Abstract
Objective
Adiponectin may play a role in the development of type 2 diabetes and cardiovascular disease (CVD). However, little is known about the relationship between adiponectin and impaired glucose tolerance (IGT). We investigated the association between adiponectin and IGT and between adiponectin and cardiovascular risk factors among subjects with IGT.
Research Design and Methods
Subjects with normal glucose tolerance (NGT)(n = 571) and impaired glucose tolerance (n = 167) were recruited from the Chennai Urban Rural Epidemiology Study in south India. Serum total adiponectin levels were measured using a radioimmunoassay (Linco Research, St. Charles, MO). High sensitivity C-reactive protein (hsCRP) was estimated by nephelometry.
Results
In sex-stratified analyses, adiponectin was significantly associated with IGT in females [odds ratio (OR): 0.93, 95% confidence interval (CI): 0.872–0.991, p = 0.026] after controlling for age, waist circumference, blood pressure, alcohol consumption, smoking, lipid profile, and glycemic indices; in males there was no significant association (OR = 0.90, 95% CI: 0.798–1.012, p = 0.078). In prediabetic females, adiponectin was not associated with any CVD risk factors (age, waist circumference, blood pressure, cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, fasting glucose, fasting insulin, and insulin resistance level), but was associated negatively with 2-hour postplasma glucose levels (r = –0.243, p < 0.05) and hsCRP (r = –0.219, p < 0.05) after adjusting for demographic and biomedical indices. No associations with CVD risk factors were observed in males with IGT.
Conclusion
Serum total adiponectin levels are associated with IGT, 2-hour postplasma glucose, and hsCRP in Asian Indian females but not in males.
Keywords: adiponectin, Asian Indians, female, impaired glucose tolerance
Introduction
Adiponectin, a polypeptide hormone produced exclusively by adipocytes, has been suggested to play a major role in the development of type 2 diabetes and its associated pathological events by sensitizing insulin and improving lipid metabolism.1 Low adiponectin levels have been reported to be closely associated with hyperinsulinemia, type 2 diabetes, obesity, metabolic syndrome, atherosclerosis, and dyslipidemia.1,2 Gender differences in adiponectin levels have also been reported.2 Several epidemiological studies suggest that high adiponectin appears to be protective against the development of type 2 diabetes and atherosclerosis.3–5 In addition, it has been reported that adiponectin has an anti-inflammatory effect and its concentration is associated negatively with the degree of inflammatory markers, such as high sensitivity C-reactive protein (hsCRP), interleukin-6, and tumor necrosis factor-α.1 A study from Trinidad demonstrated that adiponectin levels were lower in type 2 diabetic subjects than in nondiabetic subjects but there was no correlation between adiponectin levels and inflammatory markers.6 However, the majority of these studies were done in individuals with normal glucose tolerance (NGT) or in those with diagnosed type 2 diabetes. The relationship between adiponectin and impaired glucose tolerance (IGT) is not clear.
Impaired glucose tolerance is a term used to differentiate individuals with a high risk for developing diabetes and is an intermediate stage between NGT and overt diabetic subjects.7 In India, the incidence of IGT was 13.1/1000 person-year; about 40% of these patients go on to develop type 2 diabetes within 8 years.8 We investigated the association between adiponectin and IGT and the relationship between adiponectin and risk factors for cardiovascular diseases (CVD) in subjects with IGT.
Research Design and Methods
Human Subjects
Data for this study were from the Chennai Urban Rural Epidemiology Study (CURES), an epidemiological study conducted in Chennai (formerly Madras), the fourth largest city in India with a population of five million people. CURES was done in three phases and has been described in detail elsewhere.9 Briefly, a door-to-door survey of 26,001 individuals (age ≥20 years at the time of study entry) who represented the urban and rural Indian population was conducted in phase 1. Subjects completed a questionnaire that collected information on demographic, socioeconomic, behavioral, and health status. In addition, fasting capillary blood glucose, blood pressure, and basic anthropometric data were obtained in all subjects.9 In a random sample of 2600 of these subjects (of whom 2350 responded; a response rate of 90.4%), measures of biochemical parameters, obesity and anthropometric indices, and markers of diabetes-associated complications (atherosclerosis and eye disorders) were obtained.
In phases 2 and 3, detailed demographic and socio-economic information, including age, gender, educational status, family income, and smoking status, were obtained. Anthropometric measurements and biochemical tests were performed. Phase 2 subjects (excluding known diabetic subjects) and all subjects in phase 3 received a 2-hour oral glucose tolerance test using a 75-gram oral glucose load. Plasma adiponectin levels were determined in a subgroup of subjects from phases 2 and 3. Data from subjects with NGT (n = 571) and confirmed IGT (n = 167) were analyzed for this study. Institutional ethical committee approval was obtained from the Madras Diabetes Research Foundation ethical committee, and written informed consent was obtained from all study subjects.
Anthropometric Measurements
Subjects' height, weight, waist and hip circumferences, and blood pressure were measured using standardized methods.8 Body mass index (BMI) was calculated as weight (kg)/height (m2).
Biochemical Parameters
Methods to determine biochemical parameters were introduced previously.2 Concentrations of fasting plasma glucose (using glucose oxidase–peroxidase method), serum cholesterol (using cholesterol oxidase–peroxidase–amidopyrine method), serum triglycerides (using glycerol phosphate oxidase–peroxidase–amidopyrine method), and high-density lipoprotein (HDL) cholesterol (using poly-ethylene glycol-pretreated enzyme method) were determined on a Hitachi-912 autoanalyzer (Hitachi, Germany) using kits supplied by Roche Diagnostics (Basel, Switzerland). Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula. The intra- and interassay coefficients of variation for the biochemical assays ranged between 3.1 and 7.6%.
Serum insulin (Dako, Glostrup, Denmark) concentrations were measured by an enzyme-linked immunosorbent assay. The intra- and the interassay coefficients of variation for the insulin assay were 5.7 and 8.9%, respectively. The lowest detection limit of insulin assay was 0.5 μU/ml. The degree of insulin resistance was calculated using homeostasis model assessment–insulin resistance [HOMA-IR = fasting insulin (μIU/ml) × fasting plasma glucose (mg/dl)/405].10 Glycated hemoglobin (HbA1c) was measured by a Variant machine (Bio-Rad, Hercules, CA). The intra- and interassay coefficients of variation of HbA1c were both <10%.
Measurement of hsCRP and Adiponectin
The plasma concentrations of hsCRP were measured by a highly sensitive nephelometric assay using a mono-clonal antibody to CRP coated on polystyrene beads (Dade Behring, Marburg, Germany). The intra- and the interassay coefficients of variation for hsCRP were 4.2 and 6.8%, respectively, and the lower detection limit was 0.17 mg/liter.
Fasting adiponectin levels were measured using a radio-immunoassay (Cat. No. HADP-61HK, Linco Research, St. Charles, MO). The intra- and the interassay coefficients of variation were 3.8 and 7.4%, respectively, and the lower detection limit was 1 ng/ml.
Definitions
Impaired glucose tolerance was defined as a fasting plasma glucose level of <126 mg/dl, and the 2-hour postplasma glucose level was 140–199 mg/dl.11
Statistics
Data were analyzed using SPSS version 16.0 (Chicago, IL). Values of adiponectin, fasting insulin, HOMA-IR, and triglyceride were log transformed to achieve normality. Because it is possible that adiponectin may function differently between males and females,12 analyses were stratified by gender. Student t-tests (for continuous variables) and χ2 tests (for categorical variables) were used to compare differences between subjects with NGT and IGT. Multiple logistic regression analysis was used to determine the relationship between adiponectin and IGT, adjusting for demographic characteristics, lipid profile, and glycemic indices. Spearman's correlation and partial correlation tests were performed to examine the relationship between adiponectin and risk factors for CVD in prediabetic males and females. P values less than 0.05 were considered statistically significant.
Results
Table 1 shows clinical and biochemical characteristics of the study groups. Subjects with IGT were older (p < 0.05) compared to subjects with NGT in both males and females. Waist circumference, BMI, systolic and diastolic blood pressure, HOMA-IR, fasting insulin, HbA1c, serum cholesterol, serum triglycerides, and LDL cholesterol were significantly higher among subjects with IGT compared to subjects with NGT (p < 0.05) in both males and females. hsCRP levels were significantly higher in prediabetic females (4.0 ± 2.6 mg/liter; p < 0.05) compared to NGT subjects (3.0 ± 3.1 mg/liter). Among prediabetic males, hsCRP levels were higher but did not reach statistical significance. The log adiponectin values in subjects with IGT were similar to those who were normoglycemic in both males and females.
Table 1.
Clinical and Biochemical Characteristics of Study Population (Mean ± Standard Deviation)
| Female (n = 438) | Male (n = 300) | |||
|---|---|---|---|---|
| NGT (n = 319) | IGT (n = 119) | NGT (n = 252) | IGT (n = 48) | |
| Age (years) | 43 ± 12 | 49 ± 9a | 42 ± 13 | 53 ± 11a |
| BMI (kg/m2) | 24.7 ± 5.2 | 26.2 ± 4.5a | 23.2 ± 4.2 | 24.5 ± 3.5 |
| Waist circumference (cm) | 84.5 ± 12.3 | 90.6 ± 11.3a | 86.3 ± 11.7 | 93.0 ± 9.9a |
| Smoking (n, %) | 0 | 0 | 102 (40.5%) | 13 (27.1%) |
| Drinking (n, %) | 3 | 1 | 115 (45.6%) | 15 (31.3%) |
| Systolic blood pressure (mm Hg) | 120 ± 18 | 131 ± 21a | 118 ± 15 | 128 ± 17a |
| Diastolic blood pressure (mm Hg) | 74 ± 10 | 80 ± 12a | 75 ± 11 | 79 ± 10a |
| Log HOMA-IR | 0.18 ± 0.28 | 0.41 ± 0.28a | 0.13 ± 0.27 | 0.35 ± 0.25a |
| Log fasting insulin | 0.86 ± 0.28 | 1.04 ± 0.29a | 0.81 ± 0.26 | 0.97 ± 0.26a |
| HbA1c | 5.66 ± 0.51 | 6.10 ± 0.62a | 5.61 ± 0.52 | 6.21 ± 0.59 |
| Total cholesterol (mg/dl) | 186 ± 38 | 200 ± 35a | 178 ± 36 | 189 ± 36 |
| Log triglyceride | 2.00 ± 0.20 | 2.09 ± 0.17a | 2.04 ± 0.21 | 2.15 ± 0.21a |
| LDL cholesterol (mg/dl) | 117 ± 32 | 129 ± 31a | 112 ± 32 | 116 ± 33 |
| HDL cholesterol (mg/dl) | 46.6 ± 9.8 | 44.7 ± 9.7 | 41.0 ± 9.5 | 41.1 ± 9.4 |
| Log adiponectin | 0.89 ± 0.25 | 0.87 ± 0.16 | 0.82 ± 0.24 | 0.82 ± 0.13 |
| hsCRP (mg/liter)b | 3.0 ± 3.1 | 4.0 ± 2.6a | 1.94 ± 2.3 | 2.46 ± 2.1 |
The mean value was significantly different from NGT subjects analyzed by student t tests.
The hsCRP was determined in a subgroup of subjects (n = 94 and 98 in female NGT and IGT, respectively; n = 64 and 37 in males NGT and IGT, respectively).
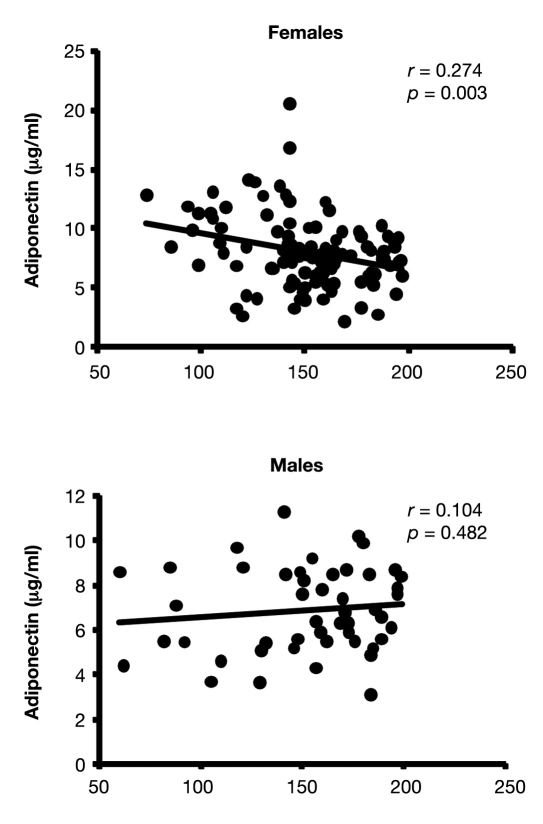
The relationship between adiponectin and risk factors for CVD was then examined by gender. Among total subjects (subjects with both NGT and IGT), adiponectin was correlated positively with age and HDL cholesterol and negatively with BMI, waist circumference, triglyceride, fasting and postload 2-hour postplasma glucose, fasting insulin, HOMA-IR, HbA1c, and hsCRP (data not shown). However, in subjects with IGT, adiponectin was not correlated with any anthropometric or biochemical parameters, except a negative relationship with 2-hour postplasma glucose levels in female subjects (r = –0.274, p < 0.001) (Table 2) (Figure 1). This relationship remained statistically significant after being adjusted for demographic and clinical indices (r = –0.243, p < 0.05). In addition, after adjustments, adiponectin became significantly associated negatively with hsCRP levels in females (r = –0.219, p < 0.05). In prediabetic males, adiponectin was not associated with any CVD risk factors.
Table 2.
Partial Correlation between Adiponectin and Anthropometric and Biochemical Parameters in IGT
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | Model 4d | Model 1a | Model 2b | Model 3c | Model 4d | |
| Age | 0.071 | 0.143 | ||||||
| Waist circumference | –0.061 | 0.151 | ||||||
| Cholesterol | 0.126 | 0.045 | 0.125 | 0.026 | ||||
| Triglyceride | –0.105 | –0.166 | 0.108 | 0.060 | ||||
| HDL | 0.129 | 0.184 | –0.045 | –0.046 | ||||
| LDL | 0.170 | 0.072 | 0.144 | 0.012 | ||||
| Fasting glucose | 0.029 | –0.032 | –0.044 | –0.032 | –0.103 | –0.099 | ||
| Two-hour postplasma glucose | –0.274e | –0.240f | –0.177 | –0.243f | 0.104 | –0.021 | –0.010 | –0.033 |
| Fasting insulin | –0.025 | 0.009 | 0.056 | –0.024 | –0.105 | –0.136 | ||
| HbA1c | 0.049 | 0.151 | 0.137 | 0.058 | –0.139 | –0.155 | ||
| HOMA-IR | –0.038 | 0.019 | 0.070 | –0.024 | –0.098 | –0.128 | ||
| hsCRP | –0.188 | –0.245f | –0.213f | –0.219f | 0.190 | 0.302 | 0.306 | 0.351 |
Unadjusted.
Adjusted for age, waist circumference, and systemic and diastolic blood pressure (and alcohol consumption and smoking in males).
Adjusted for Model 2 + cholesterol, triglyceride, and HDL levels.
Adjusted for Model 3 + fasting insulin, fasting glucose, and HbA1c.
p < 0.01
p < 0.05
Figure 1.
Correlations between plasma glucose and adiponectin levels in IGT.
Table 3 shows the association between adiponectin and IGT using multivariate logistic regression analyses. In unadjusted models, adiponectin was associated negatively with IGT in females [odds ratio (OR): 0.93, 95% confidence interval (CI): 0.887–0.988, p = 0.016] but not in males. After adjusting for demographic and clinical factors, adiponectin remained associated negatively with IGT in females (OR = 0.93, 95% CI: 0.872–0.991, p = 0.026). Moreover, in addition to adiponectin, factors associated with IGT included age (β = 0.04, OR = 1.041, 95% CI: 1.016–1.066), fasting insulin (β = 0.055, OR = 1.056, 95% CI: 1.018–1.096), and HOMA-IR (β = 0.299, OR = 1.349, 95% CI: 1.151–1.582) scores in females and age (β = 0.065, OR = 1.067, 95% CI: 1.033–1.102), triglyceride (β = 0.007, OR = 1.007, 95% CI: 1.002–1.012), and HOMA-IR (β = 0.383, OR = 1.467, 95% CI: 1.098–1.960) scores in males.
Table 3.
Multivariate Logistic Regression between Adiponectin and IGT (Dependent Variable) in Females and Males
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| β | OR | 95% CI | P | β | OR | 95% CI | P | |
| Model 1a | −0.066 | 0.93 | 0.887 – 0.988 | 0.016 | −0.057 | 0.94 | 0.865 – 1.031 | 0.20 |
| Model 2b | −0.096 | 0.91 | 0.854 – 0.967 | 0.002 | −0.099 | 0.91 | 0.813 – 1.009 | 0.072 |
| Model 3c | −0.084 | 0.92 | 0.863 – 0.980 | 0.01 | −0.116 | 0.89 | 0.791 – 1.002 | 0.055 |
| Model 4d | −0.073 | 0.93 | 0.872 – 0.991 | 0.026 | −0.107 | 0.90 | 0.798 – 1.012 | 0.078 |
Adiponectin; unadjusted.
Adjusted for age, waist circumferences, blood pressure, alcohol consumption, and smoking.
Adjusted for Model 2 + cholesterol, triglyceride, and HDL levels.
Adjusted for Model 3 + fasting insulin levels.
Discussion
Our study showed that adiponectin in Asian Indian women is associated negatively with IGT, 2-hour postplasma glucose, and hsCRP levels. However, no association with any CVD risk factors was observed in male IGT.
Literature suggests that adiponectin may play a role in glucose metabolism.1 Low adiponectin levels are frequently reported in type 2 diabetes and metabolic syndrome in different ethnic groups.2,13–16 Adiponectin levels are improved after the administration of insulin-sensitizing agents, such as thiazolidinedione and pioglitazone.17,18 Compared to subjects with normal fasting glucose, Yaturu and colleagues19 found that adiponectin levels were low in prediabetic subjects and were lower in those with concomitant coronary artery diseases. In our study, log-transformed adiponectin values were similar between IGT and NGT subjects. However, in logistic regression analyses, adiponectin was associated negatively and independently with IGT. Each microgram per milliliter of increase in adiponectin concentration was associated with a 7% decrease in IGT risk in females (p = 0.026) and 6% in males (p = 0.078). This was similar to the observation of Spranger and associates4 in subjects who developed diabetes 2–3 years later, suggesting that adiponectin may be a marker for the progression of glucose intolerance in early prediabetic stages.20
Similar to previous findings,1 we found associations between adiponectin and age, BMI, waist circumference, triglyceride, HDL cholesterol, fasting and postload 2-hour postplasma glucose, fasting insulin, HOMA-IR, and HbA1c levels in all the nondiabetic subjects. However, these associations were not found in prediabetic subjects, except that adiponectin was associated negatively and independently with 2-hour postplasma glucose levels in females. It is unclear why adiponectin was not correlated to other demographic and biomedical indices in our IGT subjects. In the Western New York Health Study, Donahue and colleagues12 found that women who developed IGT from normoglycemia after a 6-year follow-up had lower adiponectin levels at baseline compared to females who did not develop IGT. In addition, a greater level of endothelial dysfunction was observed in female prediabetic subjects compared to males.12 Therefore, it is reasonable to postulate that the pathological changes following abnormal glucose metabolism may be different between females and males, although this is purely speculative.
In addition to its insulin-sensitizing effect, adiponectin has been shown to have anti-inflammatory properties.1 It has been suggested that chronic inflammation is one of the contributing factors of glucose intolerance, type 2 diabetes, and associated CVD, possibly related to compromised insulin sensitivity, hyperglycemia, and dyslipidemia.21 In subjects with abnormal glucose metabolism, negative associations have been observed between adiponectin and inflammatory biomarkers independent of glycemic indices and lipid profile.22,23 Consistent with such findings, data from the current study found that females with IGT had elevated hsCRP levels compared to those with normal glucose tolerance. In addition, adiponectin was related negatively to hsCRP levels in females with IGT after controlling for lipid profile and glycemic indices, suggesting the anti-inflammatory effect of adiponectin in females.
One of the limitations of the study is that being a cross-sectional design, we were unable to demonstrate a cause-and-effect relationship between adiponectin and glucose intolerance. However, the strength of the study is that it is a large population-based sample in Asian Indians who have a high prevalence of premature coronary artery disease.
In summary, our study suggests that adiponectin is associated with IGT in Asian Indian females and not in males.
Acknowledgment
The CURES field studies were supported by the Chennai Willingdon Corporate Foundation, Chennai. This is the 73rd report from CURES (CURES-73). This is the 2nd publication from the MDRF-Emory Global Diabetes Research Centre (GDRC-2).
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CURES
Chennai Urban Rural Epidemiology Study
- CVD
cardiovascular disease
- HbA1c
glycated hemoglobin
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment–insulin resistance
- hsCRP
high sensitivity C-reactive protein
- IGT
impaired glucose tolerance
- LDL
low-density lipoprotein
- NGT
normal glucose tolerance
- OR
odds ratio
References
- 1.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V, Deepa R, Pradeepa R. Association of low adiponectin levels with the metabolic syndrome–the Chennai Urban Rural Epidemiology Study (CURES-4) Metabolism. 2005;54(4):476–481. doi: 10.1016/j.metabol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Knobler H, Benderly M, Boyko V. Adiponectin and the development of diabetes in patients with coronary artery disease and impaired fasting glucose. Eur J Endocrinol. 2006;154(1):87–92. doi: 10.1530/eje.1.02054. [DOI] [PubMed] [Google Scholar]
- 4.Spranger J, Kroke A, Mohlig M. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361(9353):226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Funahashi T, Tanaka S. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 6.Nayak S, Soon SQ, Kunjal R, Ramadoo R, Baptiste O, Persad J, Temull V, Diptee L, Balgobin S. Relationship between adiponectin, inflammatory markers and obesity in type 2 diabetic and non-diabetic Trinidadians. Arch Physiol Biochem. 2009;115(1):28–33. doi: 10.1080/13813450902758785. [DOI] [PubMed] [Google Scholar]
- 7.Aroda VR, Ratner R. Approach to the patient with prediabetes. J Clin Endocrinol Metab. 2008;93(9):3259–3265. doi: 10.1210/jc.2008-1091. [DOI] [PubMed] [Google Scholar]
- 8.Mohan V, Deepa M, Anjana RM. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19) J Assoc Physicians India. 2008;56:152–157. [PubMed] [Google Scholar]
- 9.Deepa M, Pradeepa R, Rema M. The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–870. [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. [DOI] [PubMed] [Google Scholar]
- 12.Donahue RP, Rejman K, Rafalson LB. Sex differences in endothelial function markers before conversion to prediabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care. 2007;30(2):354–359. doi: 10.2337/dc06-1772. [DOI] [PubMed] [Google Scholar]
- 13.Deepa M, Farooq S, Deepa R. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47) Eur J Clin Nutr. 2009;63(2):259–267. doi: 10.1038/sj.ejcn.1602920. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Li H, Franco OH. Adiponectin and metabolic syndrome in middle-aged and elderly Chinese. Obesity (Silver Spring) 2008;16(1):172–178. doi: 10.1038/oby.2007.42. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay RS, Funahashi T, Hanson RL. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360(9326):57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 16.Shaibi GQ, Cruz ML, Weigensberg MJ, Toledo-Corral CM, Lane CJ, Kelly LA, Davis JN, Koebnick C, Ventura EE, Roberts CK, Goran MI. Adiponectin independently predicts metabolic syndrome in overweight Latino youth. J Clin Endocrinol Metab. 2007;92(5):1809–1813. doi: 10.1210/jc.2006-2294. [DOI] [PubMed] [Google Scholar]
- 17.Yu JG, Javorschi S, Hevener AL. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10):2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 18.Teranishi T, Ohara T, Maeda K, Zenibayashi M, Kouyama K, Hirota Y, Kawamitsu H, Fujii M, Sugimura K, Kasuga M. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2007;56(10):1418–1424. doi: 10.1016/j.metabol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Yaturu S, Bridges JF, Reddy DR. Decreased levels of plasma adiponectin in prediabetes, Type 2 diabetes and coronary artery disease. Med Sci Monit. 2006;12(1):17–20. [PubMed] [Google Scholar]
- 20.Thamer C, Haap M, Heller E, Joel L, Braun S, Tschritter O, Haring H, Fritsche A. Beta cell function, insulin resistance and plasma adiponectin concentrations are predictors for the change of postprandial glucose in non-diabetic subjects at risk for type 2 diabetes. Horm Metab Res. 2006;38(3):178–182. doi: 10.1055/s-2006-925204. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65(12 Pt 2):S152–S156. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107(5):671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 23.Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27(7):1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]