Abstract
Background
Glucose management in an intensive care unit (ICU) is labor-intensive. A continuous glucose monitoring system (CGMS) has the potential to improve efficiency and safety in this setting. The goal of this study was to determine if the Medtronic Guardian® REAL-Time CGMS was accurate and tolerated by patients in a rural hospital ICU unit.
Method
Differences between individual finger stick blood glucose (FSBG) and CGMS values were compared to American Diabetes Association (ADA) and International Organization for Standardization (ISO) standards. Continuous glucose monitoring system accuracy was evaluated over four ranges: <75, 75–140, 140–200, and >200 mg/dl. Other accuracy measures [mean absolute deviation (MAD), mean absolute relative difference (MARD), and coefficient of linear regression of CGMS on FSBG] were calculated. Nursing staff and patients were surveyed regarding use of the CGMS in the ICU.
Results
Twenty-nine participants had 320 FSBG and corresponding CGMS readings. Sixty-two percent of participants were admitted with diabetic ketoacidosis (DKA). Two hundred and thirteen (66.6%) were accurate within the ISO standard, whereas only 70 out of 320 (21.9%) were within the 5% ADA standard. The CGMS was most accurate in euglycemia. Technical difficulties, such as adequate time for “wetting” and calibration of electrodes, arose with the sensors. The MAD was 28.3 mg/dl, the MRAD was 17.4%, and the linear regression coefficient of CGMS on FSBG was 0.834 (p < 0.001).
Conclusions
The CGMS is well tolerated by ICU patients but, at present, is not sufficiently accurate to be used for therapeutic decisions in the acute setting, particularly in patients with diabetic ketoacidosis. There is a need to find resolution to the technical issues regarding electrode “wetting” and calibration if CGMS use in the ICU setting is to provide an effective means of diabetes care and management.
Keywords: continuous glucose monitor, diabetes, glucose sensor, intensive care unit
Introduction
Use of a real-time continuous glucose monitoring system (CGMS) in the outpatient setting is now generally accepted as common practice for improving glucose control by detecting abnormal glucose excursions in patients of all ages with type 1 and 2 diabetes mellitus (DM), including pregnant women.1–3 In addition to improved glucose control, CGMS with high and low alarms have been very beneficial in preventing hypoglycemia in children.4,5 This is even more critical in people who experience nocturnal hypoglycemia and in individuals with hypoglycemia unawareness. However, studies of the utility of a CGMS in the hospital setting are limited.6–9 In these studies, the greatest utility of a CGMS has been for perioperative surgical monitoring of stable diabetic patients; yet there are many concerns about its use in the unstable critically ill.8
Hyperglycemia is a commonly observed problem in critically ill hospitalized patients, even in individuals without a previous history of diabetes mellitus.10,11 An elevated glucose level upon admission to a hospital is an important indicator of illness severity and risk for death, particularly in persons with no previous history of diabetes. In addition, mortality increases as the mean glucose level increases.10 Although the molecular mechanisms responsible for increased mortality and morbidity are only conjectural, they include cellular hypoxia and endothelial damage, leading to cytopathic death. Clinically, hyperglycemia is thought to result in an increased susceptibility to infection, critical-illness polyneuropathy, increased coronary artery events, and renal failure.12
Multiple clinical trials have indicated that intensive glucose control in the intensive care unit (ICU) is associated with decreased morbidity and mortality and in improved outcomes compared to individuals with less stringent glucose control.13,14 However, publication of the Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation trial15 demonstrated increased mortality in patients targeted to glucose levels of 81–108 mg/dl compared to those treated to target glucose levels <180 mg/dl. Further, this trial showed an increased frequency of insulin-induced hypoglycemia in the intensively treated subset of patients in the ICU. This may be responsible for the increased mortality in that group and has led to concern over what actually are optimal glucose target levels in critically ill patients or even different clinical illnesses.16
In order to attain and maintain tight glucose control in the hospital setting, an accurate and practical bedside glucose monitoring method is necessary. This should be accompanied by clinical pathways that are understood and followed by the nursing staff. Use of computerized algorithms may be beneficial in this regard and have been shown to reduce the frequency of hypoglycemia.17–19 Intensive glucose control requires frequent (usually hourly) venous or finger stick blood sugar (FSBS) glucose determinations, which can cause patient discomfort and increases the workload on nurses and other hospital personnel.20 Use of a CGMS to monitor interstitial glucose levels in hospitalized patients offers the potential to determine glucose levels every 5 minutes with less patient discomfort and a reduced nurse workload. Increased frequent monitoring by a CGMS with high/low alarms would also allow detection of upward or downward trends in glucose levels earlier than conventional hourly monitoring, thereby reducing the risk of insulin-induced hypoglycemia in these critically ill patients.
The Medtronic Guardian® REAL-Time CGMS is currently approved by the Federal Drug Enforcement Agency for monitoring glycemic trends in an outpatient setting. There have been limited clinical trials of CGMS in the hospital setting.6–9 Use of a CGMS in a hospital setting demonstrated utility in monitoring patients undergoing surgical procedures.7,21 However, in community hospitals, this technology has had limited use. There are major concerns about the physiologic delay between venous glucose levels and interstitial tissue glucose levels22 and the time delay between sensor insertion and the availability of accurate CGMS data.23 This is especially true in the ICU/coronary care unit (CCU) setting with critically ill patients who have rapidly changing glucose levels.
The purpose of this study was to determine if the Medtronic Guardian REAL-Time CGMS was accurate, safe, and tolerated by patients and hospital staff in a rural hospital ICU unit. This study also assessed patient and nurse satisfaction with use of the CGMS in this setting with regard to tolerability and workload.
Methods
Subject Recruitment
Subjects between the ages of 18 and 99 were recruited from a six-bed ICU in a rural community hospital. Daily rounds were conducted every morning in the ICU to find potential candidates for the study. Inclusion criteria were a FSBS or serum glucose level greater than 150 mg/dl and an ability to give informed consent. Exclusion criteria included any person who was unable to give consent, patients with coagulopathies, or current use of antico-agulants such as warfarin, heparin, or enoxaparin. This study was approved by the Ohio University and Hospital Institutional Review Board.
Data Collection Methods
A Medtronic MiniMed® glucose electrode was inserted subcutaneously into the patient's lower abdomen 2 inches from the umbilicus. After electrode insertion, at least 5 minutes was allowed before the MiniLink™ REAL-Time transmitter was connected to the sensor, allowing time for the electrode to be wetted properly by interstitial fluid. After a 2-hour initialization period, a nurse was instructed to conduct a FSBG measurement and to enter it into the CGMS for the first calibration. The FSBG reading needed to be within the 40- to 400-mg/dl range for calibration to be successful. After the initial calibration, the device was calibrated before breakfast, lunch, and dinner throughout the duration the patient wore the sensor. If the patient was not eating, the FSBG was determined at 8 am, noon, and 5:30 pm. The monitor was positioned by the patient's bedside and received data every 5 minutes from the transmitter. Nursing staff logged CGMS blood glucose values next to the FSBS on the bedside flow sheet every time a FSBS was completed. Finger stick blood glucose measurements were performed using a Roche® Accu-Chek Inform. Subjects in the ICU were treated with insulin protocols established by the hospital and as directed by their physician. The study was designed so that no clinical action was to be taken from glucose data produced by the CGMS during this study. If an alarm occurred in the study, the glucose value was confirmed by a FSBG reading. The glucose electrode was used for up to 3 days or throughout the duration of the ICU stay (whichever came first) or if there was a problem with the sensor itself and it was deemed medically necessary for the principal investigator to remove it.
Data Analysis
Continuous glucose monitoring system devices were downloaded using the Medtronic ComLink™, which sent data from the Guardian REAL-Time CGMS to the Medtronic CareLink™ therapy software via a serial port and cable. This study included the data table, the quick view summary, and the sensor daily overlay to review measurements collected by the CGMS. These reports were used to validate information such as calibration frequency, timing of FSBG, and concurrent CGMS values recorded by nursing staff on the bedside chart.
Differences between individual FSBG and CGMS values were evaluated to determine the accuracy of the CGMS. An Accu-Chek glucose meter FSBG was used as the glucose standard instead of venipuncture or arterial draw to evaluate the accuracy of the CGMS. This decision was made because point-of-care glucose meter data are typically the method hourly blood glucose levels are monitored in routine clinical care. Comparisons between FSBG and CGMS values were used to determine the accuracy of the CGMS in this study. Percentage error was calculated to assess the accuracy of the CGMS device. The initial FSBG used for calibration of the CGMS was not included in accuracy determination data. Current International Organization for Standardization (ISO) criteria used by glucose meter manufacturers to gain regulatory approval require that 95% of glucose values measured by the meter fall within 15 mg/dl (0.83 mmol/liter) of the manufacturer's reference method for glucose concentrations <75 mg/dl (<4.2 mmol/liter) or within 20% for glucose concentrations >75 mg/dl (>4.2 mmol/dl).24–26 These values were also compared to the 5% standard recommended by the American Diabetes Association (ADA).26 The accuracy of the CGMS was also evaluated over four different FSBG ranges—less than 75 mg/dl (low), 75–140 mg/dl (normal), 140–200 mg/dl (high normal), and greater than 200 mg/dl (high)—to determine if there were differences in accuracy at various glucose concentrations. Other accuracy measures or indicators, such as mean absolute deviation (MAD), mean relative absolute deviation (MRAD), and linear regression coefficient of CGMS on FSBG, were calculated. Rates of change in reference glucose, FSBG, and average 6-hour FSBG were calculated for diabetic ketoacidosis (DKA) patients as well as non-DKA patients.
Results
Cohort Description
Forty-one patients participated in this study between February 26, 2008 and August 15, 2009. Within this group, 11 patients had sensor failures and 1 patient's bedside chart was not completed correctly, resulting in an absence of data points. Therefore, 29 patients had usable data for this study. Twenty eight (96.5 %) had a history of diabetes mellitus. Eighteen patients had type 1 DM (62.1%) and 10 had type 2 DM (34.5%). Eighteen patients had a primary diagnosis of DKA (62.1%). Other primary diagnoses included uncontrolled hyperglycemia (3), hypoxia and renal insufficiency (1), unstable angina (1), hypertensive urgency (1), congestive heart failure (1), delirium tremens (1), pneumonia (1), septicemia (1), and malignant breast cancer (1). Fifteen patients were female (51.7%). Of the 29 patients, 28 were white (96.6%) and 1 was black (3.4%). The participants' age ranged from 19 to 85 years (mean age of 44). The body mass index (BMI) ranged from 17.0 to 46 kg/m2, and the mean BMI was 25.8 kg/m2. None of the patients were on pressor support or mechanically ventilated. None of the patients in this trial were surgical patients, although perioperative patients were eligible.
Patient Characteristics
Data were analyzed further to determine if patient characteristics (Table 1) were a predictor of accuracy of the CGMS. Cross-tabulations of gender, diabetes status (no diabetes, type 1 or 2), and accuracy criteria (5 and 20% of the FSBG value) revealed no statistically significant associations. Using the nonparametric Mann–Whitney U test, BMI did not have any statistical significant relationship with accuracy. For 5% ADA criteria, there was no accuracy (Mann–Whitney U = 80.0, p = 0.637), and there was no relationship for 20% ISO criteria (Mann–Whitney U = 74.0, p = 0.878). There were no statistically significant differences between DKA and non-DKA patients with regard to the rate of change in reference glucose, FSBG (Mann–Whitney U = 51, p = 0.591), and average FSBG over 6 hours (Mann–Whitney U = 55, p = 0.776).
Table 1.
Patient Characteristics and Rate of Change of Glucose
| Primary diagnosis | Gender | Age | Diabetes status | BMI | Initial calibration glucose | Hours between initial glucose and rate of change | Rate of change | |
|---|---|---|---|---|---|---|---|---|
| 1. | DKA | M | 31 | Type 1 | 26.8 | 189 | 6 | –2.5 |
| 2. | DKA | M | 48 | Type 2 | 27.3 | 199 | 6 | 7.67 |
| 3. | Hyperglycemia | M | 46 | Type 2 | 31.5 | 224 | 5 | 5.6 |
| 4. | DKA | M | 31 | Type 1 | 25.2 | 268 | 6 | –2 |
| 5. | Hyperglycemia | F | 33 | Type 1 | 23.3 | 191 | 6 | –1.67 |
| 6. | DKA | M | 24 | Type 1 | 23.9 | 186 | 6 | –50.33 |
| 7. | Hypoxia | F | 84 | Type 2 | 28.6 | 411 | 5 | 0.6 |
| 8. | Angina | F | 56 | Type 2 | 46 | 224 | 6 | 16.17 |
| 9. | DKA | F | 30 | Type 1 | 18.1 | 151 | 5 | –2.6 |
| 10. | DKA | F | 42 | Type 1 | 31 | 89a | — | — |
| 11. | DKA | F | 53 | Type 1 | 35 | 103 | 6 | –4.5 |
| 12. | DKA | M | 29 | Type 1 | 24.4 | 194 | 4.25 | –18.92 |
| 13. | DKA | F | 34 | Type 1 | 17.3 | 196a | — | — |
| 14. | DKA | F | 34 | Type 1 | 17.3 | 211 | 6 | 18.17 |
| 15. | DKA | M | 19 | Type 1 | 22 | 186 | 8 | 1.0 |
| 16. | DKA | F | 30 | Type 1 | 18.1 | 155 | 6 | –1.83 |
| 17. | Hypertensive urgency | F | 77 | Type 2 | 21.6 | 163a | — | — |
| 18. | DKA | F | 31 | Type 1 | 24.5 | 317 | 4.5 | 19.78 |
| 19. | Congestive heart failure | F | 85 | Type 2 | 28.5 | 328 | 5.5 | 10.91 |
| 20. | DKA | M | 32 | Type 1 | 24 | 143 | 6.25 | –3.52 |
| 21. | DKA | M | 46 | Type 1 | 22.1 | 176 | 2 | 10 |
| 22. | DKA | M | 46 | Type 1 | 19.3 | 119a | — | — |
| 23. | Hyperglycemia | M | 41 | Type 2 | 34.7 | 175a | — | — |
| 24. | Delirium tremens | F | 55 | Type 1 | 18.91 | 460 | 10.25 | 13.95 |
| 25. | Pneumonia | M | 51 | Type 2 | 31 | 245 | 8 | 3.63 |
| 26. | DKA | F | 34 | Type 1 | 23.3 | 418 | 8.25 | 25.09 |
| 27. | Septicemia | M | 62 | Type 2 | 36.6 | 103 | 5.5 | –7.45 |
| 28. | DKA | M | 47 | Type 2 | 18.11 | 141 | 4.5 | 17.11 |
| 29. | Metastatic breast cancer | F | 46 | None | 29.1 | 130a | — | — |
Rate of change not calculated due to patient only having one reading.
Finger Stick Blood Glucose and CGMS Measurements
Continuous glucose monitoring system and FSBG measurements were compared to evaluate the accuracy of CGMS based on the reference standard of ISO standardization and ADA recommendations (Table 2). In this study, a total of 320 FSBG readings were collected that had corresponding CGMS readings. Using ISO standardization for a glucometer, 213 out of 320 readings (66.6%) were found to be accurate with values above 75 mg/dl falling within 20% of the correlating FSBG and values below 75 mg/dl falling within 15 mg/dl of the FSBG. However, only 70 out of 320 readings (21.9%) had values falling within 5% of the associated FSBG, which are the current ADA recommendations.
Table 2.
Accuracy of the CGMS as Compared to ADA and ISO Reference Standards
| CGMS accuracy | |||
|---|---|---|---|
| 5% ADA standard | 20% ISO standard (values >75 mg/dl) | Values <75 mg/dl within 15 mg/dl of FSBG | |
| FSBG and CGMS data pairs | 21.9% | 66.3% | 41.7% |
The accuracy of the CGMS was also evaluated within specific blood glucose ranges: less than 75 mg/dl (low), 75–140 mg/dl (normal), 140–200 mg/dl (high normal), and greater than 200 mg/dl (high). The frequency of FSBG values that fell within each range is illustrated in Table 3, as well as the number of associated CGMS readings that fell within ADA and ISO recommended standards. Twelve values were in the less than 75-mg/dl range, comprising 3.7% of total study values. One of 12 (8.3%) was accurate within 5%. Five of 12 (41.7%) were accurate, falling within 15 mg/dl of the correlating FSBG. There were 86 values (26.9% of all values) in the 75- to 140-mg/dl range. Twenty one (24.4%) were accurate within 5% of the FSBG, and 61 (70.9%) were accurate according to 20% of the FSBG ISO standards. In the 140- to 200-mg/dl range, there were 118 values (36.9% of all values) with 19 (16.1%) accurate within 5% of the FSBG and 74 (62.7%) accurate within 20% of the FSBG. Finally, 104 values (32.5% of all values) were greater than 200 mg/dl, with 29 (27.9%) accurate within 5% of the FSBG and 73 (70.2%) accurate within 20% of the FSBG.
Table 3.
Accuracy of CGMS at Different Glucose Ranges
| Ranges based on individual CGMS readings | ||||||
|---|---|---|---|---|---|---|
| Range | Frequency | Percent | Frequency of CGMS readings within 5% ADA standards | % of CGMS readings within 5% ADA standards | Frequency of CGMS readings accurate by ISO standards | % of CGMS readings accurate by ISO standards |
| <75 | 12 | 3.7 | 1 | 8.3 | 5 | 41.7 |
| ≥75–140 | 86 | 26.9 | 21 | 24.4 | 61 | 70.9 |
| ≥140–200 | 118 | 36.9 | 19 | 16.1 | 74 | 62.7 |
| ≥200 | 104 | 32.5 | 29 | 27.9 | 73 | 70.2 |
| Total | 320 | 100.0 | 70 | 21.9 | 213 | 66.6 |
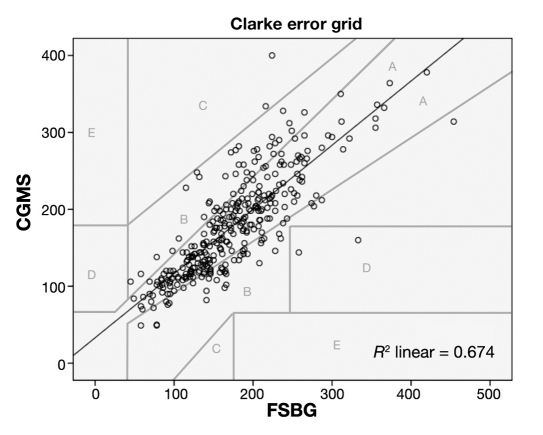
Regarding the other accuracy measures, MAD was 28.3 mg/dl, MRAD was 17.4%, and the linear regression coefficient of CGMS on FSBG was 0.834 (p < 0.001). When applying this to the Clarke error grid, the majority of readings fell into Clarke A zone (accurate) or Clarke B zone (clinically acceptable)27 (See Figure 1).
Figure 1.
Linear regression analysis of CGMS vs FSBS (mg/dl).
Calibration
There were a total of 187 opportunities for calibration at the determined breakfast, lunch, and dinner schedules. Out of these, only 24 (12.8%) were missed. The calibration missed most often was the predinner time. In addition, nurses did an additional 122 calibrations that were not scheduled. The reason these calibration were added most often was sensor alarm for a “weak signal.”
Technical Difficulties
Several technical issues (Table 4) were encountered during this study utilizing the Guardian REAL-Time CGMS in the ICU/CCU setting. A very high failure rate of sensors was observed when they were initially inserted, along with frequent “calibration errors.” A “lost sensor” alert was the most frequent problem encountered. This happened when the monitor did not receive a signal from the transmitter and was caused by either an improper connection between the transmitter and the sensor or an insufficient “wetting” of the sensor by interstitial fluid before the transmitter was connected and calibrated. At the beginning of the study we only waited 5–10 minutes between insertion of the sensor and connection to the transmitter as per the manufacturer's instruction. The manufacturer subsequently recommended waiting 30–60 minutes after sensor insertion before connecting the transmitter in order to allow proper “wetting” by interstitial fluid.
Table 4.
Technical Difficulties Experienced with CGMS System in ICU
| Problem identified | Possible etiologies |
|---|---|
| Sensor failure | Improper connection between transmitter and sensor |
| Inadequate time for “wetting” of sensor | |
| Sensor accuracy | Improper calibration protocols |
| Unstable patient glucose levels | |
| Signal interference | Electronic product interference using same frequency band |
“Calibration errors” were also encountered frequently in this study. This was again seen most frequently at initiation of the CGMS when the patients' FSBG levels were most unstable. Often the Guardian REAL-Time CGMS would not accept the initial calibration glucose value for up to an hour after initial insertion. It was also observed that other electronic devices used in an intensive care unit, including cell phones that transmit in the same frequency band used by the MiniLink transmitter, could interfere with the monitor receiving glucose information sent by the transmitter.
Patient and Nurse Surveys
Fourteen patients and 13 nurse surveys were completed during the course of this study. Patient and nurse feedback on their perspectives of the CGMS in the ICU are listed in Table 5. In general, the device was comfortable (92.8%) and patients were confident in the accuracy of the device (85.7%). Eighty-six percent of patients had a good impression of the device, and nearly 93% recommended use of the device for future admissions.
Table 5.
Nurses and Patient Opinions of CGMS
| Strongly agree | Agree | Neither agree nor disagree | Disagree | Disagree | |
|---|---|---|---|---|---|
| I was confident in the accuracy of this device | |||||
| Nurses | 0% | 53.8% | 30.8% | 7.7% | 7.7% |
| Patients | 28.6% | 57.1% | 14.3% | 0% | 0% |
| The device was useful and helped keep the patient's sugars in good control | |||||
| Nurses | 7.7% | 30.8% | 38.5% | 23.1% | 0% |
| Patients | 35.7% | 21.4% | 35.7% | 7.1% | 0% |
| My overall impression of the device was good | |||||
| Nurses | 15.4% | 53.8% | 7.7% | 23.1% | 0% |
| Patients | 42.9% | 42.9% | 14.3% | 0% | 0% |
| I recommend the future routine use of the device in the ICU | |||||
| Nurses | 38.5% | 30.8% | 15.4% | 15.4% | 0% |
| Patients | 71.4% | 21.4% | 7.1% | 0% | 0% |
| I would like to use this device should I require a future admission to the hospital | |||||
| Nurses | 15.4% | 46.2% | 15.4% | 0% | 23.1% |
| Patients | 64.3% | 21.4% | 14.3% | 0% | 0% |
| I felt the use of this device would decrease my workload | |||||
| Nurses | 15.4% | 38.5% | 23.1% | 15.4% | 7.7% |
| The device was comfortable | |||||
| Patients | 71.4% | 21.4% | 7.1% | 0% | 0% |
Two-thirds of nurses had a positive overall impression (69.2%) and recommended future use of the CGMS (69.3%). Sixty-one percent would like to have the device if they were a patient. However, less than half (43.9%) felt that this would decrease their workload, and only 53.8% were confident in the accuracy of the device.
Discussion
Clinical use of an interstitial CGMS to guide potential changes in insulin doses in unstable, critically ill patients is not reliable or accurate enough at the present time. Major factors limiting the reliability of interstitial CGMS in the hospital setting are (1) physiological delay or lag time between acute changes in blood glucose levels and interstitial fluid glucose levels; (2) delay between electrode insertion, adequate “wetting,” and time to calibration prior to clinical use; and (3) finally the accuracy/reliability of the sensor technology itself.
In the few studies performed to determine the physio-logical delay between either venous or FSBG, glucose levels and interstitial glucose levels have ranged between 8 and 15 minutes22,27 and were done in an outpatient setting. Many studies utilized venous glucose readings as a comparison to CGMS. However, in clinical care, capillary blood testing is used more frequently for point-of-care monitoring. The physiologic delay between changes in blood glucose and interstitial glucose would be expected to be more pronounced in a hospital setting, especially in critically ill patients. This may be particularly true in this population in which nearly two-thirds of the participants had DKA.28 The MAD of the CGMS as an outpatient was 16.38 mg/dl and the MARD was 15.2%.29 Our MAD was significantly different, which may be reflected in the overall condition and level of hydration of patients in this trial.
Despite these difficulties, monitoring of an interstitial CGMS could be useful in a hospital setting to detect rapidly changing trends in glucose levels (upward or downward) in patients on insulin infusion pathways. Alarms available with the Guardian REAL-Time CGMS and the Medtronic CareLink can be transmitted easily to a central nursing station for monitoring similar cardiac telemetry units, reducing staff nurse workload, alerting staff of rapid changes in glucose levels, and increasing safety dramatically. It has been suggested that the trends on glucose change are more important than individual snapshots in time,29 and we may need to redefine how CGMS data can be interpreted in unstable patients.
Several technical issues were encountered during this study utilizing the Guardian REAL-Time CGMS in the ICU/CCU setting. We observed a very high failure rate of the sensors when they were initially inserted and frequent “calibration errors.” This may be improved with longer times for “wetting” of the sensor. Previous studies have seen sensor failure or malfunction rates of 17–18%.6,30 We are learning from personal experience in outpatient settings that allowing more time between sensor insertion and initial calibration (even up to 6 hours) reduces the number of “lost sensors” and increases the accuracy of the electrodes as well (unpublished observations). “Calibration errors” were most frequent at initiation of the CGMS when the patients' FSBG levels were most unstable. Diabetic ketoacidosis was the principal diagnosis in this study, with an average glucose level of 400 mg/dl on admission, which further complicated electrode calibrations. Calibration should occur regularly at steady intervals and during times of stable glucose. If the three FSBG values were not entered into the CGMS monitor daily, the monitor was not calibrated properly, which affected the accuracy of sensor data and this again contributed to calibrations errors and reduced accuracy of the CGMS during the study.
We also observed that other electronic devices used in an intensive care, including cell phones that transmit in the same frequency band used by the MiniLink transmitter, could interfere with the monitor receiving glucose information sent by the transmitter. Consequently, the manufacturer suggested that cellular phones should be at least 12 inches from the transmitter or receiving device while it is being used. However, this has not been reported in other studies.
The CGMS is well tolerated by patients within the ICU, but the practicality of the CGMS for the ICU staff to operate must be taken into consideration. Use of a CGMS may reduce staff workload when this technology is fully implemented. However, there was increased workload placed on nurses during this trial. System errors due to improper calibration or technical difficulties can be prevented with more thorough training and increased experience of staff, which could potentially improve the performance of the CGMS in the ICU.
Conclusion
This study demonstrated that the Medtronic Guardian REAL-Time CGMS has potential application in the ICU setting. At present, the time delay between time of sensor insertion and delivery of accurate interstitial glucose levels precludes its use in the acute setting with unstable diabetic patients, especially in patients with DKA. We envision that the most immediate use for a CGMS would be in chronic monitoring of stable ICU patients. Further studies should explore protocols for ensuring proper “wetting” of electrodes at initial insertion and improving their accuracy so that they could be used in an unstable ICU setting.
Acknowledgment
We thank the Osteopathic Heritage Foundation for funding this study, Medtronic, Inc. for donating the CGMS monitors, and the OUCOM CORE Research Office for statistical support.
Abbreviations
- ADA
American Diabetes Association
- BMI
body mass index
- CCU
coronary care unit
- CGMS
continuous glucose monitoring system
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- FSBG
finger stick blood glucose
- ICU
intensive care unit
- ISO
International Organization for Standardization
- MAD
mean absolute deviation
- MARD
mean absolute relative difference
References
- 1.Bonakdaran S. Prevalence and extent of glycemic excursions in well-controlled patients with type 2 diabetes mellitus using continuous glucose-monitoring system. Indian J Med Sci. 2009;63(2):66–71. [PubMed] [Google Scholar]
- 2.Diabetes Research in Children Network (DirecNet) Study Group. Continuous glucose monitoring in children with type I diabetes. J Pediatr. 2007;151:388–393. doi: 10.1016/j.jpeds.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MS. Real time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118(3):1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan K, Jenkins A, O'Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust NZ J Obstet Gynaecol. 2007;47(3):186–190. doi: 10.1111/j.1479-828X.2007.00716.x. [DOI] [PubMed] [Google Scholar]
- 5.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6(3):339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 7.Ryan MT, Savarese VW, Hipszer B, Dizdarevic I, Joseph M, Shively N, Joseph JI. Continuous glucose monitor shows potential for early hypoglycemia detection in hospitalized patients. Diabetes Technol Ther. 2009;11(11):745–747. doi: 10.1089/dia.2009.0071. [DOI] [PubMed] [Google Scholar]
- 8.Corstjens AM, Ligtenberg JJ, van der Horst IC, Spanjersberg R, Lind JS, Tulleken JE, Meertens JH, Zijlstra JG. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit Care. 2006;10(5):R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Block C, Manuel-Y-Keenoy B, Van Gaal L, Rogiers P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006;29(8):1750–1756. doi: 10.2337/dc05-2353. [DOI] [PubMed] [Google Scholar]
- 10.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 11.Yendarumi S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55(1):33–38. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- 12.Gabbanelli V, Pantanetti S, Donati A, Principi T, Pelaia P. Correlation between hyperglycemia and mortality in a medical and surgical intensive care unit. Minerva Anestesiol. 2005;71(11):717–722. [PubMed] [Google Scholar]
- 13.Furnary AP. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 14.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive Insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 15.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalcanti AB. A randomized controlled trial comparing a computer-assisted insulin infusion protocol with a strict and a conventional protocol for glucose control in critically ill patients. J Crit Care. 2009;24(3):371–378. doi: 10.1016/j.jcrc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Davidson PC. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28(10):2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 19.Morris AH, Orme J, Jr, Truwit JD, Steingrub J, Grissom C, Lee KH, Li GL, Thompson BT, Brower R, Tidswell M, Bernard GR, Sorenson D, Sward K, Zheng H, Schoenfeld D, Warner H. A replicable method for blood glucose control in critically ill. Crit Care Med. 2008;36(6):1787–1795. doi: 10.1097/CCM.0b013e3181743a5a. [DOI] [PubMed] [Google Scholar]
- 20.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15(4):370–377. [PubMed] [Google Scholar]
- 21.Vriesendorp TM, DeVries JH, Holleman F, Dzoljic M, Hoekstra JB. The use of two continuous glucose sensors during and after surgery. Diabetes Technol Ther. 2005;7(2):315–322. doi: 10.1089/dia.2005.7.315. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch IB. Realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94(7):2232–2238. doi: 10.1210/jc.2008-2625. [DOI] [PubMed] [Google Scholar]
- 23.Diabetes Research In Children Network (DirecNet) Study Group. Buckingham BA, Kollman C, Beck R, Kalajian A, Fiallo-Scharer R, Tansey MJ, Fox LA, Wilson DM, Weinzimer SA, Ruedy KJ, Tamborlane WV. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther. 2006;8(3):318–325. doi: 10.1089/dia.2006.8.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergenstal R. Evaluating the accuracy of modern glucose meters. Excerpta Med. 2008;3:5–14. [Google Scholar]
- 25.International Organization for Standardization. 2003. ISO 15197 in vitro diagnostic test systems– requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus.
- 26.American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1994;17(1):81–86. doi: 10.2337/diacare.17.1.81. [DOI] [PubMed] [Google Scholar]
- 27.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 28.Stout PJ, Peled N, Erickson BJ, Hilgers ME, Racchini JR, Hoegh TB. Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol Ther. 2001;3(1):81–90. doi: 10.1089/152091501750220046. [DOI] [PubMed] [Google Scholar]
- 29.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klonoff D. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28(5):1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 31.Guerci B, Floriot M, Bohme P, Durain D, Benichou M, Jellimann S, Drouin P. Clinical Performance of CGMS in type 1 diabetes patients treated by continuous insulin infusion using insulin analogs. Diabetes Care. 2003;26(3):582–589. doi: 10.2337/diacare.26.3.582. [DOI] [PubMed] [Google Scholar]