Abstract
Background
The glycemic index (GI) is routinely measured 120 minutes after food intake (GI120). The purpose of this prospective open label study was to assess (1) the dynamics of glycemia over the 210 minutes following food consumption and (2) the evolution of GIs based on 120-, 150-, 180-, and 210-minute glycemic profiles.
Method
Twenty healthy subjects (mean ± SE; 21.9 ± 1.39 years of age; body mass index 23.6 ± 0.63 kg/m2; 7 men and 13 women) completed the study. Each subject consumed 10 different foods with known GI120 on three separate occasions at four different times of day according to a defined meal plan over a 9-day period; 32 meals were evaluated. The GIs for intervals of 120, 150, 180 and 210 minutes after food consumption were determined using a continuous glucose monitoring system (CGMS) to measure glycemia. The Wilcoxon signed-rank test was applied to compare the GIs.
Results
Glycemia returned to baseline within 120 minutes for honey and tomato soup; within 210 minutes for white bread, choco-rice cookies, fish and potatoes, wafers, and meat ravioli with cheese; and later for dark chocolate, apricot dumplings, and choco-wheat cookies. The extended GIs were higher than the respective GI120s in eight of the foods.
Conclusions
The 120-minute glycemic index fails to fully account for changes in glycemia after ingestion of a mixed meal because glycemia remains above baseline for a longer period. The CGMS is a convenient method to determine the glucose response/GIs over intervals extended up to 210 minutes, which is adequate time for the absorption of most foods.
Keywords: continuous glucose monitoring, data processing software, extended glycemic index, mixed meal
Introduction
The glycemic index (GI) is a measure of the hyper-glycemic power of food relative to a standard food challenge of 50 grams of glucose in 300 ml of water. The GI is defined as the incremental area under the blood glucose response curve of a 50-gram carbohydrate portion of a test food expressed as a percentage of the response to the same amount of carbohydrate from a standard food taken by the same subject. Both areas are calculated during a 120-minute interval after the ingestion of food, ignoring areas beneath the fasting glucose concentration.1
Since the pioneering papers of Otto and colleagues2 and Jenkins and colleagues,3 the concept of the GI has become an almost integral part of meal planning in some countries.4–8 In 1998, the World Health Organization and the Food and Agricultural Organization recommended including GI values in nutritional tables,1,9 while the American Diabetes Association only states that consideration of GI may provide modest additional benefits for glycemic control over that observed when total carbohydrate is considered alone.10 To date, over 600 papers with “glycemic/glycaemic index” in the title/abstract have been filed in the PubMed database.
A new GI determination method based on a commercially available continuous glucose monitoring system (CGMS) and software Solutions™ (Medtronic MiniMed, Northridge, CA),11 together with the original software DegifXL (Palacky University Olomouc, Czech Republic),12 has been developed. The CGMS is a well-recognized tool currently used by health care professionals and persons with diabetes to identify variations in glycemia13 with accuracy similar to that of self-monitoring on glucometers.14–16 The DegifXL4 software enables easy data processing and GI determination four times per day (breakfast, lunch, afternoon snack, and dinner).17 No significant differences exist between GI values obtained by the CGMS and those obtained by more time-consuming conventional methods.18
Research of the GI addresses factors that influence the variability of postprandial glycemia (prolonged exercise, previous ingestion of high glycemic index meal, content of fiber, etc.).19–24 Special attention is paid to the method-ology of GI determination.25
After intake of 50 grams of glucose standard or some other foods, the plasma glucose concentration returns to baseline level within 120 minutes. Therefore, further determination of glycemia is not deemed necessary. However, after the ingestion of mixed meals, postprandial hyperglycemia may last longer. We hypothesized that the numerical values representing the glucose response or hyperglycemic power of some foods (i.e., their “extended” GIs) would increase when measured beyond the routinely used 120-minute interval, leaving the question of whether the GI120 is predictive of post-prandial glycemia development. The purpose of this study was to assess (1) the blood glucose dynamics during the 210 minutes after food consumption and (2) the development of GIs based on 120-, 150-, 180-, and 210-minute glycemic profiles.
Subjects and Methods
Subjects
In 2007, 25 students were enrolled in this study. The following eligibility criteria were used: good health (i.e., no abnormalities on clinical examination and laboratory screening), no medication, nonsmoker, body mass index (BMI) <30 kg/m2, willingness to perform continuous glucose monitoring, and commitment to keep to the meal plan for 9 days. The planned number of volunteers over 10 was mandatory in order to make comparison with methods and results of other groups possible. The subjects provided written informed consent and were enrolled in the study. All procedures were performed in accordance to the Helsinki Declaration of 1975 as revised in 2000 and approved by the local ethics committee at Teaching Hospital Olomouc, Czech Republic.
Subjects were tested in five nonrandomized groups over the course of five sequential 9-day periods. Each group consisted of three to five subjects. There were five drop-outs due to common cold (n = 4) and gastroenteritis (n = 1). Thus, data from 20 Caucasian persons (mean ± SE; 21.9 ± 1.39 years of age; BMI 23.6 ± 0.63 kg/m2; 7 men and 13 women) were evaluated.
Test Meals
Ten popular foods/mixed meals were tested (Table 1). The choice of foods was influenced by the amount of carbohydrates in one serving (50 grams ± 5%) as listed on the package label. All of the meals, except for white bread, were purchased at one time before the beginning of the study. White bread was supplied fresh, twice per week. The meat ravioli was canned, the fried fish and apricot dumplings were frozen, and the tomato soup and mashed potatoes were in powder form when purchased.
Table 1.
Nutrient and Energy Content in Each Portion of Tested Food (Adopted from Nutritional Labels Included with Original Products); Glycemic Index
| Carbohydrates (g) | Lipids (g) | Proteins (g) | Energya (kcal) | GI120b (%) | |
|---|---|---|---|---|---|
| Breakfast and dinner meals | |||||
| 1. Glucose solution | 50 | 0 | 0 | 200 | 100 |
| 2. Dark chocolate (70% cocoa) | 50 | 62 | 13 | 810 | 35 |
| 3. Puffed chocolate-covered rice cookies (choco-rice squares) | 50 | 19 | 7 | 399 | 105 |
| 4. White bread | 50 | 3 | 8 | 259 | 93 |
| 5. Lime blossom honey | 50 | 0 | 0 | 200 | 77 |
| Lunch meals | |||||
| 6. Meat ravioli, Edam cheese | 50 | 32 | 36 | 632 | 43 |
| 7. Fried fish, mashed potatoes, fresh butter | 50 | 34 | 16 | 570 | 94 |
| 8. Apricot dumplings, fresh butter | 50 | 27 | 20 | 523 | 75 |
| Snack meals | |||||
| 9. Wafers with chocolate filling | 50 | 31 | 4 | 495 | 79 |
| 10. Puffed chocolate-covered wheat cookies (choco-spelt squares) | 50 | 22 | 9 | 434 | 64 |
| 11. Tomato soup | 50 | 3 | 6 | 251 | 40 |
Energy amounts: 1 gram carbohydrate = 4 kcal, 1 gram lipid = 9 kcal, 1 gram protein = 4 kcal.
Mean glycemic index (GI120).26
Fifty grams of glucose powder (used as the standard) was packed in small plastic containers in the pharmacy and dissolved by the participant in 300 ml of water or tea 5 minutes before consumption.
In the defined meal plan, the foods were divided into three groups according to the time of consumption (Table 1).
Glucose and each tested food were consumed at three different times; only tomato soup was consumed twice due to technical reasons. At the breakfast test on day 9 the subjects had only one possibility to consume a test meal that could not be consumed according to the meal plan (Table 2).
Table 2.
Defined Meal Plan: A Total of 32 Servings of 10 Test Meals and a Glucose Standard
| Day | Breakfast, 7 a.m. | Lunch, 12 a.m. | Snack, 4 p.m. | Dinner, 8 p.m. |
|---|---|---|---|---|
| 1 | — | 1. Meat ravioli with cheese | 2. Puffed choco-wheat cookies | 3. Dark chocolate |
| 2 | 4. Glucose | 5. Fried fish, mashed potatoes, butter | 6. Wafers | 7. Puffed choco-rice cookies |
| 3 | 8. White bread | 9. Apricot dumplings, butter | 10. Tomato soup | 11. Lime blossom honey |
| 4 | 12. Dark chocolate | 13. Meat ravioli with cheese | 14. Puffed choco- wheat cookies | 15. Glucose |
| 5 | 16. Puffed choco-rice cookies | 17. Fried fish, mashed potatoes, butter | 18. Wafers | 19. White bread |
| 6 | 20. Lime blossom honey | 21. Apricot dumplings, butter | 22. Tomato soup | 23. Dark chocolate |
| 7 | 24. Glucose | 25. Meat ravioli with cheese | 26. Puffed choco- wheat cookies | 27. Puffed choco-rice cookies |
| 8 | 28. White bread | 29. Fried fish, mashed potatoes, butter | 30. Wafers | 31. Lime blossom honey |
| 9 | Free choice | 32. Apricot dumplings, butter | — | — |
From the stated energy values of the meals (Table 1) and meal plans (Table 2), daily intake ranged from 1233 to 2076 kcal.
Study Design
Thirty-two tests were performed on each of the 20 subjects; the test period began with lunch on the first day, continued for the next 7 days, with four test meals per day (breakfast at 7 a.m., lunch at 12 a.m., afternoon snack at 4 p.m., dinner at 8 p.m.), and ended on day 9 with lunch. On the first day of the study the following procedures were performed:
Subjects were trained to handle the CGMS.27
A CGMS sensor was inserted subcutaneously into the gluteal/lumbar region before the first test and remained inserted and connected to the CGMS Gold monitor throughout the entire 9-day test period.
Each subject received the personal glucometer Advance (Hypoguard, Woodbridge, UK) and 50 Micro-draw strips to recalibrate the CGMS according to the latest manufacturer´s instructions, i.e., at least every 12 hours.
All subjects were provided with the defined meal plan (Table 2) and a logbook for recording their actual intake of food and drink, their exercise and daily activities, their general physical condition, the function of the CGMS, and glucometer results. They were asked to fast for at least 210 minutes before and after the beginning of each meal, to consume each portion within a 30-minute period, not to consume any other food, not to drink alcohol, to avoid vigorous exercise, and to keep a logbook with an emphasis on complete honesty.
Every subject received the proper amounts of test meals in sealed, labeled packages (except for white bread, which was supplied fresh) for the entire test period. Subjects were instructed to transfer the frozen foods (fried fish and apricot dumplings with butter) to their home freezers within 1 hour and to keep them frozen until 1 hour before the appropriate meal time.
The following points were emphasized:
Lunch test meals were to be consumed according to usual habits described in cookbooks, i.e., warm after adequate preparation in a microwave oven.
At the beginning of each test meal, the subject was to enter the code for the food into the CGMS Gold monitor.
Every subject was encouraged to drink 400 ml of water or unsweetened fruit tea with each meal in order to ensure a balanced fluid intake throughout the whole study period, and no additional drinks were allowed for 1 hour before the start of the meal and for 2 hours after the meal.
A medical supervisor was available by phone at any time.
Each subject entered the actual quantities and times of meal consumption, exercise, data on calibration and function of the CGMS, and any deviations from the daily routine into his/her logbook.
At day 9, having completed the meal plan, data were downloaded from the CGMS Gold monitors into a personal computer to calculate the GIs using Solutions software v.70D and software Degif XL4. Data were then revised, corrected manually, and completed according to the subject´s logbook. The exact times of the meal start were transferred from subjects' logbooks into the DegifXL4 database. The following criteria were used for exclusion of a meal test from further processing: the fasting time before a meal was shorter than 210 minutes, a meal was not consumed completely, longer than 30 minutes was taken to consume a meal, and/or any additional food was eaten. The detailed mathematical procedures of GI calculation have been described elsewhere.12,17,18
Statistical Analysis
MS Excel 2000 (Microsoft Corporation, Redmond, WA) and SPSS v.15.0 (SPSS Inc., Chicago, IL) were used to analyze data. Any tests that did not fulfill required evaluation criteria were not processed. Individual mean GIs for each period of time (120, 150, 180, and 210 minutes) were calculated for all replicates of each tested food. Glycemic indexes exceeding 500% were excluded. Next, mean group-related GI120, GI150, GI180, and GI210 were determined. Analysis of variance (ANOVA) was used to compare plasma glucose concentrations at the start of each test meal. The Shapiro–Wilk test showed a nonnormal distribution of GI values, and the Wilcoxon signed-rank test was applied to compare GIs associated with the tested foods over the course of 210 minutes; p < 0.05 was considered statistically significant. Box graphs with quartiles were used to demonstrate the evolution of the GIs.
Results
A total of 640 tests were performed with 10 foods and glucose standard in 20 subjects. In each subject, each food except tomato soup was tested in three replicates, i.e., 60 tests were performed with each of 10 foods/glucose standard and 40 tests with tomato soup.
Because some tests did not meet defined criteria, only 481 tests (75%) were processed. The number of successfully processed tests for the particular foods ranged from 34 of 60 (57%) to 52 of 60 (87%). The percentage of successful tests in each of 11 meals was compared with the percentage of successful tests in other foods using χ2, and a significant difference was found in 9 of 66 compared pairs. There were 159 of 640 (25%) test failures: 57 of 640 (9%) were subject related (the fasting time before a meal was shorter than 210 minutes, a meal was not consumed completely, longer than 30 minutes was taken to consume a meal, additional food was eaten) and 102 of 640 (16%) were CGMS related (the sensor function was disturbed during the 210 minutes following the meal start for a longer period than 20 minutes). In addition, all tests where the calculated value of GI exceeded the empirically defined limit of 500% (26 of 640 tests, i.e., 4%) were excluded from final evaluation. Thus, from 640 performed tests, only 455 tests (71 %) were considered for statistical evaluation.
Glycemia Dynamics Following Food Intake
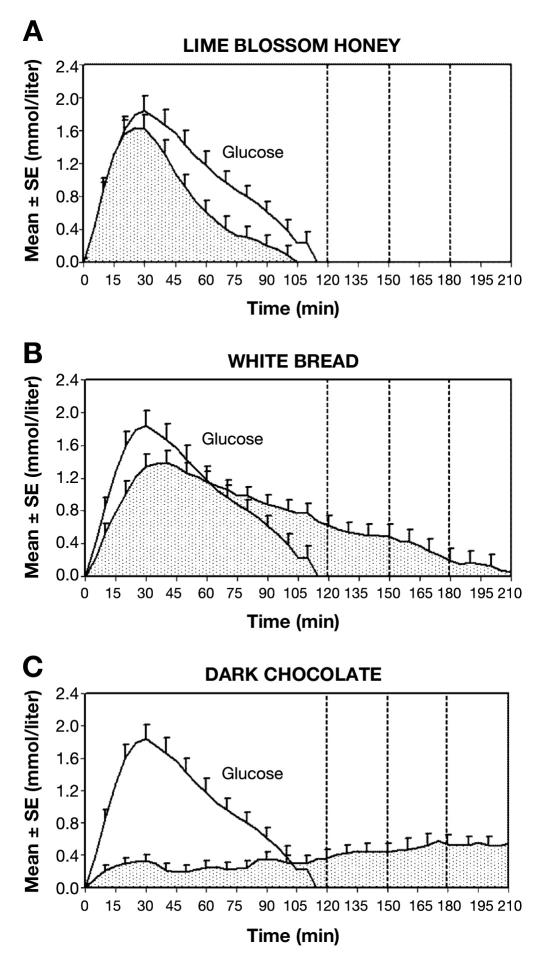
According to the evolution of glycemia during the 210 minutes after food consumption, test meals were classified into three groups. Group 1 (n = 2) consisted of foods for which glycemia returned to baseline within the same time as the glucose standard (120 minutes). These foods were lime blossom honey and tomato soup (Figure 1A). Group 2 (n = 5) contained foods for which glycemia returned to baseline within 210 minutes. These foods were white bread, choco-rice cookies, fried fish with mashed potatoes and butter, wafers, and meat ravioli with cheese (Figure 1B). Group 3 (n = 3) comprised foods for which glycemia did not return to baseline within 210 minutes. These foods were dark chocolate, apricot dumplings, and puffed choco-wheat cookies (Figure 1C).
Figure 1.
Mean glycemic change from premeal value and area under the curve after consumption of 50 grams of carbohydrates. Elapsed time from the meal start. See Figure 2 for evolution of the glycemic index between 120 and 210 minutes. (A) Lime blossom honey versus glucose standard. Glycemia returned to baseline within 120 minutes; n = 18. (B) White bread versus glucose standard. Glycemia returned to baseline within 210 minutes; n = 18. (C) Dark chocolate versus glucose standard. Glycemia did not return to baseline within 210 minutes; n = 17. Glucose, 1 mmol/liter = 18 mg/dl.
Glycemic Index Assessment Based on 120-, 150-, 180-, and 210-Minute Glycemic Profiles
Results of conventional GI120 determinations for test meals have already been reported.26 Based on classification of the Sydney University Glycemic Index Research Service,28 there were three foods with a low GI120 (≤55%), one food with a medium GI120 (56–69%), and six foods with a high GI120 (≥70%). Differences in mean plasma glucose concentrations before test meals (as compared by ANOVA) were not significant (Table 3); however, due to evaluation criteria (see earlier discussion), data from one to four subjects were excluded from analysis for each test meal such that the number of subjects evaluated in each instance (except glucose) was less than 20.
Table 3.
Glycemia (mmol/liter) at Start of Test and Glycemic Indices (%) for Test Meals at 120, 150, 180, and 210 Minutes after Start of Food Intake (Data Reported as Means ± SE); Relative Increase of GI between 120 and 210 Minutesa
| # | Meal | Glycemia Mean SE n | GI120 group | GI120 Mean SE CVbn | GI150 Mean SE CV n | GI180 Mean SE CV n | GI210 Mean SE CV n | Relative increase GI210 vs GI120% |
|---|---|---|---|---|---|---|---|---|
| 1 | Glucose | 5.5 | High | 100.0 | 100.0 | 100.0 | 100.0 | |
| 0.17 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| 19 | 20 | 20 | 20 | 20 | ||||
| 2 | Puffed choco-rice cookies | 5.0 | High | 105.3 | 121.9 | 111.2 | 114.6 | |
| 0.16 | 17.6 | 22.7 | 16.3 | 17.4 | 8.8 | |||
| 19 | 70.8 | 79.0 | 60.4 | 62.7 | ||||
| 18 | 18 | 17 | 17 | |||||
| 3 | Fried fish, mashed potatoes, butter | 5.2 | High | 94.0 | 100.1 | 101.4 | 102.8 | |
| 0.13 | 1307 | 1308 | 13.4 | 13.2 | 9.4 | |||
| 19 | 61.8 | 58.6 | 56.1 | 54.5 | ||||
| 18 | 18 | 18 | 18 | |||||
| 4 | White bread | 5.2 | High | 93.3 | 101.6 | 108.2 | 113.4 | |
| 0.15 | 7.4 | 7.6 | 8.1 | 9.0 | 21.5 | |||
| 19 | 33.5 | 31.7 | 31.8 | 33.8 | ||||
| 18 | 18 | 18 | 18 | |||||
| 5 | Wafers | 5.2 | High | 77.8 | 89.5 | 95.2 | 101.3 | |
| 0.16 | 13.2 | 15.1 | 15.4 | 17.5 | 30.2 | |||
| 18 | 72.1 | 71.7 | 68.5 | 73.3 | ||||
| 18 | 18 | 18 | 18 | |||||
| 6 | Lime blossom honey | 5.3 | High | 76.7 | 76.2 | 76.5 | 77.1 | |
| 0.21 | 8.4 | 8.7 | 9.1 | 9.5 | 0.5 | |||
| 18 | 46.1 | 48.2 | 50.6 | 52.1 | ||||
| 18 | 18 | 18 | 18 | |||||
| 7 | Apricot dumplings, butter | 4.9 | High | 75.0 | 92.1 | 105.4 | 113.8 | |
| 0.12 | 12.8 | 18.3 | 23.3 | 26.3 | 51.7 | |||
| 18 | 72.7 | 84.5 | 93.6 | 97.9 | ||||
| 18 | 18 | 18 | 18 | |||||
| 8 | Puffed choco-wheat cookies | 4.7 | Medium | 63.7 | 75.1 | 87.7 | 97.4 | |
| 0.18 | 8.1 | 8.8 | 10.6 | 12.5 | 52.9 | |||
| 17 | 53.7 | 49.5 | 51.3 | 54.3 | ||||
| 18 | 18 | 18 | 18 | |||||
| 9 | Meat ravioli with cheese | 5.0 | Low | 43.3 | 46.4 | 48.6 | 51.4 | |
| 0.19 | 6.8 | 7.5 | 8.2 | 8.6 | 18.7 | |||
| 20 | 68.1 | 70.3 | 73.5 | 73.2 | ||||
| 19 | 19 | 19 | 19 | |||||
| 10 | Tomato soup | 5.1 | Low | 38.5 | 38.4 | 38.1 | 39.7 | |
| 0.14 | 12.1 | 12.3 | 12.4 | 12.2 | 3.1 | |||
| 14 | 125.2 | 128.4 | 129.7 | 123.2 | ||||
| 16 | 16 | 16 | 16 | |||||
| 11 | Dark chocolate (70% cocoa) | 5.0 | Low | 34.7 | 43.4 | 51.1 | 62.8 | |
| 0.17 | 5.8 | 7.7 | 8.9 | 9.9 | 80.9 | |||
| 17 | 68.9 | 73.5 | 71.6 | 65.1 | ||||
| 17 | 17 | 17 | 17 |
Foods are ordered according to their GI120.
Coefficient of variation.
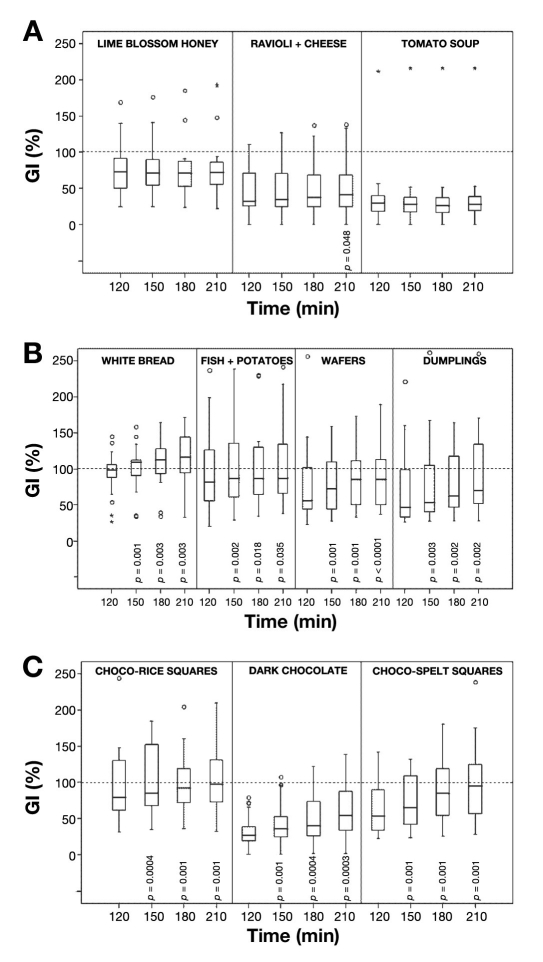
No significant GI increase could be demonstrated for lime blossom honey and tomato soup from group 1 (Figure 2A). In the eight remaining foods from groups 2 and 3 (with low, medium, or high GI120), the relative GI increase over the course of 210 minutes was significant, ranging from 9 to 81% of the respective start value (Table 3, Figure 2A, 2B, 2C).
Figure 2.
Evolution of glycemic indices over the 210 minutes following food intake. Data are presented as medians and quartiles. o, outlier; *, extreme value. See Table 3 and Figure 1 for further details. (A) Meals without marked GI evolution. The only food for which there was a significant difference between GI210 and GI120 was ravioli. (B) Various mixed meals that showed a significant increase in GI between 120 and 210 minutes. (C) Chocolate mixed meals showed significant increases in the GI at 150, 180, and 210 minutes as compared to 120 minutes postingestion. p value as compared to GI120 as calculated by Wilcoxon signed-rank test. Only numerical values of p < 0.05 are shown.
Discussion
The present study reports differences in the duration and magnitude of the glycemic response to various test foods/meals and suggests that the traditional 120-minute interval may not adequately define the entire glycemic response. An important question that was raised over 20 years ago29,30 has been addressed again.
It has been shown previously that the 2-hour period used to calculate the glycemic index of a food does not necessarily capture the entire glycemic response. The 2-hour window is used because the degree of glycemic response during this period has important and relevant implications for corresponding metabolic processes (e.g., early insulin and glucagon concentrations in the early postprandial phase and free fatty acids in the late postprandial phase). The metabolic response of the body to a food that results in a spiked glucose profile in the course of 2 hours is quite different from one that results in a flatter glucose profile in the course of 4 hours. Thus, calculating the GI from a longer time frame after meal consumption may result in more effective meal planning before physical activity in healthy persons, optimum insulin dosing at night in persons with diabetes mellitus, and so on.
However, the present approach deviates from the standard methods used,28,31 particularly with respect to the number of test meals per day (breakfast, lunch, afternoon snack, dinner), to the method of glucose estimation (CGMS), and to the increased number of subjects (n = 20) and tests.
The strength of this study is that it considers the glucose response over 210 minutes after the meal start and calculates the “extended GIs” based on 150-, 180-, and 210-minute postprandial glucose profiles. Testing the individual foods at breakfast, lunch, snack, and dinner times also increased the power of our investigational process. The subjects did not need to be present in a laboratory, and the blood glucose concentration was recorded continuously (288 values per 24 hours) by the CGMS. Final data processing was straightforward. Having tested each food three different times in 16–20 subjects, the strength of the statistical analysis in this study was greater than in other studies in which approximately 10 subjects were evaluated once with each food.31
Our results could be weakened by the facts that testing itself was performed without direct professional supervision and that glycemia was not estimated in an approved laboratory. The impact of time of day on insulin sensitivity and postprandial glycemic response was not fully explored either. In addition, the loss of subjects and the elimination of some data points (due to exclusion criteria) limit the power of the study. However, the initial training of test subjects and the confirmed long-lasting function, accuracy, and safety of the CGMS32–37 and the glucometer Advance38 were indeed adequate to approach standard testing conditions.
In addition, our results dealing with GI120 appear to be compatible with the respective GI values in nutritional tables issued previously in accepted international journals e.g., honey 77 ± 8% vs honey Canada 87 ± 8%, (item number 5869); white bread 93 ± 7% vs baguette, white, France 95 ± 15%, (item number 579); and tomato soup 39 ± 12% vs tomato soup Canada 38 ± 9%, (item number 5789). This assessment supports the CGMS as an acceptable alternative method of GI determination.
Our experimental design included withholding food for 210 minutes prior to the test meal, indicating that the fasting period before lunch, snack, and dinner tests was less than 10 hours applied in other studies28; although our design approximates “real-life” situations, it is necessary to recognize the potential effect of previous meals on the ensuing glycemic response of the subsequent test meals.39,40 Differences in establishing glucose homeostasis and the potential factors that may have influenced these differences in response to the various foods/meals need to be specified.
Figure 1 demonstrates very different responses to various foods/meals and suggests that the GI may differ between 120 and 210 minutes.
Even though the lack of significant differences among fasting glycemia before breakfast, lunch, snack, and dinner meals within the defined meal plan was demonstrated (Table 3), the influence of previously ingested fat and proteins should be recognized. This is particularly important given the fact that the present study reports that glycemic values needed mostly 210 minutes to return to baseline values with several of the test foods/meals. There are major differences in the amount of fat and protein among the tested foods/meals. However, the relationship between the GI response over time and the presence or amount of fat and protein in the foods/meals appears to correspond to the observations of others.40 The influence of fat and proteins on the rate of digestion and absorption, gastric emptying, and so on might help explain differences in the length and magnitude of the glycemic response. In our study, increased amounts of energy in one portion of tested food resulted from a larger content of fat and proteins.
In foods/meals for which glycemia returned to baseline within the same time as the glucose standard (group 1), the energy amount in one portion was ≤251 kcal; for foods for which glycemia returned to baseline within 210 minutes (group 2) it was between 259 and 632 kcal; and for portions of foods resulting in longer hyperlycemia (group 3) it was between 434 and 810 kcal per portion (Table 1). Considering “activity diaries” completed by subjects, the daily energy intake resulting from the meal plan applied in this study (1233 to 2076 kcal/day) appears to be at the lower limit of their needs. Further studies are underway to analyze the potential link among energy intake, GI, and glycemia.
Absolute increments in mean GI values between 120 and 210 minutes varied from 8 to 39%, i.e., the relative GI increase ranged from 9 to 81% of the respective start value; statistical significance was proven. The question arises as to what is the clinical importance of this GI evolution? In addition to it, coefficients of variation (Table 3) and box graphs in Figure 2 clearly indicate one of the problems with the concept of GI; i.e., the variation in glucose response within individual foods/meals is much greater than differences among foods/meals of a different glycemic index.20 This finding appears to comprise both the variability of GI between individuals and the time-of-day effect. Brand-Miller and associates41 analyzed a database of more than 1000 foods, concluding that the GI provides a good summary of postprandial glycemia, predicts the peak response, correlates highly with glucose variability, but shows only weak correlation with glycemia 120 minutes after the meal. Thus, the determination of extended glucose response and GI beyond 120 minutes may be helpful.
From a practical point of view, it is important to evaluate the postprandial glycemic profiles and benefits of low/high GI foods in healthy subjects under regular daily conditions,42 at work,43 and during exercise.44 Predicting postprandial glycemia is also useful for improving algorithms for premeal insulin boluses for persons with type 1 diabetes.6,45 However, the paradigm for recommending food with low/high GI under various conditions is complex46–49 and remains both a controversial50,51 and a challenging entity.52,53
The 120-minute glycemic index values fail to fully account for the changes in glycemia that occur after ingestion of a mixed meal because glycemia remains significantly above baseline for longer than 120 minutes. Thus, the evolution of postprandial glycemia makes determination of meal-specific GIs beyond 120 minutes worthy of further investigation. The approach adopted in this study offers extended GI estimates for foods ingested on three different occasions at four different times of day (breakfast, lunch, snack, or dinner). Use of the CGMS and the described software is an efficient and convenient method for routine measurement of GIs over extended intervals that represent the period adequate for absorption of most foods.
Acknowledgment
The authors thank all the volunteers for precise accomplishment of the study protocol and the students of the Faculty of Medicine, Palacky University Olomouc, Zuzana Fajkusova, Lucie Fajkosova, Jarmila Hucikova, Veronika Matuskova, and Helena Pribylova who gave much help in the recruitment of volunteers, providing the tested foods, and data collection.
This article is dedicated to Prof. MUDr. Jaroslav Páv, Dr.Sc., 1923–1998, IIIrd Department of Medicine, 1st Faculty of Medicine, Charles University, Prague, Czech Republic, for his enthusiastic and challenging approach to the nutrition in persons with diabetes.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CGMS
continuous glucose monitoring system
- GI
glycemic index
- GI120
glycemic index at 120 minutes
References
- 1.Food Agriculture Organization/World Health Organization. Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr Pap. 1998;66:1–140. [PubMed] [Google Scholar]
- 2.Otto H, Bleyer G, Pennartz M, Sabin G, Schauberger G, Spaethe R. Exchange of carbohydrates according to biological equivalents. In: Otto H, Spaethe R, editors. Diet in diabetes mellitus. Bern Stuttgart Wien: Verlag Hans Huber; 1973. pp. 41–50. [Google Scholar]
- 3.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 4.Ionescu-Tîrgovişte C, Popa E, Sîntu E, Mihalache N, Cheţa D, Mincu I. Blood glucose and plasma insulin responses to various carbohydrates in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1983;24(2):80–84. doi: 10.1007/BF00297385. [DOI] [PubMed] [Google Scholar]
- 5.Wolever TM, Nuttall FQ, Lee R, Wong GS, Josse RG, Csima A, Jenkins DJ. Prediction of the relative blood glucose response of mixed meals using the white bread glycemic index. Diabetes Care. 1985;8(5):418–428. doi: 10.2337/diacare.8.5.418. [DOI] [PubMed] [Google Scholar]
- 6.Chantelau E, Spraul M, Kunze K, Sonnenberg GE, Berger M. Effects of the glycaemic index of dietary carbohydrates on prandial glycaemia and insulin therapy in type I diabetes mellitus. Diabetes Res Clin Pract. 1986;2(1):35–41. doi: 10.1016/s0168-8227(86)80027-4. [DOI] [PubMed] [Google Scholar]
- 7.Bruns W. Diet treatment. In: Bibergeil H, editor. Diabetes mellitus. Guidelines for the diabetes practice. Jena: VEB Gustav Fischer Verlag; 1989. pp. 254–316. [Google Scholar]
- 8.Brand-Miller J, Wolever TMS, Colagiuri S, Foster-Powell K. The new glucose revolution. Marlowe and Company; 2002. [Google Scholar]
- 9.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76(1):5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Executive summary: standards of medical care in diabetes–2010. Clinical practice recommendations. Diabetes Care. 2010;33(Suppl 1):S4–S61. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bode BW. Clinical utility of the continuous glucose monitoring system. Diabetes Technol Ther. 2000;2(Suppl 1):S35–S41. doi: 10.1089/15209150050214104. [DOI] [PubMed] [Google Scholar]
- 12.Chlup R, Seckar P, et al. Langova K, Kudlova P, Chlupova K, Bartek J, Jelenova D. Automated computation of glycemic index for foodstuffs using continuous glucose monitoring. J Diabetes Sci Technol. 2008;2(1):67–75. doi: 10.1177/193229680800200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohnert KD, Augstein P, Zander E, Heinke P, Peterson K, Freyse EJ, Hovorka R, Salzsieder E. Glycemic variability correlates strongly with postprandial β-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009;32(6):1058–1062. doi: 10.2337/dc08-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce KL, Noakes M, Keogh J, Clifton PM. Effect of carbohydrate distribution on postprandial glucose peaks with the use of continuous glucose monitoring in type 2 diabetes. Am J Clin Nutr. 2008;87(3):638–644. doi: 10.1093/ajcn/87.3.638. [DOI] [PubMed] [Google Scholar]
- 15.Zavalkoff S, Polychronakos S. Evaluation of conventional blood glucose monitoring as an indicator of integrated values using a subcutaneous sensor. Diabetes Care. 2002;25(9):1603–1606. doi: 10.2337/diacare.25.9.1603. [DOI] [PubMed] [Google Scholar]
- 16.Peterson K, et al. Kudlova P, Matuskova V, Bartek J, Novotny D, Chlup R. Benefits of three-month continuous glucose monitoring for persons with diabetes using insulin pumps and sensors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153(1):47–51. doi: 10.5507/bp.2009.008. [DOI] [PubMed] [Google Scholar]
- 17.Pribylova H, Pallayova M, Hucikova J, Luza J. Evaluation of the new software program DegifXL4 in the determination of the glycaemic indices of foodstuffs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152(1):65–71. doi: 10.5507/bp.2008.010. [DOI] [PubMed] [Google Scholar]
- 18.Chlup R, Jelenova D, Kudlova P, Chlupova K, Bartek J, et al. Langova K, Chlupova L. Continuous glucose monitoring–a novel approach to the determination of the glycaemic index of foods (DEGIF 1)–determination of the glycaemic index of foods by means of the CGMS. Exp Clin Endocrinol Diabetes. 2006;114(2):68–74. doi: 10.1055/s-2006-923806. [DOI] [PubMed] [Google Scholar]
- 19.Sparks MJ, Selig SS, Febbraio MA. Pre-exercise carbohydrate ingestion: effect of the glycemic index on endurance exercise performance. Med Sci Sports Exerc. 1998;30(6):844–849. doi: 10.1097/00005768-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson E, Williams C, McComb G, Oram C. Improved recovery from prolonged exercise following the consumption of low glycemic index carbohydrate meals. Int J Sport Nutr Exerc Metab. 2005;15(4):333–349. doi: 10.1123/ijsnem.15.4.333. [DOI] [PubMed] [Google Scholar]
- 21.Wee SL, Williams C, Tsintzas K, Boobis L. Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise. J Appl Physiol. 2005;99(2):707–714. doi: 10.1152/japplphysiol.01261.2004. [DOI] [PubMed] [Google Scholar]
- 22.Visek J, Zourek M, Lacigova S, Rusavy Z. Influence of fiber on glycemic index of enteral nutrition. J Parenter Enteral Nutr. 2007;31(6):491–495. doi: 10.1177/0148607107031006491. [DOI] [PubMed] [Google Scholar]
- 23.Vega-López S, Ausman LM, Griffith JL, Lichtenstein AH. Inter-individual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care. 2007;30(6):1412–1417. doi: 10.2337/dc06-1598. [DOI] [PubMed] [Google Scholar]
- 24.Nell T, Venter Ch, Vorster H, Botes I, Steyn F. Intra- and inter-individual variation in glucose response to white bread and oral glucose in healthy women. South African J Clin Nutr. 2003;16:58–64. [Google Scholar]
- 25.Aston LM, Jackson D, Monshelmer S, Whybrow S, Handjieva-Darlenska T, Kreutzer M, Kohl A, Papadaki A, Martinez JA, Kunova V, Van Baak MA, Astrup A, Saris WH, Jebb SA, Lindroos AK. Developing a methodology for assigning glycaemic index values to foods consumed across Europe. Obesity Rev. 2009;11(1):92–100. doi: 10.1111/j.1467-789X.2009.00690.x. [DOI] [PubMed] [Google Scholar]
- 26.Fajkusova Z, Jadviscokova T, Pallayova M, Matuskova V, Luza J, Kuzmina G. Glycaemic index of selected foodstuffs in healthy persons. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(2):257–261. doi: 10.5507/bp.2007.043. [DOI] [PubMed] [Google Scholar]
- 27.Kudlová P, Stanislavová A. Education of healthy volunteers for the determination of glycemic index of foods. In: čáp J, Žiaková K, Nemčeková M, Holmanová E, editors. Theory, research and education in nursing. Univerzita Komenského Bratislava, Jeseniova Lekárska Fakulta Martin, Lékařská fakulta Univerzity Palackého Olomouc, čR; 2005. pp. 363–372. [Google Scholar]
- 28.Sydney University Glycemic Index Survey. Glycemic index; 2004. Available from: http://www.gisymbol.com.au/pages/index.asp.
- 29.Ganon MC, Nuttall FQ. Factors affecting interpretation of post-prandial glucose and insulin areas. Diabetes Care. 1987;10(6):759–763. doi: 10.2337/diacare.10.6.759. [DOI] [PubMed] [Google Scholar]
- 30.Nuttall FQ, Ganon MC. Plasma glucose and insulin response to macronutrients in nondiabetic and NIDDM subjects. Diabetes Care. 1991;14(9):824–838. doi: 10.2337/diacare.14.9.824. [DOI] [PubMed] [Google Scholar]
- 31.Wolever TM, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, Björck I, Brighenti F, Brown R, Brynes A, Casiraghi MC, Cazaubiel M, Dahlqvist L, Delport E, Denyer GS, Erba D, Frost G, Granfeldt Y, Hampton S, Hart VA, Hätönen KA, Henry CJ, Hertzler S, Hull S, Jerling J, Johnston KL, Lightowler H, Mann N, Morgan L, Panlasigui LN, Pelkman C, Perry T, Pfeiffer AF, Pieters M, Ramdath DD, Ramsingh RT, Robert SD, Robinson C, Sarkkinen E, Scazzina F, Sison DC, Sloth B, Staniforth J, Tapola N, Valsta LM, Verkooijen I, Weickert MO, Weseler AR, Wilkie P, Zhang J. Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr. 2008;87(1):247S–2457S. doi: 10.1093/ajcn/87.1.247S. [DOI] [PubMed] [Google Scholar]
- 32.Chlup R, Jelenova D, Chlupova K, et al. Chlupova L, Bartek J. Function and accuracy of glucose sensors beyond their stated expiry date. Diabetes Technol Ther. 2006;8(4):495–504. doi: 10.1089/dia.2006.8.495. [DOI] [PubMed] [Google Scholar]
- 33.Jadviscokova T, Fajkusova Z, Pallayova M, Luza J, Kuzmina G. Occurence of adverse events due to continous glucose monitoring. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(2):263–267. doi: 10.5507/bp.2007.044. [DOI] [PubMed] [Google Scholar]
- 34.Mlcak P, Fialova J, Trnkova K, Chlup R. A continous glucose monitoring system (CGMS)–a promising approach for improving metabolic control in persons with type 1 diabetes mellitus treated by insulin pumps. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):33–38. doi: 10.5507/bp.2004.005. [DOI] [PubMed] [Google Scholar]
- 35.Mastrototaro J, Shin J, Marcus A, Sulur G. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther. 2008;10(5):385–390. doi: 10.1089/dia.2007.0291. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch IB, Armstrong D, Bergenstal R, Buckingham B, Childs BP, Clarke WL, Peters A, Wolpert H. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM) Diabetes Technol Ther. 2008;10(4):232–245. doi: 10.1089/dia.2008.0016. [DOI] [PubMed] [Google Scholar]
- 37.Maaze RS, Strock E, Borgman S, Wesley D, Stout P, Racchini P. Evaluating the accuracy, reliability and clinical applicability of continuous glucose monitoring (CGM): is CGM ready for real time? Diabetes Technol Ther. 2009;11(1):11–18. doi: 10.1089/dia.2008.0041. [DOI] [PubMed] [Google Scholar]
- 38.Chlup R, Payne M, et al. Komenda S, Doubravova B, Reznickova M, Chlupova L, Seckar P. Results of selfmonitoring on glucometer systems Advance and Optium in daily routine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):127–139. [PubMed] [Google Scholar]
- 39.Liljeberg HG, Akerberg AK, Björck IM. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am J Clin Nutr. 1999;69(4):647–655. doi: 10.1093/ajcn/69.4.647. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson AC, Ostman EM, Granfeldt Y, Björck IM. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr. 2008;87(3):645–654. doi: 10.1093/ajcn/87.3.645. [DOI] [PubMed] [Google Scholar]
- 41.Brand-Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a diabase of more than 1000 foods. Am J Clin Nutr. 2009;89(1):97–105. doi: 10.3945/ajcn.2008.26354. [DOI] [PubMed] [Google Scholar]
- 42.Brynes AE, Adamson J, Dornhorst A, Frost GS. The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br J Nutr. 2005;93(2):179–182. doi: 10.1079/bjn20041318. [DOI] [PubMed] [Google Scholar]
- 43.Nakládalová M, Kapoun S. Occupational ability in persons with diabetes. Pracovní Lék. 2004;56:13–16. [Google Scholar]
- 44.Wu CL, Williams C. A low glycemic index meal before exercise improves endurance running capacity in men. Int J Sport Nutr Exerc Metab. 2006;16(5):510–527. doi: 10.1123/ijsnem.16.5.510. [DOI] [PubMed] [Google Scholar]
- 45.Lippaiova N, Pallayova M, Kuzmina G, Peterson K, Fajkosova L, Luza J. Safety of new algorithms for premeal insulin boluses in high glycaemic index meals in persons with type 1 diabetes mellitus using insulin pumps. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152(1):73–77. doi: 10.5507/bp.2008.011. [DOI] [PubMed] [Google Scholar]
- 46.Brand-Miller JC, Wolever TM. The use of glycaemic index tables to predict glycaemic index of breakfast meals. Br J Nutr. 2005;94(1):133–134. doi: 10.1079/bjn20041423. [DOI] [PubMed] [Google Scholar]
- 47.Monro JA, Shaw M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr. 2008;87(1):237S–2343S. doi: 10.1093/ajcn/87.1.237S. [DOI] [PubMed] [Google Scholar]
- 48.Wolever TM, Yang M, Zeng XY, Atkinson F, Brand-Miller JC. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr. 2006;83(6):1306–1312. doi: 10.1093/ajcn/83.6.1306. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266–273. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 50.Pawlak DB, Ebbeling CB, Ludwig DS. Should obese patients be counselled to follow a low-glycaemic index diet? Yes. Obes Rev. 2002;3(4):235–243. doi: 10.1046/j.1467-789x.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 51.Raben A. Should obese patients be counselled to follow a low-glycaemic index diet? Obes Rev. 2002;3(4):245–256. doi: 10.1046/j.1467-789x.2002.00080.x. [DOI] [PubMed] [Google Scholar]
- 52.Hollenbeck CB, Coulston AM. The clinical utility of the glycemic index and its application to mixed meals. Can J Physiol Pharmacol. 1991;69(1):100–107. doi: 10.1139/y91-014. [DOI] [PubMed] [Google Scholar]
- 53.Williams SM, Venn BJ, Perry T, Brown R, Wallace A, Mann JI, Green TJ. Another approach to estimating the reliability of glycaemic index. Br J Nutr. 2008;100(2):364–372. doi: 10.1017/S0007114507894311. [DOI] [PubMed] [Google Scholar]