10.1111/j.1399-5448.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 15.Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care. 1999;22(11):1779–1784. doi: 10.2337/diacare.22.11.1779. [DOI] [PubMed] [Google Scholar]
- 16.Danne T, Mortensen HB, Hougaard P, Lynggaard H, Aanstoot HJ, Chiarelli F, Daneman D, Dorchy H, Garandeau P, Greene SA, Hoey H, Holl RW, Kaprio EA, Kocova M, Martul P, Matsuura N, Robertson KJ, Schoenle EJ, Søvik O, Swift PG, Tsou RM, Vanelli M, Aman J. Hvidøre Study Group on Childhood Diabetes. Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidøre Study Group. Diabetes Care. 2001;24(8):1342–1347. doi: 10.2337/diacare.24.8.1342. [DOI] [PubMed] [Google Scholar]
- 17.Potti LG, Haines ST. Continuous subcutaneous insulin infusion therapy: a primer on insulin pumps. J Am Pharm Assoc. 2009;49(1):e1–e13. doi: 10.1331/JAphA.2009.08122. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(6):381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Research Group; Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, Orchard TJ Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005) Arch Intern Med. 2009;169(14):1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20(9):1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 21.Chen HS, Wu TE, Jap TS, Hsiao LC, Lee SH, Lin HD. Beneficial effects of insulin on glycemic control and beta-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care. 2008;31(10):1927–1932. doi: 10.2337/dc08-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]