Abstract
Reliable application of neutral protamine Hagedorn (NPH) insulin requires previous resuspension of the suspension by tipping over the cartridge 20 times. This procedure is considered annoying by patients. The goal of this investigation was to assess the efficiency of the mixing procedure when performed less frequently than recommended. Neutral protamine Hagedorn insulin cartridges from five different manufacturers (sanofi-aventis, Lilly, Berlin-Chemie, B. Braun, and Novo Nordisk) were emptied with doses of 28 IU in the morning and the evening over 5 days. While the first dose was obtained after a regular resuspension procedure (20× tipping over), the consecutive doses were obtained after 3, 6, 10, or 20 mixing procedures (12 cartridges per experimental series, two doses/day). Insulin concentrations of doses 1, 2, 6, and 10 were determined by high-pressure liquid chromatography. Between dosing, cartridges were stored at room temperature in a horizontal position. Comparable insulin concentrations were seen in the first correctly prepared doses. Pronounced and substantial deviations from the selected dose were observed with most of the cartridges, in particular when resuspending only 3 and 6 times. Mean absolute percentage deviations when tipping 3 times and maximally observed overdoses were: Insuman basal: 1.1 ± 1.0%/4 IU, Humulin N: 2.6 ± 3.4%/19 IU, Berlinsulin H basal: 4.4 ± 6.0%/26 IU, Insulin B. Braun basal: 10.4 ± 8.9%/38 IU, and Protaphane: 4.7 ± 4.1%/19 IU (all p < 0.05 vs Insuman basal). Only one cartridge with three metal mixing bullets (sanofi-aventis) was resuspended efficiently with only a few mixing procedures. All other cartridges with fewer bullets were shown to deliver potentially harmful doses if used for treatment when the mixing procedure was less frequent than demanded in the instructions for use.
Keywords: cartridge, insulin injection, mixing, NPH insulin
Introduction
Neutral protamine Hagedorn (NPH) insulin is an intermediate-acting insulin formulation that is recommended for treatment of diabetes mellitus and is used worldwide by millions of patients with type 1 or type 2 diabetes mellitus.1 It is a suspension of crystalline insulin/zinc complexes to which the positively charged polypeptide protamine is added. After subcutaneous application by injection, NPH insulin was shown to have a duration of action of 9–20 hours, which is longer than that of regular human insulin (5–6 hours) but shorter than that of the long-acting insulin analogs insulin glargine and insulin detemir (18–28 hours).2,3
All currently available NPH cartridges require a thorough mixing of the crystalline suspensions prior to injection because high-dose variability may occur without appropriate homogenization of the product.4,5 It is of utmost importance to inform the patients about this requirement, and appropriate instructions have, therefore, become a standard component of structured educational programs. Instructions for use suggest a tipping over of the pens and cartridges 20 times or rolling them for a sufficient time between the palms of the hands. Vigorous shaking of the cartridges has to be avoided because bubbles occurring after such procedure have a substantial impact on dosing accuracy.6
All manufacturers of NPH cartridges suggest that tipping over at least 10 times is sufficient for homogeneous mixing. However, this is regarded to be annoying by many patients, and a previous investigation has indicated that only 9% of insulin-treated patients perform this mixing procedure with appropriate carefulness and efficacy.7 Another investigation has confirmed that the mixing procedure is indeed of substantial importance for treatment quality in daily clinical practice.8
In order to improve the efficiency of any resuspension procedure and as a moiety for increased safety, manufac-turers have placed small bullets into the cartridges to serve as mixing aids. The bullets vary in numbers (one to three/cartridge) and are made of either glass or metal (see Table 1). The purpose of this laboratory experiment was to assess whether the different cartridge compositions (number and material of the bullets) have an influence on the accuracy of the finally applied dose in case patients perform the mixing procedure less frequently than claimed in the instructions for use.
Table 1.
Number and Material of Mixing Bodies (Bullets) in Different NPH Cartridges
| Insulin | Manufacturer | No. of bullets | Material | Weight (mg) |
|---|---|---|---|---|
| Insuman basal | Sanofi-aventis | Three | Metal | (3×) 33.4 |
| Humulin N | Lilly | One | Glass | 18.7 |
| Berlinsulin H basal | Berlin-Chemie | One | Glass | 18.7 |
| Insulin B. Braun basal | B. Braun | Two | Glass | (2×) 17.1 |
| Protaphane Penfill | Novo Nordisk | One | Glass | 17.7 |
Materials and Methods
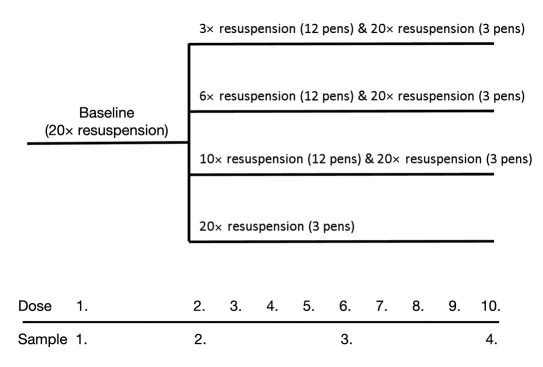
Neutral protamine Hagedorn insulin cartridges and corresponding pen devices from five different insulin manufacturers were purchased in a local pharmacy in Germany [Insuman basal and TactiPen, sanofi-aventis, Berlin; Humulin N (Huminsulin basal) and HumaPen Luxura, Lilly, Bad Homburg; Berlinsulin H basal and BerliPen, Berlin-Chemie, Berlin; Insulin B. Braun basal and Omnican pen, B. Braun, Melsungen; and Protaphane Penfill and NovoPen 4, Novo Nordisk, Mainz, Germany]. In order to mimic daily use by diabetes patients, cartridges were emptied with doses of 28 IU in the morning and the evening over 5 days. Between dosing, pens and inserted cartridges were stored at room temperature in a horizontal position. The initial dose of each application with each pen (dose 1) was performed after correct mixing (20×) and handling of the cartridges in accordance with instructions for use of the manufacturers to assess the correctness of the insulin concentrations in these U100 insulin cartridges. In each of the three experimental series, doses were applied from 12 pens after a consistent number of mixing procedures (3, 6, and 10×), mounting of a new needle, and priming of the needle with 2 IU of insulin prior to delivering a dose of 28 IU into an Eppendorf cap. As the control experiment, an additional three pens per device type were emptied in parallel after 20 mixing procedures under otherwise similar experimental conditions. Insulin concentrations were determined after delivery of doses 1, 2, 6, and 10. An overview of the experimental design is provided in Figure 1.
Figure 1.
Experimental design.
Insulin Determination
Insulin determinations were performed by means of high-pressure liquid chromatography (HPLC) in accordance with a method set forth by United States Pharmacopeia (USP).9 After dose collection, samples were diluted 1:10 with 0.01 N HCl and consecutively applied and separated on a C18 column (Phenomenex Gemini, 5 μm, HPLC column; 4.6 × 150 mm). The HPLC system (Waters, Echborn, Germany) was equipped with a water-cooled sample processing device, an ultraviolet detector (210 and 214 nm), a thermostatic column chamber (40°C), and an automated sample collection device. A USP insulin standard (insulin human 100 mg; Cat. No. 1342106; Lot I0C383, LGC-Promochem, Wesel, Germany) served as the internal reference. The area under the curve was determined to calculate the insulin concentration in comparison of the standard sample. This insulin determination method was validated to have a variability of <1%.
Statistical Analysis
The truly delivered insulin dose, when dialing a 28-IU dose with the delivery device, was calculated from the determined insulin concentrations. The mean absolute percentage deviation was calculated per NPH cartridge type, pen device, and experimental series and compared by means of a two-sided Student's t test with the concentration derived from dose 1 (i.e., the reference value for mixing 20× with each individual cartridge) and among the different pen devices. Results from 20× tipping over were combined for the analysis of correct cartridge usage. A p value <0.05 was considered to be statistically significant. In addition, minimal and maximal observed dose deviations were documented and listed for each dose, cartridge, and experimental series to obtain the theoretical dose range delivered from each of the cartridges during these experiments.
Results
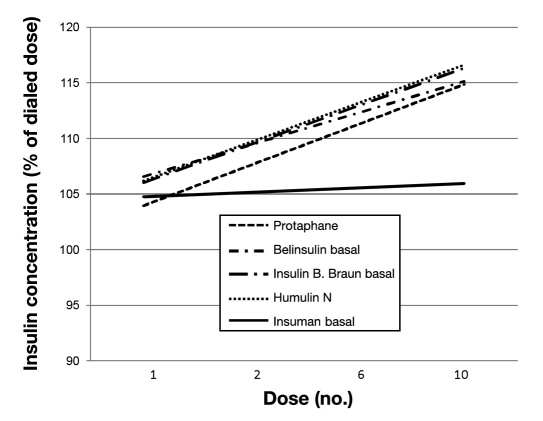
All cartridges showed comparable insulin concentrations matching the USP specification for U100 insulin when the initial dose was delivered after 20× mixing in accordance with the manufacturer's instructions. Thereafter, pronounced and substantial deviations were observed from the selected dose with most of the cartridges, in particular when mixing only 3 and 6 times. The more mixing procedures applied, the lower the overall variability. Mean absolute percentage deviations from the initial dose when emptying the cartridge with different mixing frequencies are provided in Table 2. Only two NPH cartridges (Insuman basal and Humulin N) showed overall low mean deviations in this analysis. However, with a low number of mixing procedures (3 and 6×), increasing insulin concentrations were observed during cartridge emptying for all cartridges except the Insuman basal cartridge, as shown for 6 mixing procedures in Figure 2.
Table 2.
Mean Absolute % Deviation after Different Mixing Frequencies Observed after Selection of 28 IU
| NPH cartridge | No. of mixing procedures | |||
|---|---|---|---|---|
| 3× | 6× | 10× | 20× | |
| Humulin N | 2.6 ± 3.4a | 1.4 ± 3.0 | 0.8 ± 0.8 | 0.3 ± 0.3 |
| Berlinsulin H basal | 4.4 ± 6.0a | 2.1 ± 2.3a | 1.8 ± 1.6a | 0.6 ± 0.7a |
| Insulin B. Braun basal | 10.4 ± 8.9a | 1.5 ± 1.2a | 1.1 ± 1.0 | 0.3 ± 0.3 |
| Protaphane | 4.7 ± 4.1a | 1.7 ± 1.5a | 1.5 ± 1.1 | 0.3 ± 0.2 |
| Insuman basal | 1.1 ± 1.0 | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.3 ± 0.2 |
p < 0.05 vs Insuman basal.
Figure 2.
Linear regression curve of changes in insulin concentration when emptying cartridges with only six mixing procedures and with at least 8-hour resting period in horizontal position between each dose delivery.
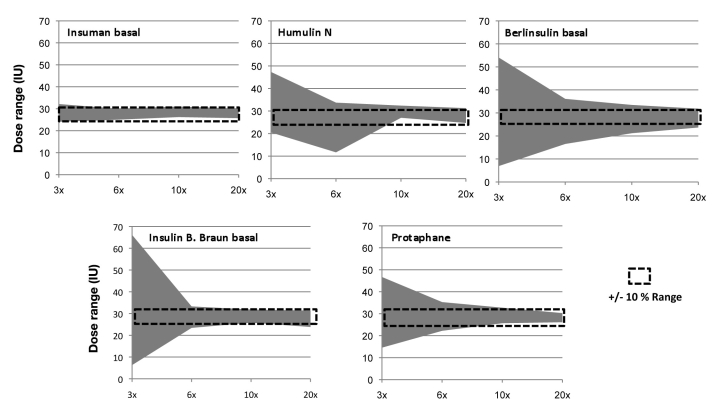
As each individual dose can be potentially harmful for patients if injected subcutaneously, we also determined minimal and maximal individual doses delivered from the cartridges for each experimental series. These data allowed us to determine the range of doses delivered from the NPH cartridges as a function of the number of mixing procedures. The graphical presentation of this analysis is provided in Figure 3.
Figure 3.
Observed dose ranges by pen when emptying cartridges with different numbers of mixing procedures. Dotted line represents an arbitrarily defined acceptable deviation range of ±10% (± 2.8 IU).
We arbitrarily defined a deviation of ±10% (∼3 IU) to be clinically acceptable at the selected dose of 28 IU. Percentages of doses outside of this range for 3, 6, 10, and 20× tipping over were 8.3, 2.1, 2.1, and 0.0% for Insuman basal; 33.3, 8.3, 2.1, and 0.0% for Humulin N; 35.4, 25.0, 12.5, and 4.2% for Berlinsulin H basal; 68.8, 10.4, 10.4, and 2.1% for Insulin B. Braun basal; and 56.3, 18.8, 4.2, and 0.0% for Protaphane.
Discussion
Much attention has been paid to injection techniques and the variability of absorption of insulin. Variability of insulin action after subcutaneous injection is influenced by many confounding factors, including, but not limited to, the injection site,10,11 variability of insulin absorption from subcutaneous tissue,12 dose accuracy of delivery devices,13 type of insulin, and environmental conditions.14
In case of injection of NPH insulin or fixed insulin mixtures containing NPH insulin, the homogeneity of the crystalline suspension creates another source of variability with an until yet unknown impact magnitude. It is surprising that only two published investigations could be found when searching the literature for investigations of this phenomenon.
Jehle and colleagues7 found a decade ago that inadequate resuspension of NPH insulin before pen injection is common among diabetes patients treated with insulin. They did not measure the insulin doses but used the observation that the cloudy component of the cartridges equates to the complexed insulin, while the clear component is the diluting fluid or soluble insulin, depending on the type of insulin used. The optical density provides a simple way of measuring cloudiness and thus indirectly on the drug composition. Only 10 of their 109 patients (9%) tipped and rolled their pen more than 10 times. After they instructed their patients on the correct resuspension technique, patients who improved their technique for insulin resuspension had significantly fewer hypoglycemic episodes than those that did not improve their technique. The authors concluded that physicians, nurses, and patients must be alerted to the difficulty of incomplete NPH insulin resuspension in order to improve diabetes control as well as the safety of insulin use. They recommended that patients using insulin pens with NPH preparations tip the pen at least 20 times and that this advice should be added to diabetes education programs.7
In a more recent study, Brown and colleagues8 explored the completeness of NPH resuspension by 180 patients in the community of Kirkcaldy by applying a similar optical density method for assessment of the efficacy of resuspension. Only 1 patient in their cohort mixed the insulin as the manufacturers recommended. In 40% of the cartridges half emptied by the patients, the opacity of the insulin varied significantly from the expected value. The authors concluded that most patients do not mix insulin adequately, which may result in their giving different incorrect doses of insulin during the use of each pen.8
From both studies it is apparent that patients find the resuspension requirement annoying. As a consequence, cartridge manufacturers have searched for technical solutions to improve the effectiveness of the resuspension procedures. Current NPH cartridges contain small bullets as mixing bodies. However, there is no product standardization and the cartridges vary regarding the amount of bullets (one to three) and material (glass or metal). Our experimental study investigated the impact of different numbers of resuspension procedures on the dosing accuracy of NPH insulin cartridges of five manufacturers in Germany. In contrast to the older clinical investigation, we standardized the tipping over method and the storage of pens between doses. We also measured the insulin content of the applied doses by means of a certified HPLC method. We found very high dose accuracy for all cartridges when resuspension was initially performed in accordance with the instructions for use. However, pronounced differences between NPH cartridges became apparent when resuspension was only performed three or six times. Only the cartridge with the heaviest and highest number of bullets (sanofi-aventis) was mixed efficiently with these few mixing procedures. All other cartridges (with one or two glass bullets) were shown to occasionally deliver doses under these conditions that would have potentially harmed the patient if injected into the subcutaneous tissue.
The Humulin N and Berlinsulin basal cartridges are the same product produced by Eli Lilly. Differences between these two brands may represent the variability of our research methods. However, it is more tempting to speculate that observed differences between the two brands of the same cartridge may be influenced by the pen devices provided by both companies. However, further research is necessary to understand the potential impact of haptic pen properties or patient dexterity regarding the mixing procedure.
All cartridges in this study performed well when used according to the instructions for use with 20× shaking. While patients should therefore be instructed clearly to use NPH insulin cartridges always in accordance with the instructions for use, a higher number of mixing bullets made of metal may be considered to be a technical measure of treatment safety for patients injecting NPH insulin or fixed mixtures containing NPH insulin formulations.
This study underlined the importance of putting more emphasis on appropriate patient training and retraining on the necessity of efficient mixing when introducing NPH insulin-containing therapies in patients with diabetes mellitus.
Acknowledgment
This work was funded by an unrestricted grant from sanofi-aventis.
Abbreviations
- HPLC
high-pressure liquid chromatography
- NPH
neutral protamine Hagedorn
- USP
United States Pharmacopeia
References
- 1.American Diabetes Association. Clinical practice recommendations. Diabetes Care. 2009;32(Suppl.1):S1–S98. [PubMed] [Google Scholar]
- 2.Scholtz HE, Pretorius SG, Wessels DH, Becker RH. Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using an euglycaemic clamp technique. Diabetologia. 2005;48(10):1988–1995. doi: 10.1007/s00125-005-1916-y. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann L, Sinha K, Weyer C, Loftager M, Hirschberger S, Heise T. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet Med. 1999;16(4):332–338. doi: 10.1046/j.1464-5491.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen JO, Flyvbjerg A, Jorgensen JT, Sorensen HH, Johansen BR, Christiansen JS. NPH insulin administration by means of a pen injector. Diabet Med. 1998;55(6):574–576. doi: 10.1111/j.1464-5491.1988.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 5.Laubach E, Schwandt P, Ritter MM. Neutral protamine Hagedorn insulin. Lancet. 2000;355(9199):236. doi: 10.1016/S0140-6736(05)72115-2. [DOI] [PubMed] [Google Scholar]
- 6.Fisken RA, Goulbourn J. Treatment of insulin-dependent diabetes using an injection pen: control, problems and patients preferences. Diabetes Res. 1989;11(4):195–197. [PubMed] [Google Scholar]
- 7.Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999;354(9190):1604–1607. doi: 10.1016/S0140-6736(98)12459-5. [DOI] [PubMed] [Google Scholar]
- 8.Brown A, Steel JM, Duncan C, Duncan A, McBain AM. An assess-ment of the adequacy of suspension of insulin in pen injectors. Diabet Med. 2004;21(6):604–608. doi: 10.1111/j.1464-5491.2004.01206.x. [DOI] [PubMed] [Google Scholar]
- 9.The US Pharmacopoeia convention. Available from: www.usp.org.
- 10.Thow JC, Johnson AB, Fulcher G, Home PD. Different absorption of isophane (NPH) insulin from subcutaneous and intramuscular sites suggests a need to reassess recommended insulin injection technique. Diabet Med. 1990;7(7):600–602. doi: 10.1111/j.1464-5491.1990.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 11.Bantle JP, Neal L, Frankamp LM. Effects of the anatomical region used for insulin injections on glycemia in type 1 diabetes subjects. Diabetes Care. 1993;16(12):1592–1597. doi: 10.2337/diacare.16.12.1592. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673–682. doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- 13.Hänel H, Weise A, Sun W, Pfützner JW, Thomé N, Pfützner A. Differences in the dose accuracy of insulin pens. J Diabetes Sci Technol. 2008;2(3):478–481. doi: 10.1177/193229680800200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger M, Cuppers HJ, Hegner H, Jorgens C, Berchtold P. Absorption kinetics and biological effects of subcutaneously injected insulin preparations. Diabetes Care. 1982;5(2):77–91. doi: 10.2337/diacare.5.2.77. [DOI] [PubMed] [Google Scholar]