Abstract
CD4+CD25+ regulatory T cells (T reg cells) expressing the transcription factor Foxp3 can be induced from peripheral T cell receptor (TCR) transgenic CD4+CD25−Foxp3− T cells stimulated with noninflammatory dendritic cells presenting low amounts of agonist cognate antigen. However, limited evidence exists for extra-thymic T reg cell generation from non-TCR transgenic T cells in unmanipulated mice. We compared events early during agonist-driven generation of Foxp3+ TCR transgenic T cells to polyclonal CD4+ T cell populations in unmanipulated mice. We identified an interleukin-2– and phosphatidylinositol-3-kinase–dependent precommitted Foxp3− precursor to Foxp3+ T reg cells in peripheral lymphoid organs. Transforming growth factor β signaling played a minor role in the generation and subsequent differentiation of these T reg precursor cells.
CD4+CD25+ regulatory T cells (T reg cells) expressing the forkhead family transcription factor Foxp3 play a nonredundant role in maintaining dominant immunological tolerance (Sakaguchi et al., 2008). Experimental evidence suggesting an important function of the thymus in producing T reg cells includes the demonstration of the presence of Foxp3+ T cells in the thymus by expression analysis in single cells (Fontenot et al., 2005b) and the reduction of peripheral T reg cell numbers by neonatal thymectomy at day 3 after birth (Sakaguchi et al., 1985). Besides thymic T reg cell generation, initially naive CD4+Foxp3− T cells can extrathymically acquire a Foxp3+ T reg cell phenotype in several in vitro and in vivo experimental settings, such as TGF-β treatment in vitro (Chen et al., 2003; Fantini et al., 2004, 2007) or homeostatic expansion in lymphopenic mice (Curotto de Lafaille et al., 2004; Knoechel et al., 2005). Under more physiological conditions, antigen (Ag)-specific T reg cells can be induced in vivo from TCR transgenic CD4+ T cells by delivering Ag under subimmunogenic conditions, such as infusion by implanted osmotic minipumps (Apostolou and von Boehmer, 2004) or injection of recombinant anti–DEC-205 fusion antibodies to target steady-state DCs (Kretschmer et al., 2005, 2006). In contrast to TGF-β–mediated in vitro generation of Foxp3+ cells (Floess et al., 2007; Polansky et al., 2008), DC-targeted T reg cell de novo generation in vivo results in efficient demethylation of conserved CpG motifs within the noncoding part of the Foxp3 gene (Polansky et al., 2008), which is a predictive parameter for the long-term stability of induced Foxp3 expression. Consequently, T reg cells generated in this manner survive for extended periods of time in the absence of the inducing Ag and maintain a stable Foxp3+ suppressor phenotype under immunogenic conditions (Kretschmer et al., 2005; Polansky et al., 2008).
Consistent with the absence of deliberate DC activation as a prerequisite for efficient DC-targeted T reg cell conversion, cells that proliferate least induce Foxp3 expression more efficiently than those that divide robustly (Kretschmer et al., 2005). The capacity of specialized subsets of steady-state DCs in lymphoid tissues to induce Foxp3 expression is further corroborated by the observation that CD103+ DCs from the small intestine and intestine-associated lymphoid organs induce Foxp3 expression in vitro in a small fraction of initially CD4+Foxp3− T cells via TGF-β– and retinoic acid–dependent mechanisms (Benson et al., 2007; Coombes et al., 2007; von Boehmer, 2007), with retinoic acid interfering with the negative effect of high levels of costimulation on T reg cell conversion (Nolting et al., 2009). Similar to intestinal DCs, CD8+DEC205+ DCs, but not CD8−DCIR2+ DCs, from spleen induce functional Foxp3+ T reg cells in vitro from yet to be identified CD4+Foxp3− precursor populations in the presence of low Ag doses but without exogenous TGF-β (Yamazaki et al., 2008).
In non-TCR transgenic animals, infusion of female mice with male transplantation antigens resulted in T reg cell–mediated long-term tolerance to transplanted male skin (Verginis et al., 2008), supporting the notion that de novo generation and population expansion of Ag-specific T reg cells may be a suitable approach toward immune-based therapies in clinical settings of unwanted immunity. However, extrathymic T reg cell de novo generation has been documented only for a limited number of TCR specificities. In addition, it remains largely unclear whether molecular and cellular pathways of T reg cell generation described in TCR transgenic model systems can be extended to non-TCR transgenic T cells under physiological conditions. Furthermore, the relative contributions of thymic and extrathymic differentiation pathways to the overall peripheral Foxp3+ T reg cell pool are unknown. Approaches, such as TCR-based lineage tracing in BDC2.5 transgenic mice (Wong et al., 2007) or comparative TCR repertoire analysis of T reg cells and conventional CD4+ T cells (Hsieh et al., 2006; Pacholczyk et al., 2006; Lathrop et al., 2008), suggested that peripheral Foxp3+ T reg cells may predominantly originate from the thymus. However, limitations inherent to the study of T cell repertoires, which are likely to be locally shaped by both tissue-specific and foreign Ags and lack of suitable surface markers to identify extrathymic T reg precursor cells or to distinguish thymically and extrathymically generated Foxp3+ cells, remain substantial experimental obstacles.
In this paper, we report on our attempts to track extrathymic generation of polyclonal T reg cells in unmanipulated mice. Analysis of early events during agonist ligand DC-targeted T reg cell generation from TCR transgenic CD4+ T cells allowed us to delineate differentiation stages to extrathymic Foxp3+ T reg cells with distinct surface marker expression. Correlating these findings with polyclonal T cells from WT mice helped identify a sizeable population of CD4+Foxp3− T cells in peripheral lymphoid organs that are precommitted to differentiate into Foxp3+ T reg cells. Our results indicate that both thymic and extrathymic T reg cell generation contributes to the overall Foxp3+ T reg cell pool in peripheral lymphoid organs of nonmanipulated mice.
RESULTS
Extrathymic differentiation of Ag-specific Foxp3− precursors to Foxp3+ T reg cells
Previous studies indicated that low-dose antigenic stimulation by noninflammatory DCs in vivo favors extrathymic conversion of naive CD4+ T cells into functional CD25+Foxp3+ T reg cells (Kretschmer et al., 2005). These experiments took advantage of an adoptive transfer model consisting of TCR-haemagglutinin (HA)107-119 transgenic CD4+ T cells, which were purified from donor mice on a recombination-activating gene (Rag) 2–deficient (Rag2−/−) background to preclude preformed Foxp3+ cells and minute amounts of recombinant anti–DEC-205-HA107-119 fusion antibodies to target DCs in immunocompetent recipients. To reassess DC targeting to de novo generate Foxp3+ T reg cells in vivo, we now asked whether this approach can be extended to CD4+ T cells from Rag-proficient Foxp3GFP reporter mice with different transgenic TCR specificities. The results show that, in addition to TCR-HA107-119 T cell activation through DC-targeted HA107-119, DC-targeted whole ovalbumin protein induced Foxp3GFP expression in H2-IAd– and H2-IAb–restricted DO11.10 and OT2 CD4+ T cells, respectively (Fig. 1 A). In addition, DC targeting of peptide mimotopes induced Foxp3 expression in 30–40% of initially Foxp3− diabetogenic BDC2.5 CD4+ T cells recognizing a NOD I-Ag7–restricted self-Ag in pancreatic β cells (unpublished data).
Figure 1.
Tracking extrathymic differentiation of Ag-specific CD4+ T cells during DC-mediated Foxp3 induction. (A and B) Using clonotypic antibodies (TCR-HA107-119: 6.5, DO11.10: KJ1-26) or anti–Vα2/-Vβ5.1/5.2 antibodies (OT2), naive TCR transgenic T cells (CD4+CD25−CD62L+Foxp3GFP−) were FACS purified from Rag-proficient Foxp3GFP mice (A, top). 2 × 106 cells were adoptively transferred i.v. into congenic recipients, which were injected i.p. with anti–DEC-205 antibodies fused to respective Ags (anti–DEC-205-HA107-119, 50 ng; anti–DEC-205-ovalbumin, 250 ng) the next day. Flow cytometry of congenic marker+ cells (A, middle) for induced Foxp3GFP expression (A, bottom) at day 7 and percentages of congenic marker+ cells at day 14 in pools of LNs (B). (C) Numbers of transferred Thy1.2+DO11.10+ (left) or Thy1.2+TCR-HA107-119 (right) cells recovered from indicated organs at day 5 after injection of anti–DEC-205-ovalbumin or anti–DEC-205-HA107-119, respectively. (D–G) Analysis of transferred Thy1.2+DO11.10+ cells at day 5 after anti–DEC-205-ovalbumin injection. (D) Flow cytometry of scLN-derived Thy1.2+ cells for Foxp3GFP, CD62L, and CD69 expression. (E) Percentages of CD4+Thy1.2+Foxp3− cells with up-regulated CD25 expression recovered from indicated organs. Dots and horizontal lines in C and E indicate individual mice and mean values, respectively. (F) CD25+Foxp3− and CD25−Foxp3− subsets among Thy1.2+DO11.10+ cells from scLNs of Foxp3GFP mice were FACS purified from recipients at day 5 after transfer (top). CD25 and Foxp3GFP expression was determined at day 3 of IL-2–containing cultures (bottom). (G) Percentages (left) and numbers (right) of Foxp3GFP-expressing cells at day 3 of cultures of initially Foxp3−DO11.10+ cells, which were isolated from spleen or mLNs irrespective of their CD25 expression (Foxp3− total), or isolated from scLNs based on CD25−Foxp3− or CD25+Foxp3− expression. Numbers in histograms and dot plots indicate the percentage of gated cells in respective quadrants or gates. Dots and horizontal lines indicate replicate wells and mean values, respectively. Data are representative of at least three independent experiments with pooled cells of three to six mice.
DC-primed congenic marker+ CD4+ T cells were maintained in recipient animals for extended periods of time, with the exception of OT2 cells which were efficiently deleted by day 14 after DC targeting (Fig. 1 B). However, OT2 T cells were maintained and readily detectable in isotype control-treated or untreated recipient mice (Fig. S1, A and B; and not depicted). Thus, DC targeting consistently resulted in extrathymic induction of Foxp3GFP expression in CD4+ T cells with transgenic TCRs to foreign as well as self-Ags, although T cell survival and Foxp3 induction efficiency may differ between individual TCRs.
Tracking TCR transgenic CD4+ T cells in peripheral lymphoid organs indicated that 5 d after adoptive transfer and DC targeting, congenic marker+ cells preferentially accumulated in subcutaneous LNs (scLNs; Fig. 1 C), with the majority of initially naive T cells exhibiting an activated phenotype, as judged by low CD62L and high CD69 expression (Fig. 1 D). In addition to high frequencies of cells with induced Foxp3GFP expression at this early time point during T reg cell conversion, congenic marker+ cells that lacked Foxp3GFP but exhibited up-regulated CD25 expression were most prominent in scLNs (Fig. 1 E). We next asked whether these Foxp3− cells that had been activated in vivo through DC-targeted Ag may have acquired the capacity to up-regulate Foxp3 expression. To this end, CD4+Thy1.2+Foxp3GFP− cells were FACS purified and cultured in the presence of IL-2, without deliberate TCR or TGF-β receptor (TGF-βR) stimulation. Flow cytometry at different time points after initiation of cultures (Fig. 1 F and not depicted) revealed that such CD4+Foxp3− T cells rapidly up-regulated Foxp3GFP expression in vitro, with CD25+ cells (72.0 ± 5.7%) being more efficient than CD25− cells (36.5 ± 5.0%; Fig. 1, F and G). We conclude that in this in vivo model system of T reg cell conversion, induced Foxp3 expression via DC-targeted Ag is preceded by early modulation of CD69, CD62L, and CD25 expression in immediate Foxp3− precursor cells.
Extrathymic differentiation of polyclonal Foxp3− precursors to Foxp3+ T reg cells
Having shown that during DC-mediated T reg cell conversion, TCR transgenic CD4+Foxp3− T cells undergo a series of differentiation steps that are characterized by differential expression of distinct surface markers, we sought to determine whether equivalent CD4+Foxp3− precursor populations to Foxp3+ T reg cells exist in peripheral lymphoid organs of nonmanipulated mice. FACS analysis of Foxp3GFP mice showed that substantial numbers of CD4+CD25+Foxp3− cells (Fig. 2 A) with differential CD69 and CD62L expression (Fig. 2 B) could be detected in peripheral lymphoid organs but were near the level of detection among peripheral blood mononuclear cells. Next, CD4+CD25+Foxp3− cells from spleen, mesenteric LNs (mLNs), and scLNs were FACS purified and tested for their capacity to differentiate into Foxp3+ cells in vitro. These experiments established conditions that resulted in sizeable numbers of initially CD4+CD25+Foxp3− cells with up-regulated Foxp3 protein expression in IL-2–supplemented cultures (Fig. 2, C–F), with differentiation of precursors to Foxp3+ cells reaching a plateau at 72 h (Fig. 2 D). FACS purification of CD4+CD25+Foxp3− cells from day-3 cultures revealed no up-regulation of Foxp3GFP expression in secondary cultures (unpublished data). In summary, secondary lymphoid tissues of nonmanipulated mice contain CD4+CD25+Foxp3− T reg precursor cells, which are reminiscent of a thymic CD4+CD8−CD25+Foxp3− T cell population (Fig. 2, A–F) which has recently been described by Lio and Hsieh (2008) to contain precursors to Foxp3+ T reg cells.
Figure 2.
Tracking extrathymic differentiation of polyclonal CD4+CD25+Foxp3− T cell precursors to Foxp3+ cells in nonmanipulated mice. (A) Flow cytometry of CD25 and Foxp3GFP expression among gated CD4+ cells of indicated origin before (top) and after (bottom) magnetic bead enrichment of CD25+ cells. Numbers in dot plots indicate the percentage of gated cells within the respective gate. (B) Flow cytometry of CD69 and CD62L expression among gated CD4+CD25+Foxp3− cells from anatomical locations as indicated in A. (C) CD4+CD25+Foxp3− cells from secondary lymphoid organs and CD4+CD8−TCRβ+CD25+Foxp3− cells from thymus were purified by flow cytometry and CD25 and Foxp3GFP expression was assessed at day 3 of IL-2–containing cultures (n.d., not detectable). (D–F) Analysis of CD4+CD25+Foxp3− cells from indicated lymphoid organs of Foxp3GFP mice for their capacity to up-regulate Foxp3GFP expression in the presence of 1,000 U/ml IL-2 at indicated time points of culture (D), in the presence of indicated amounts of IL-2 at day 3 of culture (E), and in the presence of 1,000 U/ml IL-2 and titrating amounts of LY294002 (0, 2.5, 10, or 40 µM) at day 3 of culture (F). Data are representative of at least two independent experiments. Error bars indicate SD of triplicate wells.
With some experiments yielding 40–50% of Foxp3+ cells at day 3 of culture, CD4+CD25+Foxp3− cells from scLNs were consistently superior with regard to up-regulation of Foxp3 expression compared with cells originating from other secondary lymphoid organs (Fig. 2 C and see Fig. 7 C). This also holds true for numbers of Foxp3+ cells recovered from such cultures (Fig. S2 A). Subfractionation of peripheral CD4+Foxp3− cells based on CD25 expression levels established that CD25low cells yield substantially fewer Foxp3+ cells than CD25high cells, whereas CD25−Foxp3− cells did not give rise to Foxp3+ cells (Fig. S2 A). Notably, with regard to numbers of cells with up-regulated Foxp3GFP expression, peripheral CD4+CD25+Foxp3− precursors were consistently superior to CD4+CD8−CD25+Foxp3− thymic precursors, as total cell numbers recovered at day 3 of cultures were four- to fivefold higher from cultures with peripheral than with thymic precursor populations (Fig. S2 A). Foxp3 induction in precursor populations from thymus and peripheral lymphoid organs was strictly dependent on the presence of exogenous IL-2 (Fig. 2 E and Fig. S2 A). The common gamma chain cytokines IL-4, IL-7, and IL-15 substantially improved survival of cultured cells (not depicted) but did not appreciably induce up-regulation of Foxp3GFP protein expression in precursor populations from peripheral lymphoid organs (Fig. S2 C). Consistent with previous results (Lio and Hsieh, 2008), high-dose IL-15 had some beneficial effect on thymic CD4+CD8−CD25+Foxp3− precursor differentiation (Fig. S2 C). Furthermore, addition of LY294002, a potent inhibitor of phosphatidylinositol-3-kinase activity, abrogated peripheral CD4+CD25+Foxp3− precursor differentiation to Foxp3+ cells in a dose-dependent manner (Fig. 2 F and Fig. S2 D). In addition, TCR stimulation (either via plate-bound anti-CD3 antibodies in the absence or presence of costimulatory anti-CD28 antibodies or anti-CD3/CD28–coated beads) efficiently abrogated in vitro induction of Foxp3 expression in peripheral CD4+CD25+Foxp3− precursors (Fig. S2 B and not depicted). Finally, the mTOR inhibitor rapamycin did not alter CD4+CD25+Foxp3− precursor differentiation in vitro (Fig. S2 E), even at high concentrations which in control experiments substantially diminished proliferation of conventional CD4+ T cells in response to TCR stimulation (Fig. S3).
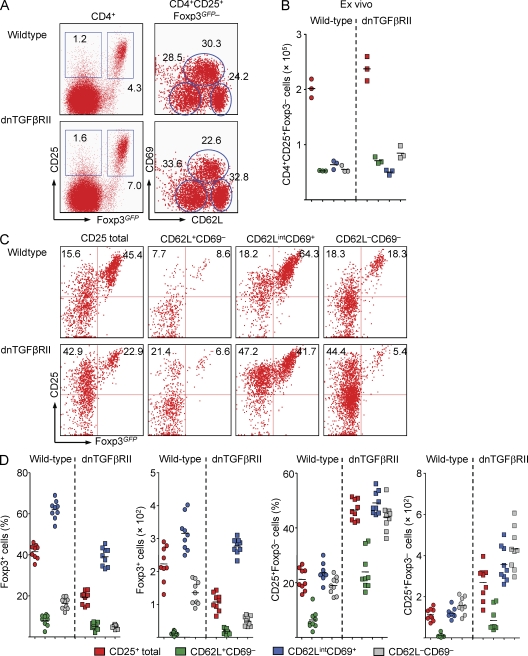
Figure 7.
Characterization of extrathymic T reg cell differentiation in mice with abrogated TGF-βR signaling. (A) Representative flow cytometry of CD69 and CD62L expression among gated CD4+CD25+Foxp3− cells from scLNs of 2-wk-old dnTGF-βRII and WT age-matched littermate control Foxp3GFP mice. Numbers in dot plots indicate the percentage of gated cells in the respective quadrant or gate. (B) Absolute numbers of CD4+CD25+Foxp3− cells and indicated subsets among gated CD4+CD25+Foxp3− cells presented in A. Each dot represents an individual mouse. (C) Differentiation of CD25+Foxp3+ cells from FACS-purified total CD4+CD25+Foxp3− populations or indicated subsets among CD4+CD25+Foxp3− cells from scLNs of WT control (top) and dnTGF-βRII (bottom) Foxp3GFP mice at day 3 of IL-2–containing cultures. (D) Percentages and absolute numbers of CD25+Foxp3+ and CD25+Foxp3− cells originating from WT and dnTGF-βRII mice presented in C. Data are representative of two independent experiments with three mice per group. Each dot represents an individual triplicate well per condition, and horizontal bars indicate mean values.
In vivo differentiation, stability, and suppressor function
Analysis of LN-derived CD4+CD25+Foxp3− cells at different time points after adoptive transfer into congenic fully immunocompetent recipients revealed rapid up-regulation of Foxp3 protein expression in ∼20% of initially Foxp3− cells (Fig. 3 A). Thus, Foxp3 up-regulation in CD4+CD25+Foxp3− cells can be achieved in vivo to an extent that is comparable to what we observed in IL-2–containing cultures. In addition, adoptive transfer of CD4+CD25+Foxp3− populations into Rag2−/− mice, which promotes T cell homeostatic proliferation in a cytokine-deprived environment, did not lead to up-regulation of Foxp3 expression (not depicted), which is consistent with strict requirements for IL-2 receptor (IL-2R) signaling (Fig. 2 E and Fig. S2 A) and limited TCR stimulation in vitro (Fig. S2 B).
Figure 3.
Characterization of CD4+CD25+Foxp3− precursor and differentiated Foxp3+ T cells. (A) Differentiation of CD4+CD25+Foxp3− precursors to Foxp3+ T cells in vivo. CD4+CD25+Foxp3−Thy1.2+ cells were FACS purified from LNs of Foxp3GFP mice. Presort analysis among gated CD4+ cells after magnetic bead enrichment of CD25+ cells (top left) and postsort analysis (bottom left) are depicted. FACS-purified cells (4 × 104) were injected i.v. into immunocompetent congenic recipients. Thy1.2+ cells were analyzed for CD25 and Foxp3GFP expression at days 3 and 6. Numbers in dot plots indicate percentages of gated cells in the respective quadrant or gate. (B and C) Stability of in vitro–differentiated CD4+CD25+Foxp3+ T cells in vivo. CD4+CD25+Foxp3−Thy1.2+ cells were cultured in the presence of IL-2. At day 3, total cells (2 × 105) were coinjected i.v. with naive CD62L+CD25−Foxp3−Thy1.1+CD4+ T cells (8 × 106) into Rag2−/− mice (B) or total cells (4 × 104) were injected alone into immunocompetent congenic recipients (C). Foxp3GFP expression of congenic marker+CD4+ cells in LNs (B and C) and spleen (C) of recipients was tracked at indicated time points. (D) Suppressor function in vitro. CFSE-labeled CD4+CD25− T responder (Tresp) cells were cultured with irradiated splenocytes and anti-CD3ε antibodies, either alone or co-cultured with CD25+Foxp3+ T cells originating from CD4+CD25+Foxp3− precursor cells (Foxp3−→Foxp3+) or freshly isolated CD4+CD25+Foxp3+ T reg cells (Foxp3+). Median fluorescence intensity (MFI) of CD25 staining (left) and CFSE dilution (right) of T responder cells was determined at day 3. Division cycles of T responder cells are indicated by arrowheads. Colors of bars mirror those of histograms. (E) Suppressor function in vivo. Naive CD62L+CD25−Foxp3−Thy1.1+CD4+ T cells (5 × 106) were injected into Rag2−/− mice alone or together with 105 total cells from IL-2–containing differentiation cultures of Foxp3− precursors as shown in B (bottom left). Data are representative of at least two independent experiments including three to four mice. Dots represent individual mice, and horizontal bars indicate mean values.
Next, we asked whether differentiation of CD4+CD25+Foxp3− cells gives rise to stable and functional CD25+Foxp3+ T reg cells. To this end, CD4+CD25+Foxp3− populations were cultured in the presence of IL-2 (Fig. 3 B, left), and day-3 cultures were injected into Rag2−/− mice, together with congenic marker–mismatched conventional CD4+ T cells as feeder cells to provide cytokines such as IL-2. Kinetic studies demonstrated that a sizeable population of CD25+Foxp3+ T cells, which originated from in vitro–differentiated CD4+CD25+Foxp3− precursors, was maintained in recipients for at least 19 d after transfer (Fig. 3 B). Adoptive transfers using day-3 cultures of peripheral CD4+CD25+Foxp3− precursors and fully immunocompetent congenic recipients further corroborated the in vivo stability of Foxp3+ cells (Fig. 3 C).
The capacity of de novo–generated CD4+CD25+Foxp3+ T cells to suppress conventional CD4+ T cells in standard co-culture assays was indistinguishable from that of freshly isolated LN-derived CD25+Foxp3+ T reg cells, as judged by abrogation of both proliferation and expression of the early activation marker CD25 on T responder cells (Fig. 3 D). Next, we wanted to assess the in vivo immunoregulatory capacity of Foxp3+ T reg cells originating from CD4+CD25+Foxp3− precursors. We therefore analyzed homeostatic expansion and accumulation of conventional CD4+ T cells that had been adoptively transferred into Rag2−/− mice either alone or together with congenic marker–mismatched Foxp3+ T reg cells from peripheral precursors at day 3 of IL-2–containing cultures. The results show that such Foxp3+ T reg cells efficiently suppressed accumulation of conventional CD4+ T cells in lymphopenic hosts (Fig. 3 E).
The CD4+CD25+CD62LintCD69+ subset is enriched for Foxp3− T reg precursor cells
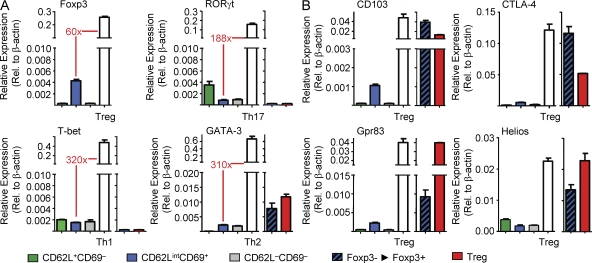
Compared with spleen or mLNs, IL-2–mediated in vitro differentiation of equivalent input numbers of scLN-derived CD4+CD25+Foxp3− cells consistently yielded substantially more CD25+Foxp3+ cells (Fig. 2 C, Fig. S2 A, and Fig. 4 A), suggesting that differences in frequencies of precommitted extrathymic T reg precursor cells in the respective microenvironments may exist. In fact, CD4+CD25+Foxp3− cells can be subdivided into distinct CD62L+CD69−, CD62LintCD69+, and CD62L−CD69− subpopulations that are differently distributed between peripheral lymphoid organs (Fig. 2 B), with CD62L−CD69− and CD62LintCD69+ cells being most prominent in spleen and LNs, respectively. When we FACS purified individual CD4+CD25+Foxp3− subpopulations from the different peripheral lymphoid organs of Foxp3GFP mice (Fig. 4 B, top) and analyzed their capacity to undergo IL-2–mediated differentiation into Foxp3+ cells in vitro, it became clear that precommitted extrathymic T reg precursor cells were enriched in the CD62LintCD69+ subpopulation (Fig. 4, B and C). This became most obvious when the in vitro conversion efficiency of the total CD4+CD25+Foxp3− population from spleen (Fig. 4 A, 3.6%) was compared with the respective CD62LintCD69+ subpopulation (Fig. 4 B, 26.2%). Overall, the CD62L+CD69− and CD62L−CD69– subpopulations exhibited modest to no capacity to up-regulate Foxp3 expression (Fig. 4, B and C). Consistently, comparative gene expression analysis of CD4+CD25+Foxp3− subsets revealed low but significant Foxp3 mRNA expression in the CD62LintCD69+ subpopulation, whereas expression levels of RORγt, T-bet, GATA-3, and T reg cell signature genes were negligible (Fig. 5, A and B). Furthermore, CD4+CD25+ T cells with in vitro up-regulated Foxp3GFP protein expression exhibited mRNA expression of so-called T reg signature genes such as CD103, CTLA-4, Gpr83, and Helios (Fig. 5 B and not depicted).
Figure 4.
Differentiation capacities of CD4+CD25+Foxp3− precursor subsets. (A and B) Total CD4+CD25+Foxp3− populations (A) or CD62L−CD69−, CD62LintCD69+ and CD62L+CD69− subsets (B) among gated CD4+CD25+Foxp3− cells were FACS purified from indicated lymphoid organs of Foxp3GFP mice, cultured in the presence of IL-2, and analyzed by flow cytometry for up-regulated Foxp3GFP expression at day 3 of cultures. Numbers in dot plots and histograms indicate the percentage of gated cells in the respective quadrant or gate. (C) Percentages (left) and absolute numbers (right) of Foxp3GFP+ cells originating from total populations of initially CD4+CD25+Foxp3− cells or populations that were subfractionated based on differential CD62L and CD69 expression, as indicated. Error bars indicate SD of triplicate wells. (D) Pacific Blue–succinimidyl ester (Pacific Blue–SE)–labeled CD4+CD25+Foxp3− cells from scLNs or thymus of Foxp3GFP mice were cultured in the presence of IL-2 and were analyzed by flow cytometry at day 3 for up-regulated Foxp3GFP expression and cell division, as revealed by dilution of Pacific Blue–succinimidyl ester. Division cycles are indicated by arrowheads. Numbers in dot plots indicate the percentages of undivided and divided cells among Foxp3+ cells. (E) Flow cytometry of CD62L and CD69 expression among gated CD25+Foxp3− and CD25+Foxp3+ cells at day 3 of IL-2–containing cultures of CD4+CD25+Foxp3− precursor cells from thymus or LNs of Foxp3GFP mice. See Fig. 2 for comparison with CD4+CD25+Foxp3− precursor cells before in vitro differentiation. Data are representative of at least two independent experiments.
Figure 5.
mRNA expression in CD4+CD25+Foxp3− precursor subsets and differentiated CD25+Foxp3+ T reg cells. Expression of mRNA encoding indicated T cell lineage transcription factors (A) and selected T reg cell signature genes (B) was determined by real-time RT-PCR in freshly isolated CD4+CD25+Foxp3− precursor subsets presented in Fig. 4 B. Freshly isolated scLN-derived CD4+CD25+Foxp3+ cells and conventional CD4+ T cells that had been cultured under Th1-, Th2-, or Th17-polarizing conditions were included as positive controls as indicated. CD25+Foxp3+ T cells that were in vitro differentiated from CD4+CD25+Foxp3− precursor cells (Foxp3−→Foxp3+) and purified by flow cytometry at day 3 of culture based on Foxp3GFP expression are also included. β-Actin was used for normalization. Shown are mean and range of duplicate samples. Data are representative of at least two independent experiments.
Importantly, >50% of Foxp3+ cells originating from thymic and peripheral CD62LintCD69+ precursor cells remained undivided in vitro (Fig. 4 D), suggesting that differences among subpopulations in their ability to up-regulate Foxp3 expression were not a result of differences in their proliferative capacity. It is of note that thymic and peripheral CD4+CD25+CD62LintCD69+Foxp3− precursors up-regulated CD62L expression to high levels during induction of Foxp3 protein expression (Fig. 2 B and Fig. 4 E).
Contribution of the thymus to the pool of peripheral Foxp3− T reg precursor cells
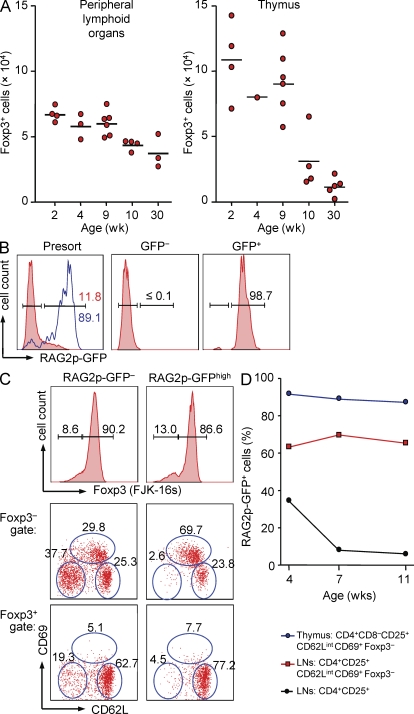
We reasoned that extrapolating numbers of in vitro–generated Foxp3+ cells to culture input and CD4+CD25+Foxp3− precursor cell numbers in vivo may provide an approximation of Foxp3+ T reg cell numbers produced in vivo. Thus, in an attempt to compare the capacity of thymus and peripheral lymphoid organs to produce Foxp3+ T reg cells, CD4+CD25+Foxp3− precursors were FACS purified from thymus, spleen, mLNs, and pools of different scLNs (for details see Materials and methods) of individual Foxp3GFP mice. Equivalent T cell numbers were seeded in the presence of IL-2 and output of Foxp3+ cell numbers was determined at day 3 of cultures. The results indicated that the thymus of young mice appeared more efficient in producing Foxp3+ cells compared with the restricted set of peripheral lymphoid tissues included in these experiments (Fig. 6 A). Overall, the efficiency of T reg cell generation from peripheral and thymic CD4+CD25+Foxp3− precursors continuously decreased with progressive thymic involution during aging of mice. To address the possibility that maintenance of the peripheral T reg precursor cell population throughout life may directly depend on the influx of newly produced T cells from the thymus, we next aimed at assessing the contribution of recent thymic emigrants (RTEs) to the peripheral pool of Foxp3− precursor cells. For this, we took advantage of mice carrying a GFP transgene driven by the Rag2 promoter (hereafter referred to as RAG2p-GFP mice; Yu et al., 1999). In these mice, high levels of GFP expression mark RTEs in peripheral lymphoid organs within 1 wk after the cells left the thymus (Boursalian et al., 2004). CD4+CD25+ T cells from LNs were isolated by flow cytometry to high purity based on absence or presence of RAG2p-GFP expression (Fig. 6 B) and subsequently subjected to intracellular staining using fluorochrome-conjugated mAbs to Foxp3. This two-step approach ensured faithful identification of RAG2p-GFPhigh RTEs among CD4+CD25+Foxp3− precursors because the RAG2p-GFP fluorescence was found to be severely abrogated by the fixation/permeabilization procedure (unpublished data). The results indicated equivalent frequencies of CD62L+CD69−, CD62LintCD69+, and CD62L−CD69− cells among the CD4+CD25+Foxp3−RAG2p-GFP− population (Fig. 6 C, left). In contrast, ∼70% of CD4+CD25+Foxp3−RAG2p-GFPhigh cells were comprised of the CD62LintCD69+ subset of peripheral Foxp3− precursor cells (Fig. 6 C, right). Notably, this enrichment of peripheral T reg precursor cells among RTEs was maintained during aging of mice, whereas the contribution of the total population of peripheral CD4+CD25+Foxp3− T cells to the RTE pool decreased in the same mice, as expected (Fig. 6 D). These data indicate that de novo production of CD4+ T cells from the thymus substantially contributes to the maintenance of the peripheral T reg precursor cell population.
Figure 6.
Contribution of the thymus to the peripheral pool of CD4+CD25+Foxp3− T reg precursor cells. (A) Approximation of Foxp3+ T reg cell numbers produced in peripheral lymphoid organs and thymus. For this, total numbers of CD4+CD25+Foxp3− cells in peripheral lymphoid organs (spleen, mLNs, and a set of scLNs; see Materials and methods) or thymus of individual Foxp3GFP mice were determined. Equivalent numbers of FACS-purified peripheral and thymic precursor populations were cultured in the presence of IL-2, and absolute numbers of Foxp3+ cells were determined at day 3 of culture. Estimated contribution of precursor populations to the peripheral T reg cell pool was calculated as follows: [total numbers CD4+CD25+Foxp3− cells ex vivo] × [absolute numbers Foxp3+ cells in vitro] ÷ [absolute numbers CD25+Foxp3− cells culture input]. Each dot represents an individual mouse of indicated age, and horizontal bars indicate mean values. (B–D) Contribution of RTEs to the peripheral pool of Foxp3− precursor cells. (B) Left histogram is gated on CD4+CD25+ T cells from LNs of 7-wk-old RAG2p-GFP mice (red filled histograms). Blue histogram depicts CD4+CD8− thymocytes. Right histograms show postsort isolation of GFP− and GFP+ subsets. Numbers in histograms show the percentages of GFPhigh cells within the indicated gate. (C) Foxp3 expression, as revealed by intracellular staining using the mAb FJK-16s, in FACS-purified peripheral CD4+CD25+ T cells that were RAG2p-GFP− or RAG2p-GFPhigh, and CD62L and CD69 expression among gated CD4+CD25+Foxp3− (middle) and CD4+CD25+Foxp3+ T cells (bottom). (D) Percentages of RAG2p-GFPhigh cells among indicated thymic and peripheral populations from RAG2p-GFP mice at indicated ages. Data are representative of at least two independent experiments.
Function of TGF-β in extrathymic T reg cell conversion in the steady state
Numerous studies have shown that in vitro activation in the presence of exogenous TGF-β induces Foxp3 expression in peripheral CD4+CD25− T cells (Fantini et al., 2007). However, intrathymic T reg cell generation can proceed by TGF-β–independent mechanisms in the absence of TGF-β (Marie et al., 2005), in the presence of a dominant-negative TGF (dnTGF)–βRII (Gorelik and Flavell, 2000) or T cell–specific deletion of TGF-βRII (Li et al., 2006; Marie et al., 2006). To address the role of TGF-β in extrathymic T reg cell de novo generation under steady-state conditions, we characterized CD4+CD25+Foxp3− precursor populations in Foxp3GFP mice expressing a dnTGF-βRII transgene in T cells (Li et al., 2006). Comparative analysis of TGF-βR signaling-proficient and -deficient animals revealed no gross differences with regard to proportions and numbers of the CD62LintCD69+ subset among CD4+CD25+Foxp3− cells (Fig. 7, A and B). Compared with TGF-βR signaling-proficient T reg precursor cells, IL-2–dependent in vitro differentiation of FACS-purified peripheral CD4+CD25+Foxp3− cells indicated an approximately twofold reduction in the percentage of CD25+Foxp3+ cells originating from the CD62LintCD69+ subset of dnTGF-βRII+ mice, whereas the CD25+Foxp3− population increased in these cultures (Fig. 7, C and D). It is of note that the numbers of Foxp3+ cells derived from CD62LintCD69+ cells that are either TGF-βR signaling proficient or deficient were comparable (Fig. 7 D, bottom). Similar results were obtained in experiments concerned with IL-2–dependent in vitro differentiation of thymic CD4+CD8−CD25+Foxp3− T reg precursor cells (Fig. S4 A). These data suggest that TGF-βR signaling plays a minor role in both generation and differentiation progression of extrathymic CD4+CD25+Foxp3− T reg precursor cells, whereas population expansion and/or survival of CD4+CD25+Foxp3− cells is enhanced. This interpretation is corroborated by the observation that SB431542, a potent TGF-βR signaling antagonist which efficiently abrogated TGF-βR–mediated up-regulation of Foxp3 expression in TCR-stimulated conventional CD4+ T cells in vitro (Fig. S4 B), has little if any impact on induction of Foxp3 expression in TGF-βR signaling-proficient extrathymic CD4+CD25+Foxp3− T reg precursor cells in vitro (Fig. 8, A and B). Again, SB431542-mediated TGF-βR signaling abrogation resulted in an approximately twofold increase in CD4+CD25+Foxp3− cell numbers (Fig. 8 C). Similar results were obtained with thymic CD4+CD8−CD25+Foxp3− T reg precursor cells (Fig. S4 C). Furthermore, increasing amounts of exogenous TGF-β to enhance TGF-βR signaling did not help IL-2–mediated up-regulation of Foxp3 expression in thymic or peripheral CD4+CD25+Foxp3−CD62LintCD69+ precursor cultures (Fig. 8 D). In addition, analysis of polyclonal populations of initially naive CD4+CD62L+CD25−Foxp3− T cells from dnTGF-βRII+ or WT Foxp3GFP mice that had been injected as individual populations into congenic recipients revealed similar efficiencies of up-regulated Foxp3 expression at day 7 after adoptive transfer (Fig. S4 D). Lastly, progression of either CD25−Foxp3− or CD25+Foxp3− TCR transgenic CD4+ T cells, which had been primed in vivo through DC-targeted Ag, to Foxp3+ cells in vitro remained largely unaffected by SB431542-mediated TGF-βR signaling abrogation (Fig. 8, E and F). However, abrogation of TGF-βR signaling during CD4+Foxp3− Ag-specific precursor differentiation to Foxp3+ cells in vitro resulted in a dose-dependent reduction of Foxp3 protein expression levels (Fig. 8 G).
Figure 8.
Impact of TGF-βR signaling deprivation or enhancement on extrathymic T reg precursor cell differentiation in vitro. (A–C) CD4+CD25+ CD62LintCD69+Foxp3− cells were FACS purified from pools of spleen, mLNs, and scLNs of Foxp3GFP mice and cultured with IL-2 in the absence or presence of titrating amounts (2.5, 10, and 40 µM) of the Smad kinase inhibitor SB431542. (A) Representative flow cytometry of CD25 and Foxp3GFP expression at day 3 of cultures. Numbers in dot plots indicate the percentage of gated cells in the respective quadrant. (B and C) Percentages (top) and absolute numbers (bottom) of CD25+Foxp3+ (B) and CD25+Foxp3− (C) cells recovered from these cultures. Dots and horizontal lines indicate individual replicate wells and mean values, respectively. Efficient abrogation of TGF-β–mediated Foxp3 induction in TCR-stimulated conventional naive CD4+ T cells by SB431542 is shown in Fig. S4 B. (D) FACS-purified CD4+CD25+Foxp3− cells from scLNs and CD4+CD8−TCR-β+CD25+Foxp3− cells from thymus of Foxp3GFP mice were cultured with IL-2 in the presence of titrating amounts of human TGF-β1, as indicated. At day 3 of cultures, the impact of TGF-β was calculated as fold difference of numbers of Foxp3+ cells as follows: [absolute numbers Foxp3+ cells from cultures with added TGF-β] ÷ [absolute numbers Foxp3+ cells from cultures without added TGF-β]. (E–G) CD25−Foxp3− and CD25+Foxp3− cells among Thy1.2+DO11.10+ T cells were FACS purified at day 5 after adoptive transfer into congenic recipients and subsequent anti–DEC-205-ovalbumin injection. IL-2–containing cultures with indicated amounts of SB431542 were analyzed at day 3 for CD25 and Foxp3GFP expression. (E) Representative flow cytometric analysis. (F) Percentages (top) and absolute numbers (bottom) of CD25+Foxp3− (left) and CD25+Foxp3+ (right) cells recovered from these cultures. (G) Median fluorescence intensities (MFI) of Foxp3GFP expression. Shown are the mean and SD of triplicate wells. Data are representative of at least two independent experiments.
Multiple lines of evidence suggest reciprocal developmental pathways for Th17 and T reg cell generation. Although TGF-β alone promotes both RORγt and Foxp3 expression, IL-6 in concert with inflammatory cytokines is thought to have a pivotal function in promoting Th17 but inhibiting T reg cell generation (Bettelli et al., 2006; Ichiyama et al., 2008; Korn et al., 2008; Manel et al., 2008; Zhang et al., 2008; Zhou et al., 2008). Therefore, we asked whether abrogation of IL-6 levels enhances anti–DEC-205–mediated extrathymic T reg cell generation in vivo. The results indicated that DC-targeted T reg cell conversion of adoptively transferred TCR transgenic CD4+ T cells is not enhanced in IL-6 gene-targeted recipients or upon treatment of IL-6–proficient recipients with neutralizing anti–IL-6 mAbs (unpublished data). To address the possible involvement of IL-6 in extrathymic T reg cell conversion in nonmanipulated mice, we next analyzed CD4+CD25+Foxp3− precursor populations in polyclonal Foxp3GFP mice that were either IL-6 proficient or deficient. This comparative analysis of IL-6−/− and IL-6+/− mice revealed no significant differences with regard to precursor numbers at different anatomical locations (Fig. S5 A and not depicted) or their capacity to up-regulate Foxp3GFP expression in IL-2–supplemented cultures (Fig. S5 B). Consistently, CD4+CD25+Foxp3− T reg precursor cells efficiently induced Foxp3 expression in cultures supplemented with high dose IL-6 (Fig. S5 C). We conclude that accumulation of CD4+CD25+Foxp3− T reg precursor cells and their differentiation into Foxp3+ T reg cells in the steady state is independent of IL-6.
DISCUSSION
TCR transgenic model systems have been invaluable for the elucidation of basic mechanisms of agonist ligand-mediated extrathymic T reg cell generation (Apostolou et al., 2002; Apostolou and von Boehmer, 2004; Kretschmer et al., 2005). Tetramer visualization of Ag-specific CD4+ T cells revealed that agonist ligand administration to WT mice can result in T reg cell induction from a polyclonal T cell repertoire (Verginis et al., 2008). However, in addition to the question of whether extrathymic T reg cell generation occurs in the steady state, a better understanding of the exact mechanisms of non-TCR transgenic T cell conversion has been hampered by the lack of suitable markers to identify extrathymic T reg precursor cell populations and their products in peripheral lymphoid organs. Consequently, relative contributions of thymic and extrathymic T reg cell generation to the overall peripheral T reg cell pool under physiological conditions remained unknown.
In this study, we have analyzed activation marker expression and differentiation capacity of Ag-primed CD4+Foxp3– T cells during extrathymic Foxp3 induction and have then correlated our findings with polyclonal T cells from nonmanipulated Foxp3GFP mice. These experiments provided evidence for the existence of a sizable population of precommitted CD4+Foxp3− precursors to Foxp3+ T reg cells in the periphery of steady-state mice. We demonstrated that these precursors up-regulated Foxp3 expression and acquired a T reg cell phenotype in vivo in adoptive transfer settings, as well as in cultures without deliberate TCR and TGF-βR stimulation. Foxp3 up-regulation was strictly dependent on IL-2R and phosphatidylinositol-3-kinase signaling pathways. Foxp3+ T reg cells that originated from peripheral Foxp3– precursors exhibited potent in vitro and in vivo suppressive capacity and were maintained for extended periods of time in both lymphopenic Rag−/− mice and normal lympho-replete recipient mice.
We have further shown that the CD62LintCD69+ subset among CD4+CD25+Foxp3− T cells in spleen and LNs was highly enriched for precursor cells to Foxp3+ T reg cells. Our comparative analysis of intermediate Foxp3− precursor populations from polyclonal nonmanipulated mice and TCR transgenic T cells during DC-targeted T reg cell conversion revealed striking similarities with regard to activation marker phenotype and requirements to up-regulate Foxp3 expression. In addition, the CD62LintCD69+ subset among CD4+CD25+Foxp3− T cells appeared to be underrepresented in blood mononuclear cells compared with peripheral lymphoid tissues. This observation, together with the notion that splenic CD8+DEC-205+ DCs induce Foxp3 expression in vitro in initially CD4+Foxp3– T cells without added TGF-β (Yamazaki et al., 2008), suggests that steady-state generation of CD4+CD25+Foxp3− T reg precursor cells is likely to be mediated by lymphoid tissue–residing CD8+DEC-205+ DCs. At present, we can only speculate on the nature of involved antigens, but because the peripheral CD4+CD25+Foxp3− T reg precursor cell compartment appeared fully established in mice as early as 2 wk after birth (Fig. 6 A), it is reasonable to hypothesize that self-antigens play a prominent role in this process. In addition, our experiments on DC-targeted T reg cell conversion of TCR transgenic CD4+ T cells indicated that both foreign and self-antigens can efficiently induce Foxp3 expression.
Reminiscent of hen egg lysozyme-specific 3A9 CD4+ T cells (Hawiger et al., 2001), anti–DEC-205–mediated DC targeting resulted in nearly complete deletion of adoptively transferred TCR transgenic OT2 cells. This was in striking contrast to what we observed for DO11.10 cells recognizing the same ovalbumin peptide. In an attempt to address this issue, we have performed initial experiments on the role of transgenic TCR expression in peripheral CD4+ T cell maturation using aforementioned RAG2p-GFP mice to track RTEs (Boursalian et al., 2004). The results showed that the vast majority of OT2 cells (> 80%) in peripheral lymphoid organs of adult mice exhibited an immature phenotype, as indicated by high GFP expression levels. In contrast, the peripheral CD4+ T cell pool in TCR-HA107-119 transgenic and non-TCR transgenic mice comprised 20–30% RTEs (unpublished observation). Thus, it appears that transgenic TCR expression in OT2 cells resulted in aberrant T cell maturation, which might be the underlying cause of enhanced DC targeting-induced apoptosis.
Our results indicated that thymic and extrathymic T reg cell generation pathways may be more similar than previously recognized. In fact, the CD4+CD25+Foxp3− surface marker phenotype of peripheral T reg precursor cell populations presented in this study is equivalent to that of CD4+CD8−CD25+Foxp3− thymocytes, which have recently been suggested to represent T reg precursor cells (Lio and Hsieh, 2008). It is important to note that previous studies have indicated that Foxp3+ T reg cell lineage commitment can also occur at the double-positive (DP) stage of thymic development and that Foxp3+CD4+CD8+ DP thymocytes represent precursor cells to Foxp3+ T reg cells (Fontenot et al., 2005a,b; Wan and Flavell, 2005; Liston et al., 2008). This view has recently been challenged by the notion that numbers of Foxp3+ cells in the DP stage may have been overestimated in previous studies as a result of a propensity of Foxp3−CD4+CD8+ DP cells to form doublets with Foxp3+CD4+ single-positive cells (Lee and Hsieh, 2009), which is consistent with our own unpublished observation. Lee and Hsieh (2009) further demonstrated that faithful flow cytometric isolation of Foxp3+CD4+CD8+ thymocytes from Foxp3GFP mice is hampered by exceedingly low numbers of DP thymocytes that are truly Foxp3+.
We thus restricted our attempts to quantify thymic and extrathymic T reg cell generation to the CD4+CD8−CD25+Foxp3− precursor populations. The results provided evidence that both thymic and peripheral T reg cell developmental pathways may substantially contribute to the overall peripheral T reg cell pool in young mice. However, it is important to note that the calculation is based on controlled in vitro conditions in IL-2–complemented cultures, whereas at this stage we can only speculate that the availability of cytokines promoting the induction of Foxp3 expression in vivo is comparable in thymus and peripheral lymphoid organs. Thus, additional experiments that may involve (surface) markers differentially expressed on the products of thymic and peripheral T reg precursor cells are required to more directly assess the relative contribution of thymic and peripheral T reg precursor cells to the overall T reg cell pool in the periphery.
Previous studies established that T cell numbers produced in the thymus reach a peak at ∼6 wk of age and continuously decrease thereafter (Boursalian et al., 2004). Consistently, our comparative analysis of young and adult mice suggested that production of CD25+Foxp3+ T reg cells from thymic precursors dropped early during age-associated thymic involution, whereas T reg cell differentiation from peripheral precursors from the same mice appeared to remain more stable. Thus, peripheral T reg cell generation may be particularly important in settings of reduced thymic T reg cell production. Interestingly, our preliminary experiments using BAC transgenic mice expressing a diphtheria toxin (DT) receptor–GFP fusion protein under the control of the foxp3 gene locus (Lahl et al., 2007) indicated that DT administration to reduce T reg cell numbers resulted in a rapid increase of peripheral CD4+CD25+Foxp3− precursors and peripheral CD25+Foxp3+ T reg cell generation (unpublished data). This raises the possibility that thymic and peripheral T reg cell generation are linked by yet-to-be-defined feedback circuits to control peripheral T reg cell homeostasis.
Previous studies concerned with modalities of efficient DC-targeted peripheral T reg cell conversion from TCR transgenic CD4+ T cells established an inverse relationship of cell division and induced Foxp3 expression (Thorstenson and Khoruts, 2001; Kretschmer et al., 2005). This link between proliferation and conversion was supported by the notion that conditions that limit proliferation (low Ag dose, immature DC maturation stage, reduced IL-2R, and enhanced TGF-βR signaling) of converting cells increased Foxp3 up-regulation and acquisition of a T reg cell phenotype. This interpretation was recently corroborated in studies by Josefowicz et al. (2009) using DNA methyltransferase 1–deficient T cells, which revealed that one function of TGF-β in T reg cell generation is to facilitate Foxp3 induction by opposing cell cycle–dependent Dnmt1-mediated silencing of the gene locus encoding Foxp3. Consistently, we have shown in this paper that IL-2R signaling-dependent up-regulation of Foxp3 in initially CD4+CD25+Foxp3− polyclonal T reg precursor cells did not require proliferation and was abrogated by augmented TCR signaling. With regard to a function of TGF-β in addition to cell cycle regulation, the present study suggested that TGF-βR signaling in vivo plays a minor role in both generation and differentiation progression of extrathymic CD4+CD25+Foxp3− T reg precursor cells. Complementary in vitro experiments showed that abrogated or enhanced TGF-βR signaling had limited if any impact on Foxp3+ cell numbers from CD4+CD25+Foxp3− T reg precursor cell differentiation cultures. Thus, reminiscent of intrathymic T reg cell generation (Lio and Hsieh, 2008), extrathymic T reg cell generation from polyclonal precursor populations can proceed by TGF-β–independent mechanisms. However, pharmacological abrogation of TGF-βR signaling resulted in somewhat reduced Foxp3 expression levels, which is consistent with the notion that TGF-β enhances Foxp3 expression (Marie et al., 2005), perhaps by promoting Smad3 and NFAT binding to a Foxp3 enhancer region to support Foxp3 promoter activity (Tone et al., 2008).
With regard to cytokines involved in the reciprocal developmental pathways of Th17 and T reg cell generation, our experiments provided evidence that abrogation of systemic IL-6 levels, either by neutralizing anti–IL-6 mAbs or using gene-targeted mice, did not lead to enhanced accumulation of peripheral Foxp3− T reg precursor cells or differentiated Foxp3+ T reg cells from both TCR transgenic or polyclonal CD4+ T cells. Although these data clearly indicate that IL-6 may have limited impact on peripheral T reg cell generation in the steady state, further studies are required to address a possible role of IL-6 in T reg cell conversion during inflammation.
In summary, our data have provided evidence for the existence of an extrathymic CD4+CD25+Foxp3− immediate precursor population to Foxp3+ T reg cells in peripheral lymphoid organs of nonmanipulated mice that is likely to play an important role in maintaining immune homeostasis under physiological conditions. A detailed molecular and functional analysis of thymic and peripheral precursor populations and their products should provide useful insights into intra- and extrathymic pathways of dominant tolerance.
MATERIALS AND METHODS
Mice.
Thy-1.2 BALB/c and CD45.2 C57BL/6 mice were purchased from Janvier or Taconic. Congenic Thy1.1 BALB/c and CD45.1 C57BL/6 mice, as well as BALB/c Rag2−/− and TCR-HA(107–119) mice, were kindly provided by H. von Boehmer (Dana-Farber Cancer Institute, Boston, MA). TCR-HA107-119, DO11.10 (recognizing the ovalbumin peptide 323–339; Ova323-339), and dnTGF-βRII (Gorelik and Flavell, 2000) mice were backcrossed to Foxp3IRES-GFP BALB/c mice (Haribhai et al., 2007). RAG2p-GFP mice (Yu et al., 1999) were backcrossed to BALB/c mice. IL-6-deficient and OT2 (recognizing Ova323-339) mice (The Jackson Laboratory) were backcrossed to Foxp3GFP C57BL/6 mice (Fontenot et al., 2005b). The mice described in this section were bred and maintained at the Experimental Center of the Medizinisch-Theoretisches Zentrum (Dresden University of Technology, Germany) or at the animal facility of Max Planck Institute of Molecular Cell Biology and Genetics (Dresden, Germany) under specific pathogen-free conditions. Animal experiments were performed as approved by the Regieriungspräsidium Dresden.
Flow cytometry and cell sorting.
Single cell suspensions of thymus, spleen, mLNs (Lnn. mesenterici) and scLNs (Lnn. mandibularis, Lnn. cervicales superficiales, Lnn. axillares et cubiti, Lnn. inguinales superficiales, and Lnn. subiliaci) were prepared using 70-µm cell strainers (BD). mAbs to CD4 (GK1.5 or RM4-5), CD25 (PC61), CD62L (MEL-14), CD69 (H1.2F3), TCR-β (H57-597), DO11.10 (KJ1-26), Vα2 (B20.1), Vβ5.1, 5.2 (MR9-4), CD8 (53–6.7), Thy 1.2 (53–2.1), and CD45.1 (A20), as well as Fc receptor–blocking mAb against CD16/32 (93) and Pacific blue– or PE-conjugated streptavidin, were purchased from eBioscience or BD. The mAb to TCR-HA107-119 (6.5) was purified and conjugated with Alexa Fluor 647 (Invitrogen) in our laboratory according to standard protocols. Where indicated, intracellular Foxp3 expression was analyzed using the mAb Foxp3 (FJK 16s) and the Foxp3 staining buffer set (both eBioscience) according to the manufacturer’s protocol. Before FACS, for some experiments CD4+ or CD25+ cells were enriched from single cell suspensions using biotinylated antibodies directed against CD4 or CD25, respectively, streptavidin-conjugated microbeads, and the AutoMACS magnetic separation system (Miltenyi Biotec). Samples were analyzed on an LSRII or FACSCalibur or sorted using a FACSAria (BD). Data were analyzed using FlowJo software (Tree Star, Inc.).
T cell proliferation measurement.
Where indicated, CD4+ T cells were labeled with 50 µM Pacific Blue succinimidyl ester or 1 or 10 µM CFSE (Invitrogen) for in vitro and in vivo proliferation measurements, respectively, by incubation for 10 min at 37°C in the dark at a density of 106 cells/ml in PBS containing 0.1% BSA.
Adoptive cell transfer.
Indicated FACS-purified, Thy1.2, or CD45.1 T cell populations were injected into lateral tail veins of Thy1.1 BALB/c or CD45.2 C57BL/6 congenic recipient mice, respectively. For some experiments, mixtures of congenic marker mismatched conventional CD4+ T cell and T reg precursor cell populations were adoptively transferred into BALB/c Rag2−/− recipient mice.
Anti–DEC-205–mediated DC targeting.
The plasmid vectors containing the cDNA of aa 107–119 of HA (HA107-119; Kretschmer et al., 2005) or of the whole ovalbumin protein (Shakhar et al., 2005) added to the C terminus of cloned anti-DEC-205 and III/10 control antibody have been described previously (Hawiger et al., 2001). Hybrid antibodies were produced using the FreeStyle MAX 293 Expression System (Invitrogen) according to the manufacturer’s recommendations. In brief, suspension cultures of FreeStyle 293-F Cells were maintained in serum-free FreeStyle 293 Expression Medium and transiently transfected with the respective IgH chain and Igκ chain plasmid vectors using FreeStyle MAX Reagent. All antibodies were purified on Prepacked HiTrapTM Protein G HP columns (GE Healthcare). Protein concentrations were determined spectrophotometrically by measuring the absorbtion at 280 nm. The amount and the presence of full-length recombinant fusion protein were verified by SDS-PAGE with an IgG1/IgLκ antibody used as reference. For in vivo DC targeting, 50 or 250 ng of recombinant anti–DEC-205 antibodies fused to either HA107–119 peptide or whole ovalbumin protein, respectively, were injected intraperitoneally at day 1 after adoptive T cell transfers.
T cell culture and in vitro suppression assays.
T cells were cultured in 96-well round-bottom plates (Greiner) and RPMI 1640 supplemented with 10% FCS, 1 mM sodium pyruvate, 1 mM HEPES, 2 mM Glutamax, 100 U/ml Penicillin-Streptomycin, 0.1 mg/ml Gentamycine, 0.1 mM nonessential aminoacids, and 0.55 mM β-mercaptoethanol (Invitrogen). Unless otherwise stated, 1,000 U/ml recombinant human IL-2 (Teceleukin; Roche) was added to Foxp3 induction cultures and Foxp3 expression was analyzed at day 3. Mouse IL-6 (PeproTech), mouse IL-4 (eBioscience), mouse IL-7 (R&D Systems), mouse IL-15 (PeproTech), and human TGF-βI (PeproTech) was added to the medium at final concentrations indicated. For T cell stimulation, 10 µg/ml of plate-bound anti-CD3ε (145-2C11; BD) and 1 µg/ml of soluble anti-CD28 (37.51; eBioscience), anti-CD3/CD28–coated Dynabeads (four beads/cell; Invitrogen), or 30 Gy irradiated T cell–depleted splenocytes and 1 µg/ml of soluble anti-CD3ε was used. Stock solutions of 10 mM LY294002, 250 µM Rapamycin, and 10 mM SB431542 (Sigma-Aldrich) were prepared in DMSO (Sigma-Aldrich). For standard in vitro suppression assays, FACS-purified CD4+CD25−CD62L+ T responder cells were CFSE-labeled and cultured alone or co-cultured at a 2:1 ratio with indicated Foxp3+ T cell populations, together with 30 Gy irradiated T cell–depleted splenocytes and soluble anti-CD3ε. Dilution of CFSE and CD25 expression on T responder cells was monitored at day 3 after initiation of cultures.
Gene expression analysis.
Total RNA was extracted from FACS-purified T cell populations using the miRNeasy kit and DNase I digestion, and cDNA was synthesized according to the manufacturer’s recommendations (miScript reverse transcription kit; QIAGEN). QuantiFast SYBR Green PCR kit (QIAGEN) and a Mastercycler ep realplex thermal cycler (Eppendorf) were used to analyze cDNA in duplicate reactions. The following primers were used: β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′; Foxp3, 5′-GGCCCTTCTCCAGGACAGA-3′ and 5′-GCTGATCATGGCTGGGTTGT-3′; T-bet, 5′-CAACAACCCCTTTGCCAAAG-3′ and 5′-TCCCCCAAGCAGTTGACAGT-3′; GATA-3, 5′-AGAACCGGCCCCTTATGAA-3′ and 5′-AGTTCGCGCAGGATGTCC-3′; RORγt, 5′-CACGGCCCTGGTTCTCAT-3′ and 5′-CAGATGTTCCACTCTCCTCTTCTCT-3′; CD103, 5′-GCCGTGATCCAGACTGAGTTTGAT-3′ and 5′-ATGGCTGAGGCGGTCTTAGTGACT-3′; Gpr83, 5′-ACCCTCCCCAGTTCCTTCCTTCAG-3′ and 5′-GGCCACAACGGGTTCCACAGAT-3′; Helios, 5′-GCCCCCAAGGGCTCTCT-3′ and 5′-GACTCGGCAGTGCTCACACTT-3′; CTLA-4, 5′-TGGATCCTTGTCGCAGTTAGCT-3′ and 5′-ACTTCTTTTCTTTAGCATCTTGCTCAA-3′.
Online supplemental material.
Fig. S1 shows the phenotype of adoptively transferred TCR transgenic CD4+ T cells in untreated recipients. Fig. S2 depicts the IL-2–dependent differentiation capacity of CD4+Foxp3− T reg precursor cells with low or high CD25 expression and the effects of TCR stimulation, common gamma chain cytokines, and mTOR inhibition on precursor differentiation. Fig. S3 shows the biological activity of rapamycin. Fig. S4 provides additional information on the impact of TGF-βR signaling on T reg precursor cell differentiation. Fig. S5 depicts the role of IL-6 in T reg cell differentiation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100045/DC1.
Acknowledgments
The authors are indebted to M. Nussenzweig (Laboratory of Molecular Immunology, The Rockefeller University, New York, NY) for providing the RAG2p-GFP mice and the plasmid vectors of the IgH and respective Ig-κ light chain cDNA of cloned anti–DEC-205 NLDC-145 and III/10 isotype control antibodies. We thank T. Sparwasser (Institute for Infection Immunology, Twincore, Hanover, Germany) for providing DEREG mice, G. Pearce for expert help in cell sorting, and T. Koenig and C. Friebel for excellent technical assistance. We are indebted to A. I. Garbe for critically reading the manuscript and helpful advice.
This work was supported by the Kompetenznetz Diabetes mellitus (Competence Network for Diabetes mellitus) funded by the Federal Ministry of Education and Research (FKZ 01GI0805-07), by the Dresden International Graduate School for Biomedicine and Bioengineering, funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation), and the FZT 111 (DFG, Center for Regenerative Therapies Dresden, Cluster of Excellence).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- Ag
- antigen
- dnTGF
- dominant-negative TGF
- DP
- double positive
- HA
- haemagglutinin
- mLN
- mesenteric LN
- Rag
- recombination-activating gene
- RTE
- recent thymic emigrant
- scLN
- subcutaneous LN
- T reg cell
- regulatory T cell
References
- Apostolou I., von Boehmer H. 2004. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199:1401–1408 10.1084/jem.20040249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou I., Sarukhan A., Klein L., von Boehmer H. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763 [DOI] [PubMed] [Google Scholar]
- Benson M.J., Pino-Lagos K., Rosemblatt M., Noelle R.J. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204:1765–1774 10.1084/jem.20070719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Boursalian T.E., Golob J., Soper D.M., Cooper C.J., Fink P.J. 2004. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5:418–425 10.1038/ni1049 [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille M.A., Lino A.C., Kutchukhidze N., Lafaille J.J. 2004. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 173:7259–7268 [DOI] [PubMed] [Google Scholar]
- Fantini M.C., Becker C., Monteleone G., Pallone F., Galle P.R., Neurath M.F. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153 [DOI] [PubMed] [Google Scholar]
- Fantini M.C., Dominitzki S., Rizzo A., Neurath M.F., Becker C. 2007. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat. Protoc. 2:1789–1794 10.1038/nprot.2007.258 [DOI] [PubMed] [Google Scholar]
- Floess S., Freyer J., Siewert C., Baron U., Olek S., Polansky J., Schlawe K., Chang H.D., Bopp T., Schmitt E., et al. 2007. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 5:e38 10.1371/journal.pbio.0050038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Dooley J.L., Farr A.G., Rudensky A.Y. 2005a. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202:901–906 10.1084/jem.20050784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005b. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Gorelik L., Flavell R.A. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181 10.1016/S1074-7613(00)80170-3 [DOI] [PubMed] [Google Scholar]
- Haribhai D., Lin W., Relland L.M., Truong N., Williams C.B., Chatila T.A. 2007. Regulatory T cells dynamically control the primary immune response to foreign antigen. J. Immunol. 178:2961–2972 [DOI] [PubMed] [Google Scholar]
- Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J.V., Steinman R.M., Nussenzweig M.C. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779 10.1084/jem.194.6.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.S., Zheng Y., Liang Y., Fontenot J.D., Rudensky A.Y. 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7:401–410 10.1038/ni1318 [DOI] [PubMed] [Google Scholar]
- Ichiyama K., Yoshida H., Wakabayashi Y., Chinen T., Saeki K., Nakaya M., Takaesu G., Hori S., Yoshimura A., Kobayashi T. 2008. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J. Biol. Chem. 283:17003–17008 10.1074/jbc.M801286200 [DOI] [PubMed] [Google Scholar]
- Josefowicz S.Z., Wilson C.B., Rudensky A.Y. 2009. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J. Immunol. 182:6648–6652 10.4049/jimmunol.0803320 [DOI] [PubMed] [Google Scholar]
- Knoechel B., Lohr J., Kahn E., Bluestone J.A., Abbas A.K. 2005. Sequential development of interleukin 2–dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 202:1375–1386 10.1084/jem.20050855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Mitsdoerffer M., Croxford A.L., Awasthi A., Dardalhon V.A., Galileos G., Vollmar P., Stritesky G.L., Kaplan M.H., Waisman A., et al. 2008. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 105:18460–18465 10.1073/pnas.0809850105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M.C., von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- Kretschmer K., Heng T.S., von Boehmer H. 2006. De novo production of antigen-specific suppressor cells in vivo. Nat. Protoc. 1:653–661 10.1038/nprot.2006.105 [DOI] [PubMed] [Google Scholar]
- Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., Sparwasser T. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63 10.1084/jem.20061852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop S.K., Santacruz N.A., Pham D., Luo J., Hsieh C.S. 2008. Antigen-specific peripheral shaping of the natural regulatory T cell population. J. Exp. Med. 205:3105–3117 10.1084/jem.20081359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.M., Hsieh C.S. 2009. Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J. Immunol. 183:2261–2266 10.4049/jimmunol.0901304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.O., Sanjabi S., Flavell R.A. 2006. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 25:455–471 10.1016/j.immuni.2006.07.011 [DOI] [PubMed] [Google Scholar]
- Lio C.W., Hsieh C.S. 2008. A two-step process for thymic regulatory T cell development. Immunity. 28:100–111 10.1016/j.immuni.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., Nutsch K.M., Farr A.G., Lund J.M., Rasmussen J.P., Koni P.A., Rudensky A.Y. 2008. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc. Natl. Acad. Sci. USA. 105:11903–11908 10.1073/pnas.0801506105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N., Unutmaz D., Littman D.R. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9:641–649 10.1038/ni.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J.C., Letterio J.J., Gavin M., Rudensky A.Y. 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201:1061–1067 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J.C., Liggitt D., Rudensky A.Y. 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 25:441–454 10.1016/j.immuni.2006.07.012 [DOI] [PubMed] [Google Scholar]
- Nolting J., Daniel C., Reuter S., Stuelten C., Li P., Sucov H., Kim B.-G., Letterio J.J., Kretschmer K., Kim H.-J., von Boehmer H. 2009. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J. Exp. Med. 206:2131–2139 10.1084/jem.20090639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk R., Ignatowicz H., Kraj P., Ignatowicz L. 2006. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 25:249–259 10.1016/j.immuni.2006.05.016 [DOI] [PubMed] [Google Scholar]
- Polansky J.K., Kretschmer K., Freyer J., Floess S., Garbe A., Baron U., Olek S., Hamann A., von Boehmer H., Huehn J. 2008. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 38:1654–1663 10.1002/eji.200838105 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Fukuma K., Kuribayashi K., Masuda T. 1985. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 161:72–87 10.1084/jem.161.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance. Cell. 133:775–787 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Shakhar G., Lindquist R.L., Skokos D., Dudziak D., Huang J.H., Nussenzweig M.C., Dustin M.L. 2005. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat. Immunol. 6:707–714 10.1038/ni1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstenson K.M., Khoruts A. 2001. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188–195 [DOI] [PubMed] [Google Scholar]
- Tone Y., Furuuchi K., Kojima Y., Tykocinski M.L., Greene M.I., Tone M. 2008. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9:194–202 10.1038/ni1549 [DOI] [PubMed] [Google Scholar]
- Verginis P., McLaughlin K.A., Wucherpfennig K.W., von Boehmer H., Apostolou I. 2008. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc. Natl. Acad. Sci. USA. 105:3479–3484 10.1073/pnas.0800149105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. 2007. Oral tolerance: is it all retinoic acid? J. Exp. Med. 204:1737–1739 10.1084/jem.20071251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.Y., Flavell R.A. 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 102:5126–5131 10.1073/pnas.0501701102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Mathis D., Benoist C. 2007. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J. Exp. Med. 204:2039–2045 10.1084/jem.20070822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Dudziak D., Heidkamp G.F., Fiorese C., Bonito A.J., Inaba K., Nussenzweig M.C., Steinman R.M. 2008. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181:6923–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Misulovin Z., Suh H., Hardy R.R., Jankovic M., Yannoutsos N., Nussenzweig M.C. 1999. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5′ of RAG2. Science. 285:1080–1084 10.1126/science.285.5430.1080 [DOI] [PubMed] [Google Scholar]
- Zhang F., Meng G., Strober W. 2008. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 9:1297–1306 10.1038/ni.1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Lopes J.E., Chong M.M., Ivanov I.I., Min R., Victora G.D., Shen Y., Du J., Rubtsov Y.P., Rudensky A.Y., et al. 2008. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 453:236–240 10.1038/nature06878 [DOI] [PMC free article] [PubMed] [Google Scholar]