Abstract
The determination of C-reactive protein (CRP) by means of a highly sensitive laboratory method as an independent biomarker for assessment of chronic systemic vascular inflammation and cardiovascular risk is recommended by therapeutic guidelines for diabetes and cardiovascular disease in the United States and in Europe. The purpose of this investigation was to investigate the specificity and sensitivity of a newly developed lateral-flow-based point-of-care (POC) rapid test with semi-quantitative visual reading in comparison with a laboratory reference standard method.
The high-sensitivity CRP concentrations of 66 samples were determined by means of turbidimetry and the POC test (5 μl serum/10 μl capillary whole blood, 10 minutes) was independently performed by three investigators blinded to each other's results. The visual readings were classified, as recommended by the American Heart Association, to represent a low risk (0–1 mg/liter), moderate risk (>1–3 mg/liter), or high risk (>3–10 mg/liter) or to indicate an unspecific inflammation (>10 mg/liter).
According to the reference method, there were 17 samples in the low-risk group, 19 samples in the moderate-risk group, and 26 samples in the high-risk group, and 4 samples showed an unspecific inflammation. All three investigators reached very conclusive results. The range of agreement between the visual readings of the investigators and the laboratory method ranged between 94% and 97%. The sensitivity for assessment of moderate-to-high cardiovascular risk was 100% (45/45 were detected), and the specificity ranged between 90% and 95%.
The newly developed lateral-flow-based POC rapid test showed an excellent agreement between individual visual reading and the laboratory reference method. It may therefore be suitable for a fast and convenient screening, which, after laboratory test confirmation, may help to identify patients with elevated risk of macrovascular disease.
Keywords: cardiovascular disease, chronic systemic inflammation, high-sensitivity C-reactive protein, point-of-care rapid test
Introduction
Over the past decades, evidence has accumulated that a chronic systemic inflammation of the vessel wall is a key driver of atherosclerosis and cardiovascular disease (CVD).1,2 This finding has supported the identification of clinically useful inflammatory biomarkers to improve CVD risk prediction. A prominent member of these glucose -independent risk indicators is C-reactive protein (CRP) as measured by a high-sensitivity (hs) assay.3 C-reactive protein represents the classical acute-phase protein produced in the liver in response to an inflammation, and plasma levels of hs-CRP provide a sensitive marker of increased inflammatory activity in the arterial wall.4 This biomarker has to date the best evidence among the other discussed markers to support its use as an independent predictor of increased CVD risk in diabetes patients and patients without diabetes.5–7 In addition, numerous clinical trials have established elevated CRP levels to be predictive of the development of insulin resistance, the metabolic syndrome, and type 2 diabetes.8–14
In daily practice, it is important to distinguish between standard and high sensitivity assays for the measurement of CRP. The traditional CRP assay, using a polyclonal antibody, assists in the diagnosis and assessment of acute inflammation, i.e., associated with infections and neoplastic diseases. A more sensitive CRP test, the hs-CRP assay, allows for detection and linear quantification of CRP levels down to and below 3 mg/liter. These lower levels toward the upper end of normal (10 mg/liter) reflect low-grade inflammation and have predictive value of future risk for CVD events.14 Measurement of hs-CRP should be done twice, optimally two weeks apart, to provide a more stable estimate of the level of this biomarker. The relative cardiovascular risk categories for average hs-CRP levels are low risk (<1 mg/dl), average/moderate risk (1.0–3.0 mg/liter), high risk (3.0–10 mg/liter), and unspecific elevation (>10 mg/liter); these should be reevaluated for acute inflammatory conditions.15
The possibility to assess individual cardiovascular risk has increased the desire for fast and reliable methods to determine hs-CRP directly in clinical practice. Here, we report on the results of a laboratory evaluation of a new point-of-care (POC) rapid test developed to allow for visual reading and semi-quantitative determination of hs-CRP levels in accordance with the previously mentioned guideline classifications. In a previous investigation for regulatory approval in Europe (CE-mark), 44 out of 49 tests (90%) gave a correct result compared with a reference method. All samples (13/13) with low risk were correct (100%), 2 samples out of 15 with a moderate risk were analyzed as low risk (87% correct results), and 3 samples out of 18 with high risk were determined as unspecifically elevated (83% correct results). The current study evaluated the test performance under daily routine conditions.
Materials and Methods
The samples used for this analysis were derived from clinical studies in diabetic and nondiabetic patients previously performed at the Institute for Clinical Research and Development, Mainz, Germany. The participants in these trials had authorized the further use of their blood samples for laboratory research in their written informed consent.
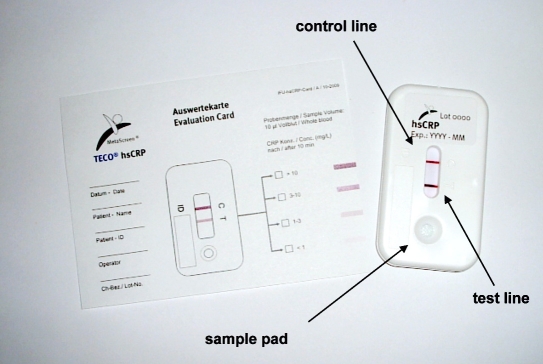
High-sensitivity CRP was initially assessed by means of a turbimetric method (Olympus, Hamburg, Germany). The semi-quantitative POC hs-CRP rapid test was kindly provided by IR2Dx Inc., Moraga, CA (in Europe, MetaScreen®, TecoMedical AG, Sissach, Switzerland). This hs-CRP test is already approved and CE marked in Europe. It uses a cassette format (Figure 1) and consists of a system of overlapping membranes containing the dried components needed for the test performance. These membranes are assembled to small strips that are placed into a plastic housing for better handling. The patient's material (5 μl of serum or 10 μl of capillary whole blood) is loaded to the ‘sample application pad.’ Thereafter, two drops of a reagent buffer are added. Figure 1. Picture of the new point-of-care hs-CRP rapid test.
Figure 1.
Picture of the new point-of-care hs-CRP rapid test.
In the case of whole blood/capillary blood samples, a separation of blood cells and plasma takes place. The liquid fraction of the patients sample diffuses through the so-called ‘conjugate release pad’ containing labeled detection antibodies for CRP. The conjugate molecules are specifically directed against CRP. The conjugate is redissolved, and CRP is specifically bound by the gold conjugate.
The CRP–gold conjugate complex further diffuses through the ‘analytical membrane.’ On this membrane, two lines are arranged one after the other: (i) the ‘test line’ containing hs-CRP-specific molecules responsible for immobilizing the CRP–gold conjugate complexes and (ii) the ‘control line’ fixing nonbound gold conjugate particles indicating that conjugate has flown over the ‘test line.’ The color intensity of the test line is directly proportional to the CRP concentration in the patient's sample enabling (semi-)quantitative interpretation of the test result. If CRP is concentrated above the upper threshold limit (10 mg/liter), the ‘test line’ and the ‘control line’ are clearly visible; if CRP is below 10 mg/liter, different color intensities of the ‘test line’ help to determine the patient's risk group (Figure 2). A reference card indicating the color intensities of the different thresholds is provided with each individual test to support the visual analysis and to enable documentation of the test results for filing into the patient files. The characteristics of this test are provided in Table 1. For this evaluation, 5 μl of serum sample was used, and the test was interpreted after 10 minutes. Three investigators analyzed the test independently and were blinded to the reference results and to the evaluation of the other investigators.
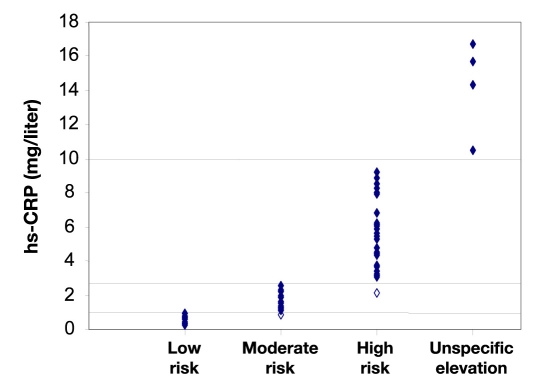
Figure 2.
Laboratory reference values for hs-CRP and readings of the best performing investigator. (Highlighted data points indicate the two false readings.)
Table 1.
Characteristics of the MetaScreen High-Sensitivity C-Reactive Protein Point-of-Care Rapid Test
| Parameter | Specification |
|---|---|
| Sample material | serum, plasma, or (capillary) whole blood |
| Volume | 5 μl serum or plasma, 10 μl whole blood |
| Time requirement | 10 minutes |
| Read out | semi-quantitative, visual comparison with a reference color card |
| Kit components | test cassette, 10 μl pipette, reagent buffer |
| Additional material required | Lancets for blood draw from the fingertip, paper pads |
For the statistical analysis, the results of the individual investigators were compared with the laboratory reference results and the percentage of agreement was determined for all different risk groups. Sensitivity for moderate-to-high CVD risk determination was calculated as the correctly identified samples ranging between 1 and 10 mg/liter. Specificity was determined as the amount of wrong positive samples (i.e., readings between 1 and 10 mg/liter) in samples with values below 1 mg/liter (low risk) or >10 mg/liter (unspecific elevation).
Results
From the selected samples, 17 were in the low-risk group, 19 were in the moderate-risk group, and 26 were in the high-risk group. Four samples had hs-CRP levels >10 mg/liter, indicating unspecific inflammation. All applied POC rapid tests went technically well and delivered a result feasible for immediate visual analysis. In general, all three investigators had a high agreement between their reading and the result from the laboratory analysis. One investigator classified 2/66 samples to be in a higher risk group than indicated by the reference measurement (one in the low-risk group and one in the moderate-risk group, i.e., 97% agreement). The two other investigators classified four samples into a higher risk group (in both cases, three from the low-risk group and one from the moderate-risk group, i.e., 94% agreement) (see Table 2). The results of the laboratory reference values and the readings by the better investigator are provided in Figure 2.
Table 2.
Results of the Laboratory Reference Tests and the Readings by the Three Investigators
| Risk group | hs-CRP | n | Reading | Investigator 1 | Investigator 2 | Investigator 3 |
|---|---|---|---|---|---|---|
| Low risk | <1 mg/liter | 17 | Too high | 1 | 3 | 3 |
| Correct | 16 | 14 | 14 | |||
| Moderate risk | 1–3 mg/liter | 19 | Too high | 1 | 1 | 1 |
| Correct | 18 | 18 | 18 | |||
| Too low | 0 | 0 | 0 | |||
| High risk | 3–10 mg/liter | 26 | Too high | 0 | 0 | 0 |
| Correct | 26 | 26 | 26 | |||
| Too low | 0 | 0 | 0 | |||
| Unspecifically elevated | >10 mg/liter | 4 | Correct | 4 | 4 | 4 |
| Too low | 0 | 0 | 0 |
When combining moderate- and high-risk group samples (n = 45), all three investigators classified all samples to be in either of the groups achieving a sensitivity for elevated risk assessment of 100%. All samples with unspecifically elevated hs-CRP were correctly classified by all investigators. One investigator wrongly classified one of the samples <1 mg/liter to be in the moderate-risk groups (three samples by the other investigators) achieving a specificity of 95% (90%, respectively).
Discussion
Research of the past decades revealed that low-grade inflammation is significantly linked to insulin resistance, type 2 diabetes, and increased risk of CVD. Elevated levels of hs-CRP emerged as a reliable biomarker for the subclinical inflammatory state. Current evidence supports the usefulness of hs-CRP measurement for vascular risk and treatment efficacy assessment in insulin-resistant diabetes patients and nondiabetic individuals.7,11,12,14 A stratification into four risk groups has been identified and is recommended in current clinical guidelines.15
In this investigation, we evaluated the performance of a fast and convenient POC rapid test with visual reading and suitable for risk screening in daily practice. We demonstrated that the test has an excellent sensitivity, a very high specificity, and a very low variability of the test interpretation when read out by different investigators. With this performance, the test can be recommended for routine screening in daily practice. A positive test should lead to laboratory reference confirmation and further exploration of the individual cardiovascular risk of the patient.
Clinical trials (Action to Control Cardiovascular Risk in Diabetes; Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation; and Veterans Affairs Diabetes Trial) have demonstrated that improved glycemic control with reduction of hemoglobin A1c levels into the target range has no significant influence on cardiovascular mortality.16–18 At the same time, prospective interventional studies have shown that drugs that reduce chronic systemic vascular inflammation, such as aterovastatin or pioglitazone, may lead to improved macrovascular outcome, indicating the crucial role of this inflammation for the atherosclerotic process and outcome.19–23 Both drugs reduced not only hs-CRP levels, but also intima-media thickness, another well-established clinical surrogate marker for macrovascular disease. This was not the case with other more commonly prescribed drugs for treatment of metabolic syndrome such as metformin or sulfonylurea drugs.24–27 These findings indicate that a more individualized approach to diabetes therapy may provide a better way to improve the macrovascular prognosis and outcome for a given patient. Characterization of the individual risk situation of patients by means of hs-CRP and other biomarkers (e.g., adiponectin to assess the activity of the visceral adipose tissue and the related insulin resistance28 or intact proinsulin to determine the degree of β-cell dysfunction29) may allow for selection and monitoring of more individually tailored therapies. However, future research is required to elucidate the impact of such an approach on final patient outcome. Follow-up data will demonstrate how the test results evolve over time for any given patient. In any case, the different biomarker tests must become accessible to physicians and patients to be used in daily routine, and the POC format tested in this trial, with its short testing time of 10 minutes, may provide an attractive option to address this need.
In conclusion, the new lateral-flow-based POC rapid test for semi-quantitative determination of hs-CRP from IR2Dx/TecoMedical is a reliable screening method and may serve as a practical tool for rapid identification of diabetic patients and nodiabetic patients with elevated chronic systemic inflammation and higher risk of macrovascular disease.
Abbreviations
- CRP
C-reactive protein
- CVD
cardiovascular disease
- hs
high sensitivity
- POC
point of care
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 3.Clearfield MB. C-reactive protein: a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc. 2005;105(9):409–416. [PubMed] [Google Scholar]
- 4.Pf¨tzner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol Ther. 2006;8(1):28–36. doi: 10.1089/dia.2006.8.28. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285(19):2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 9.Festa A, D'Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O'Reilly DS, Packard CJ, Sattar N. West of Scotland Coronary Prevention Study. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51(5):1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23(4):650–655. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 13.Pf¨tzner A, Standl E, Strotmann HJ, Schulze J, Hohberg C, L¨bben G, Pahler S, Schöndorf T, Forst T. Association of high-sensitive C-reactive protein with advanced stage beta-cell dysfunction and insulin resistance in patients with type 2 diabetes mellitus. Clin Chem Lab Med. 2006;44(5):556–560. doi: 10.1515/CCLM.2006.108. [DOI] [PubMed] [Google Scholar]
- 14.Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in C-reactive protein from low-fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism. 2010;59(1):54–61. doi: 10.1016/j.metabol.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson TA, Mensah GA, Hong Y, Smith SC., Jr CDC, AHA. CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: overview. Circulation. 2004;110(25):e543–e544. doi: 10.1161/01.CIR.0000148979.11121.6B. [DOI] [PubMed] [Google Scholar]
- 16.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. Erratum in: N Engl J Med. 2009;361(10):1028. [DOI] [PubMed] [Google Scholar]
- 19.Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39(12):3297–3302. doi: 10.1161/STROKEAHA.108.516450. [DOI] [PubMed] [Google Scholar]
- 20.Sever PS, Poulter NR, Dahlof B, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes G, Mehlsen J, Nieminen MS, O'Brien ET, Ostergren J. ASCOT Investigators. The Anglo-Scandinavian Cardiac Outcome Trial lipid lowering arm extended observations 2 years after trial closure. Eur Heart J. 2008;29(4):499–508. doi: 10.1093/eurheartj/ehm583. [DOI] [PubMed] [Google Scholar]
- 21.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 22.Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM. PROactive Investigators. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007;49(17):1772–1780. doi: 10.1016/j.jacc.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox R, Bousser MG, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S, Dormandy J. PROactive Investigators. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38(3):865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- 24.Pf¨tzner A, Marx N, L¨bben G, Langenfeld M, Walcher D, Konrad T, Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the Pioneer Study. J Am Coll Cardiol. 2005;45(12):1925–1931. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 25.Langenfeld MR, Forst T, Hohberg C, Kann P, L¨bben G, Konrad T, F¨llert SD, Sachara C, Pf¨tzner A. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation. 2005;111(19):2525–2531. doi: 10.1161/01.CIR.0000165072.01672.21. [DOI] [PubMed] [Google Scholar]
- 26.Hanefeld M, Marx N, Pf¨tzner A, Baurecht W, Luebben G, Kargiannis E, Stier U, Forst T. Anti-inflammatory effect of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitive CRP: the PIOSTAT study. J Am Coll Cardiol. 2007;49:291–298. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 27.Forst T, Wilhelm B, Pf¨tzner A, Fuchs W, Lehmann U, Schaper F, Weber M, M¨ller J, Konrad T, Hanefeld M. Investigation of the vascular and pleiotropic effects of atorvastatin and pioglitazone in a population at high cardiovascular risk. Diab Vasc Dis Res. 2008;5(4):298–303. doi: 10.3132/dvdr.2008.043. [DOI] [PubMed] [Google Scholar]
- 28.Schöndorf T, Maiworm A, Emmison N, Forst T, Pf¨tzner A. Biological background and role of adiponectin as marker for insulin resistance and cardiovascular risk. Clin Lab. 2005;51(9-10):489–494. [PubMed] [Google Scholar]
- 29.Pf¨tzner A, Pf¨tzner AH, Larbig M, Forst T. Role of intact proinsulin in diagnosis and treatment of type 2 diabetes mellitus. Diabetes Technol Ther. 2004;6(3):405–412. doi: 10.1089/152091504774198124. [DOI] [PubMed] [Google Scholar]