Abstract
The sphingosine 1-phosphate receptor 1 (S1P1) promotes lymphocyte egress from lymphoid organs. Previous work showed that agonist-induced internalization of this G protein–coupled receptor correlates with inhibition of lymphocyte egress and results in lymphopenia. However, it is unclear if S1P1 internalization is necessary for this effect. We characterize a knockin mouse (S1p1rS5A/S5A) in which the C-terminal serine-rich S1P1 motif, which is important for S1P1 internalization but dispensable for S1P1 signaling, is mutated. T cells expressing the mutant S1P1 showed delayed S1P1 internalization and defective desensitization after agonist stimulation. Mutant mice exhibited significantly delayed lymphopenia after S1P1 agonist administration or disruption of the vascular S1P gradient. Adoptive transfer experiments demonstrated that mutant S1P1 expression in lymphocytes, rather than endothelial cells, facilitated this delay in lymphopenia. Thus, cell-surface residency of S1P1 on T cells is a primary determinant of lymphocyte egress kinetics in vivo.
Sphingosine 1-phosphate (S1P), a multifunctional lipid mediator that signals via five G protein–coupled receptors (GPCRs), regulates vascular maturation, permeability, and angiogenesis (Hla, 2004; Cyster, 2005). Recently, interest in the roles of S1P and its receptors in the immune system has been prompted in part by the identification of the immunomodulator FTY720 (Brinkmann et al., 2002; Mandala et al., 2002; Chiba, 2005), which upon phosphorylation by Sphk2 to FTY720-P (Sanchez et al., 2003; Zemann et al., 2006) acts as a strong agonist for four out of five S1P receptors (Brinkmann et al., 2004). FTY720 induces profound lymphopenia by inhibiting the egress of lymphocytes from the thymus, peripheral lymph nodes, and Peyer’s patches (Chiba, 2005). Indeed, it is now appreciated that S1P signaling modulates the trafficking of not only naive and central memory T cells, but also B cells, dendritic cells, NK cells, osteoclasts, and hematopoietic progenitor cells (Allende and Proia, 2002; Kabashima et al., 2006; Massberg et al., 2007; Schwab and Cyster, 2007; Walzer et al., 2007; Ledgerwood et al., 2008; Rivera et al., 2008; Sebzda et al., 2008; Ishii et al., 2009). These studies suggest that S1P regulates hematopoietic and immune cell trafficking under homeostatic and disease conditions; however, it is unclear precisely how S1P receptor signaling modulates cellular responses to egress cues.
The mechanism of how S1P regulates T cell trafficking has been intensively investigated; T cell–specific deletion of S1p1r or hematopoietic reconstitution using S1p1r−/− fetal liver cells resulted in profound lymphopenia, suggesting that the T cell–intrinsic S1P receptor 1 (S1P1) is essential for their egress from the thymus and secondary lymph nodes (Allende et al., 2004; Matloubian et al., 2004). This observation, coupled with the finding that FTY720-P induces the loss of cell-surface S1P1 from lymphocytes in an irreversible manner (Gräler and Goetzl, 2004; Matloubian et al., 2004), suggests that functional antagonism of S1P1 in the lymphocyte compartment is essential for the inhibition of T cell egress.
However, other studies have led to the proposal of an alternative mechanism by which S1P1 regulates lymphocyte egress. Immunofluorescence microscopy demonstrated high expression levels of S1P1 in endothelial cells, whereas staining of lymphocytes was weaker (Singer et al., 2005; Sinha et al., 2009). Moreover, administration of SEW2971, a selective S1P1 agonist, does not induce irreversible receptor loss from the cell surface but causes significant lymphopenia in vivo (Jo et al., 2005). Two-photon microscopy of explanted lymph nodes containing labeled lymphocytes suggested that S1P1 agonists may modulate barrier function and closure of vascular portals in the medulla, through which T cells egress into efferent lymphatics (Wei et al., 2005). Thus, this alternative proposal favors endothelial cells as the primary target cell type for S1P1 agonists to inhibit lymphocyte egress (Rosen et al., 2008).
Close interactions between immune and vascular cells may underlie the ability of S1P1 to promote lymphocyte egress. In lymph node cortical sinuses, egress of T and B cells required S1P1-dependent transendothelial traverse (Grigorova et al., 2009; Sinha et al., 2009). Indeed, competing chemotactic signaling between the egress-promoting S1P–S1P1 system and the retention-promoting CXCL21–CCR7 chemokine receptor system of T cells appears to determine the rate and extent of their egress from secondary lymphoid organs (Pham et al., 2008). Whether S1P1 signaling in lymphocytes, endothelial compartments, or both is important in the process of egress is not known.
S1P1 is a type I GPCR that is rapidly phosphorylated upon agonist stimulation. Although several protein kinases are involved in the phosphorylation of S1P1 (Lee et al., 2001), phosphorylation at the C-terminal domain is particularly relevant to receptor desensitization and internalization (Hla, 2001). Because FTY720-P is degraded less efficiently than S1P by S1P lyase and S1P phosphatases (Bandhuvula et al., 2005; Mechtcheriakova et al., 2007; Yamanaka et al., 2008), its ligation likely induces sustained receptor activation kinetics. Presumably, this underlies the FTY720-P–induced irreversible internalization and proteosomal degradation of S1P1 and resultant lymphopenia (Oo et al., 2007). The GRK-2 enzyme is capable of phosphorylating the serine-rich motif in the C-terminal tail of S1P1 (Watterson et al., 2002), and we recently demonstrated that mutation of the five serines in the C terminus of S1P1 to nonphosphorylatable alanines inhibited S1P- and FTY720-P–induced receptor internalization in transfected HEK293 cells (Oo et al., 2007). Although previous studies of GPCR signaling and chemotaxis have provided some insights into the role of internalization in these processes, the results appear to be receptor specific. For example, a CXCR4 superagonist induced greater chemotaxis than the native ligand stromal cell–derived factor–1α (SDF-1α) with no perceptible receptor internalization (Sachpatzidis et al., 2003). Conversely, mutations in the C terminus of CXCR2 resulted in defective receptor internalization concomitant with impaired chemotaxis (Sachpatzidis et al., 2003). In the case of S1P1, it is unknown whether internalization is required for lymphocyte egress and recirculation.
To address the role of S1P1 internalization in the control of lymphocyte egress during homeostasis and FTY720 treatment, we developed a mouse model in which WT S1P1 is replaced by the internalization-deficient mutant (S5A-S1P1). We show that although T cell trafficking under homeostasis is unaltered, S1p1rS5A/S5A mice display kinetic resistance to lymphopenia induced by the S1P1 modulator (FTY720-P) or disruption of the S1P gradient. Adoptive transfer of S1p1rWT/WT and S1p1rS5A/S5A lymphocytes and S1P1 surface staining of lymph node endothelial cells demonstrate that the T cell S1P1, and not endothelial cell S1P1 expression, regulates the rate of lymphocyte egress in vivo. These data support a T cell–intrinsic model of S1P1 signaling in egress kinetics wherein the internalization of S1P1 is a crucial modulator of the cues for T cell migration.
RESULTS
Derivation of the S1p1rS5A/S5A mouse strain
To determine the in vivo role of S1P1 internalization in receptor function, we generated a knockin mouse expressing a mutant internalization-deficient S1P1 under the control of its natural promoter. Specifically, we replaced the five clustered C-terminal tail serine residues, which are known to be phosphorylated upon agonist-induced desensitization with nonphosphorylatable alanines (Oo et al., 2007). This mutant S5A-S1P1 was expressed efficiently on the cell surface of transfected HEK293 cells and displayed decreased internalization kinetics compared with WT S1P1 in response to FTY720-P or S1P treatment (Oo et al., 2007). However, the kinetics of S5A-S1P1 signaling was similar to WT S1P1, as demonstrated by extracellular signal-regulated kinase (ERK) phosphorylation assays in transfected cells (Fig. S1). This is consistent with previous studies from our laboratory showing that deletion of the C-terminal tail of S1P1 did not interfere with ligand binding or signal transduction (Liu et al., 1999).
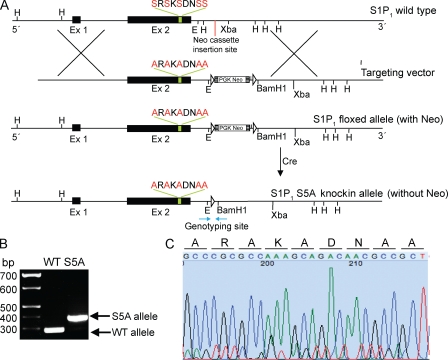
Given that the S5A-S1P1 exhibits a specific deficiency in ligand-induced internalization but not G protein–dependent signaling, we rationalized that this mutant receptor would demonstrate extended cell-surface residency as compared with the WT receptor, even after agonist-induced activation, and would therefore reveal phenotypes that depend on receptor internalization. Thus, the corresponding mutation was knocked into the genome of mouse embryonic stem (ES) cells by homologous recombination. The resulting ES cells were used to derive chimeric mice, which were then bred to homozygosity and backcrossed five to six generations onto the C57BL/6 background. Sequence analysis of genomic DNA from S1p1rS5A/S5A mice showed that the corresponding mutation was knocked in to the genome (Fig. 1). The S1p1S5A/S5A mice were viable, reproduced normally, and gave rise to healthy offspring. This suggests that the development of the vascular system was not perturbed in the S1p1rS5A/S5A mice.
Figure 1.
Construction of the S1p1rS5A/S5A mice. (A) Schematic representation of the S5A-S1P1 knockin construct. E, EcoRI; Ex, exon; H, HindIII. (B) Genotyping analysis of tail DNA. (C) DNA sequence of the mutated site from the tail DNA of S1p1S5A/S5A mice. Nucleotides are represented by black (G), blue (C), green (A), and red (T), with each respective codon shown above.
Delayed internalization of S5A-S1P1 after FTY720 treatment
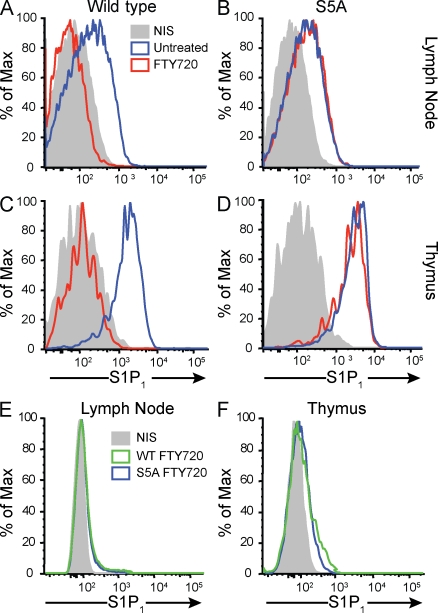
We next examined the cell-surface expression of S1P1 in vivo in lymph node and thymic T cells. Mice were treated with FTY720, which is rapidly converted to FTY720-P (Mandala et al., 2002; Brinkmann et al., 2004). Lymphocytes were isolated from mice treated with FTY720 for 0, 2, or 24 h, and expression of T cell–surface S1P1 was assessed by flow cytometry using an antibody specific for S1P1 (Pham et al., 2008). As shown in Fig. 2, WT T cells expressed significant levels of S1P1 on the cell surface. 2 h after FTY720 treatment, the receptor was not detectable on WT T cells, suggesting that it was internalized (Fig. 2, A and C). In sharp contrast, FTY720-treated S5A-S1P1 T cells showed robust receptor expression on the cell surface. Similar results were obtained using thymic T cells (Fig. 2, B and D). 24 h after FTY720 treatment, surface S1P1 staining was not detectable on either WT or mutant lymph node or thymic T cells (Fig. 2, E and F). At no time was the S1P1 detected on the surface of peripheral blood T cells (unpublished data). These data suggest that surface expression of S1P1 under homeostatic conditions is not affected by the S5A mutation. However, the internalization rate of mutant S5A-S1P1 is significantly slower than that of the WT counterpart upon FTY720 treatment in vivo.
Figure 2.
Slow internalization of S1P1 in vivo. (A–D) Internalization of S5A-S1P1 is delayed in vivo in T cells from the lymph nodes (A and B) and thymus (C and D). S1P1 levels on CD4+CD62Lhi cells are shown. S1p1WT/WT (WT) and S1p1S5A/S5A (S5A) mice were untreated or treated with 0.5 mg/kg FTY720, and 2 h later cells were stained with the S1P1-specific antibody. Blue tracings show untreated cells, red tracings show FTY720-treated cells, and gray graphs show staining with nonimmune serum (NIS). (E and F) S1P1 staining in lymph node cells (E) and thymocytes (F) isolated from WT and S5A mice 24 h after FTY720 treatment. Green tracings show WT cells, blue tracings show S5A cells, and gray graphs show staining with nonimmune serum. Surface S1P1 staining was not detectable in either WT or S5A cells. Data represent FACScan profiles from a typical experiment that was repeated twice with similar results.
Sustained chemotactic response of S5A-S1P1 T cells
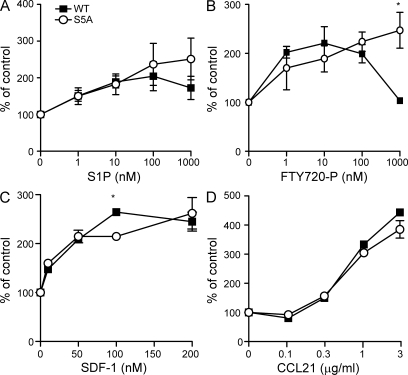
Next, we tested the ability of lymphocytes isolated from WT and S5A-S1P1 mutant mice to respond to chemotactic stimuli, including agonists of S1P1. Both WT and mutant lymphocytes migrated toward S1P and FTY720-P. However, at high doses of both S1P and FTY720-P, mutant cells displayed persistent cell migration, whereas the WT counterparts were refractory to continued chemotactic stimulation. Thus, a bell-shaped response was obtained in WT cells, whereas mutant cells exhibited a sigmoidal response at high doses of receptor agonist (Fig. 3). This phenotype was more pronounced in response to FTY720-P than to S1P, consistent with the fact that FTY720-P induces more effective receptor internalization in WT cells. In contrast, both WT and mutant cells responded to other chemokines—CXCL12 (SDF-1) and CCL21—in a similar manner, suggesting that the lack of desensitization in the S5A-S1P1 cells is specific for S1P1 and not a general effect on other chemotactic GPCRs.
Figure 3.
Lymphocytes bearing S5A-S1P1 show aberrant chemotactic response to S1P1 agonists. (A–D) Lymphocytes were isolated from lymph nodes of S1p1WT/WT and S1p1S5A/S5A mice and were stimulated with S1P (A), FTY720-P (B), SDF-1 (C), or CCL21 (D) in a chemotaxis assay as described in Materials and methods (n = 3; *, P < 0.01). Values are expressed as the percentage of cells that migrate at the given concentration of chemoattractant compared with the number of cells that migrate in the absence of chemoattractant after 3 h at 37°C. Data are means ± SE and are representative of two experiments performed.
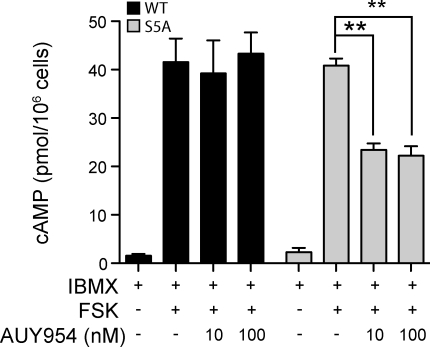
To further establish the involvement of altered receptor desensitization in S5A-S1P1 cells, WT and mutant mice were treated with FTY720 and ex vivo receptor-activated Gi responses were quantified 2 h later. S1P1 activation was measured by quantifying intracellular cAMP levels after ex vivo stimulation of T cells with forskolin (a direct activator of adenylate cyclase), IBMX (3-isobutyl-1-methylxanthine; a phosphodiesterase inhibitor), and AUY954 (an S1P1-specific agonist; Pan et al., 2006). As shown in Fig. 4, WT S1P1 was effectively desensitized in vivo, as these cells did not display a decrease in cAMP, indicative of S1P1 agonist-activated Gi responses. Conversely, S5A-S1P1 cells exhibited robust Gi activation in response to S1P1 agonism with AUY954. These data establish that in sharp contrast to WT S1P1, S5A-S1P1 is not desensitized after exposure to FTY720-P in vivo.
Figure 4.
Defective desensitization of the mutant S5A-S1P1. S1p1WT/WT and S1p1S5A/S5A mice were treated with 0.5 mg/kg FTY720 or vehicle, and 2 h later thymocytes were isolated. Cells were incubated with the adenylate cyclase activator forskolin (FSK) and the phosphodiesterase inhibitor IBMX in the presence or absence of 10 or 100 nM of the S1P1-specific agonist AUY954 for 15 min as described in Materials and methods. Intracellular cAMP concentrations were quantified by ELISA (n = 4; **, P < 0.01) and are expressed as picomoles per 106 cells. Error bars represent means ± SE. Data are representative of two experiments performed.
The S5A-S1P1 mutation exhibits delayed lymphopenia kinetics
Lymphoid architecture can be altered as a result of changes to cell homing and migration within the structures (Weninger and von Andrian, 2003). To identify any alterations in the T cell compartment of lymphoid organs in S1p1rS5A/S5A mice, lymph node structures were compared between S1p1WT/WT and S1p1S5A/S5A mice by immunofluorescence microscopy. As shown in Fig. S2, B cell follicles (B220+), T cell zones (CD4+, CD8+), LYVE-1+ lymphatic vessels, platelet/endothelial cell adhesion molecule 1+ (PECAM-1+) high endothelial venules, and dendritic cell (CD11c+) staining were similar. Thymic architecture was also similar, suggesting that the S5A-S1P1 mutation had no discernable impact on the development of primary or secondary lymphoid organ architecture. Flow cytometric analysis of T cells from both S1p1rWT/WT and S1p1rS5A/S5A mice revealed similar numbers of CD4+CD8+ double-positive, CD4−CD8− double-negative, and CD4+ and CD8+ single-positive (SP) T cells in the thymus, suggesting that T cell development is normal in S1p1S5A/S5A mice under homeostatic conditions (Fig. S3).
In the spleen, SP C4+ and SP CD8+ cell numbers were modestly elevated in S1p1rS5A/S5A mice, whereas no significant differences were found in the numbers of circulating blood or lymph node and lung tissue SP CD4+ or CD8+ cells. Expression of the lymphocyte maturation and activation markers CD69, CD44, CD25, and CD24 in the thymus; CD69, CD44, and CD11a in the lymph node, spleen, and lung; and CD69, CD62L, and CD11a in blood were indistinguishable between S1p1WT/WT and S1p1S5A/S5A mice (Figs. S4, S5, S6, S7, and S8). Collectively, these data suggest that T cell development was largely unaffected by the S5A-S1P1 mutation. T cell numbers, maturation, and activation states in different compartments were largely unchanged, except for a slight expansion of the CD4+ and CD8+ compartments in the spleens of S1p1S5A/S5A mice.
Although homeostatic T cell trafficking appeared to be unchanged, an effect on T cell transit into lymph nodes could not be ruled out. To address the possibility that the S5A mutation was altering lymphocyte homing into the lymph node, fluorescently labeled S1p1WT/WT and S1p1S5A/S5A splenocytes were transferred into naive WT mice treated with integrin-blocking antibodies (Lo et al., 2003; Pham et al., 2008). We did not observe a difference in the fraction of transferred lymphocytes retained in the lymph nodes 8 h after integrin blockade (Fig. S9), suggesting that lymphocyte egress from lymph nodes under homeostasis is not affected by the S5A-S1P1 mutation.
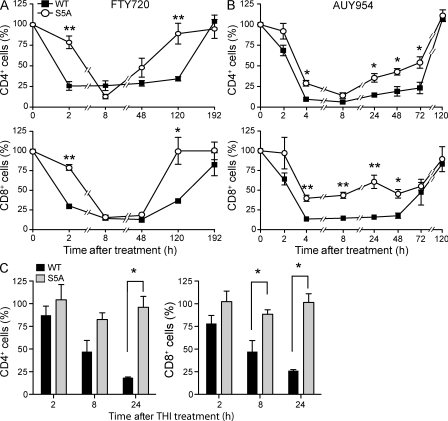
Given that S1P1 is critical for the inhibition of lymphocyte egress resulting in FTY720-induced lymphopenia, we compared the kinetics of the lymphopenic response in WT and S5A-S1P1 mice. As shown in Fig. 5 A, treatment of mice with 0.5 mg/kg FTY720 led to a rapid decrease in CD4+ and CD8+ cells in the blood of WT mice. In contrast, the disappearance rate of blood CD4+ and CD8+ cells in the mutant mice was significantly slower. The lymphopenic response was maintained in WT mice for up to 120 h. In contrast, the recovery of CD4+ and CD8+ cells in the blood to untreated levels occurred much more rapidly in S5A-S1P1 mice compared with their WT counterparts. To confirm that S1P1 was responsible for these effects, we treated mice with the S1P1-specific agonist AUY954 (Pan et al., 2006). We observed that lymphopenia kinetics were significantly delayed, attenuated, and recovered faster in S5A-S1P1 mice compared with the WT counterparts (Fig. 5 B). These data indicate that S1P1 internalization in vivo plays a direct role in the inhibition of lymphocyte egress from lymphoid organs into the circulation.
Figure 5.
S5A-S1P1 animals exhibit delayed lymphopenia. (A and B) S1p1WT/WT and S1p1S5A/S5A mice were treated with either 0.5 mg/kg FTY720 (A) or 1 mg/kg AUY954 (B). Peripheral blood samples were collected from mice at various time points and were processed for FACS analysis. (n = 3; *, P < 0.05; **, P < 0.01). Values are expressed as a percentage of the number of CD4+ (top) or CD8+ (bottom) cells at each time point compared with untreated values. Symbols represent means ± SE. Data are representative of two experiments. (C) 10 mg/kg THI was administered to WT and S5A mice by oral gavage; peripheral blood was collected at 0, 2, 8, and 24 h after treatment; and cells were processed for FACS analysis as described in Materials and methods. Values are expressed as the percentage of CD4+ (left) or CD8+ (right) detected in peripheral blood at the indicated time point after THI treatment as compared with untreated controls (n = 3; *, P < 0.05; **, P < 0.01). Error bars represent means ± SE and are representative of three experiments performed.
Although S1P1 internalization was clearly required for the inhibition of T cell egress in response to FTY720 treatment, we asked whether the S5A mutation would display altered kinetics in response to the natural agonist, S1P. Inhibition of S1P lyase with 2-acetyl-4-tetrahydroxybutylimidazole (THI) increases S1P concentrations, thereby disrupting the S1P gradient between vascular and extravascular spaces, and results in profound lymphopenia by blocking lymphocyte egress (Schwab et al., 2005). To obtain further evidence for the role of S1P1 internalization in effecting lymphocyte egress, we increased S1P concentrations by THI treatment in WT and S5A-S1P1 mice. As shown in Fig. 5 C, S1p1WT/WT mice demonstrated a dramatic disappearance of both CD4+ and CD8+ T cells from circulating blood within 24 h of THI treatment. In sharp contrast, S1p1S5A/S5A mice exhibited a strong resistance to THI-induced lymphopenia, suggesting that receptor internalization is critical for the response to the natural S1P1 ligand, as well as to FTY720 agonism.
Lymphocyte but not vascular S1P1 is critical for determining egress kinetics
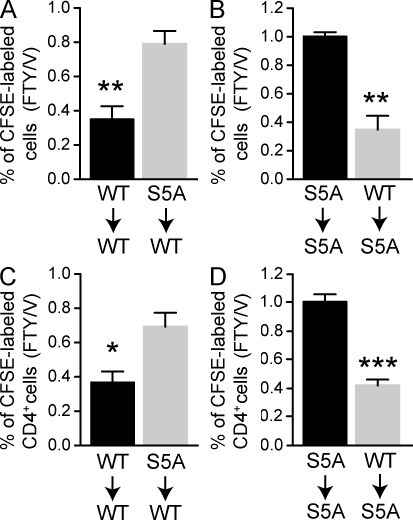
Because of the competing models regarding the respective roles of vascular versus lymphoid S1P1 expression in the modulation of lymphocyte egress, we sought to determine whether the effect on lymphopenia kinetics observed in S5A-S1P1 cells could be attributed to T cell or endothelial cell expression of S1P1. We performed adoptive transfer of CFSE-labeled WT or S5A-S1P1 bulk lymphocytes into recipient WT or mutant mice and measured the effect of FTY720 administration on T cell numbers in the blood. If T cell S1P1 surface residency determines egress rates, then mutant T cells would be resistant to FTY720-induced lymphopenia at 2 h when transferred into WT recipients, and WT T cells transferred into mutant animals would demonstrate faster kinetics of lymphopenia. Conversely, if the endothelial S1P1 was the major determinant, then WT T cells transferred into mutant recipients would exhibit resistance to FTY720 treatment and mutant T cells in WT recipients would demonstrate the rapid lymphopenia response. As shown in Fig. 6, S5A-S1P1 total lymphocytes (A and B) or CD4+ T cells (C and D) were not responsive to FTY720 when transferred into the WT or mutant backgrounds, whereas WT lymphocytes or T cells responded equally well to FTY720 in both WT and mutant recipients. Furthermore, examination of S1P1 expression on lymph node endothelial cells demonstrated that although WT surface S1P1 was dramatically reduced within 2 h and remained low for 24 h after FTY720 treatment, endothelial cells expressing tS5A-S1P1 displayed resistance to surface down-regulation that quickly recovered to pretreatment levels (Fig. S10). Thus, the S5A-S1P1 mutation in the vascular elements did not influence lymphocyte egress rates, suggesting that the rate of S1P1 internalization on T cells is the primary determinant of lymphopenia kinetics in vivo.
Figure 6.
Lymphocyte but not vascular S1P1 is critical in determining egress kinetics. (A–D) 107 lymphocytes from S1p1WT/WT and S1p1S5A/S5A mice were labeled with CFSE and injected i.v. into mice of the indicated genotype. 24 h later, mice were treated with 0.5 mg/kg FTY720, and 2 h later, peripheral blood was collected and analyzed by FACS for total CFSE (A and B) or CD4+ CFSE+ (C and D). Data are represented as the ratio of FTY720-treated to vehicle-treated mice (n = 3; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Data are from one representative experiment out of three.
DISCUSSION
The S1P–S1P1 signaling axis represents one of the most critical regulators of lymphocyte trafficking, yet little is known about the in vivo dynamics of S1P1 in response to ligand binding. Studies have identified a cyclical pattern of lymphocyte S1P1 expression: increased in lymphoid organs, decreased or absent in peripheral circulation. However, the signaling properties within this pattern, i.e., whether S1P1 surface expression is a necessary determinant of lymphoid residence, have been difficult to elucidate in the in vivo context. The diversity of GPCR signal regulation further complicates the issue. Agonist-induced internalization of GPCRs is believed to be important in a variety of signaling modalities (Hanyaloglu and von Zastrow, 2008; Marchese et al., 2008). In the case of some receptors, cells become unresponsive to agonists after a significant fraction of receptors are internalized (Vroon et al., 2006). Additionally, endocytosed receptors may carry out unique signaling functions in the different subcellular compartments (i.e., early versus late endosome; Hanyaloglu and von Zastrow, 2008; Marchese et al., 2008). Although S1P1 is known to undergo agonist-induced surface down-regulation, it is unclear what functional consequences result from this event.
In this study, we demonstrate that the C-terminal serine cluster, which corresponds to the GRK-2 phosphorylation site (Watterson et al., 2002; Oo et al., 2007), is important in vivo for determining the rate of internalization of S1P1 in T cells after FTY720 administration. In contrast, the receptor level on the surface of T cells under homeostatic conditions (high plasma S1P levels) was not affected by the S5A mutation. Similarly, ligand-induced activation of the ERK signaling pathway is not dependent on receptor internalization, because S5A-S1P1–expressing cells exhibited kinetics of ERK phosphorylation similar to those of WT cells. Although the S5A-S1P1 mutant showed delayed internalization at 2 h after FTY720 treatment, it was undetectable on the surface of T cells by 24 h after FTY720 treatment, suggesting that additional posttranslational modifications on the receptor mediate endocytic events.
GPCR signals are the primary modulators of T cell lymphoid transit (Pham et al., 2008). In vitro studies using T cells demonstrated that S5A-S1P1 did not undergo agonist-induced desensitization at high ligand concentrations, as illustrated by the linear curve of the chemotaxis responses to both FTY720-P and the natural ligand S1P. Similarly, T cells from FTY720-treated mice did not show agonist-induced desensitization in cAMP levels. These observations suggest that receptor internalization limits cellular responses during supramaximal agonism.
Changes in lymphocyte homing can alter the architecture of the lymphoid organs and the cognate vasculature. The S1p1S5A/S5A mice did not exhibit developmental or structural defects in the vascular or immune systems, indicating that changes in the rate of S1P1 internalization do not result in gross signaling defects. Furthermore, homeostatic trafficking of T cells was not significantly different between S1p1WT/WT and S1p1S5A/S5A mice, as demonstrated by cell retention rates within lymph nodes after integrin blockade. In sharp contrast, lymphopenia induced by the S1P1 agonists FTY720 and AUY954 was substantially blunted. Additionally, we assessed the response of S5A-S1P1 animals to THI administration, an inhibitor of S1P lyase known to increase S1P levels in lymphoid organs and disrupt the S1P gradient essential for normal lymphocyte egress (Schwab et al., 2005). Although S1p1WT/WT mice exhibit profound lymphopenia after THI administration, the S1p1S5A/S5A animals were refractory to THI-induced lymphopenia, suggesting that THI-induced supraphysiological S1P levels in the lymphoid organs suppress lymphocyte egress by inducing S1P1 internalization. These observations suggest that the primary function of S1P1 internalization is to limit exaggerated receptor signaling at supramaximal agonist activation. In addition, the concept that the S1P1 level on the plasma membrane is important in efficient egress of T cells is reinforced.
Given that S5A-S1P1 exhibits a clear difference in lymphocyte egress kinetics in response to FTY720 treatment, we wished to clarify whether S1P1 function in the lymphocyte or endothelial cell compartment is critical to the determination of egress kinetics. Adoptive transfer experiments indicated that S5A-S1P1 T cells were refractory to FTY720-induced lymphopenia, even when transferred into WT hosts. Furthermore, S1P1 surface staining of lymph node endothelial cells isolated from FTY720-treated S1p1S5A/S5A animals revealed that the mutant receptor undergoes minimal down-regulation by 24 h, in contrast to the receptor on T cells, consistent with the idea that the lymphocyte and not endothelial cell S1P1 modulates egress inhibition by FTY720. Recent work has shown that lymphocyte egress from lymph nodes occurs at cortical sinuses, where lymphocytes exhibit a “probing” behavior dependent on S1P1 activation, and that agonism of the receptor is necessary for the successful traverse of the lymphocyte between the lymphatic endothelial cells that line the cortical sinusoids (Grigorova et al., 2009; Sinha et al., 2009). Studies of the leukocyte extravasation suggest that a specific membrane domain termed lateral border recycling compartment, which contains PECAM and CD99, is critical in the transcytotic process (Mamdouh et al., 2009; Muller, 2009). Whether S1P1 signaling utilizes a similar process is unknown and should be the subject of future investigations. Nevertheless, our data suggest that upon FTY720 administration, accumulation of FTY720-P in the lymphoid tissue, followed by sustained internalization of S1P1 in the lymphocyte compartment, is needed to arrest egress and generate lymphopenia.
In summary, with the ability to dissect signaling versus internalization of S1P1 in the context of lymphocyte homing, these studies demonstrate that internalization of S1P1 makes a profound impact on lymphopenia kinetics in vivo, a mechanism that may be important in the regulation of lymphocyte egress in pathological conditions. The finding that supraphysiological agonism is necessary to visualize a difference in trafficking by cells expressing S5A-S1P1, whether with FTY720 or the elevation of S1P concentrations by THI, is of considerable importance physiologically, not only in the context of FTY720, which has completed phase III clinical trials for the treatment of multiple sclerosis (Cohen et al., 2010), but also in numerous other inflammatory disease states. Changes in S1P concentrations in blood and at tissue-specific sites accompany many diseases, such as coronary artery disease, asthma, rheumatoid arthritis, and certain cancers (Ammit et al., 2001; Deutschman et al., 2003; Kitano et al., 2006; Bandhuvula and Saba, 2007). During these conditions, exaggerated production of S1P and subsequent signaling may be limited by receptor internalization. We anticipate that further studies examining the role of S1P1 trafficking in various physiological and pathological contexts may be revealed by this novel mouse model.
MATERIALS AND METHODS
Generation of S1p1S5A/S5A mice.
Using a homologous recombineering method with mouse genomic bacterial artificial chromosomes (Liu et al., 2003), a mutant S1P1 (SRSKSDNSS to ARAKADNAA) was constructed and introduced into 129S6 × C57BL/6 F1 hybrid ES cells by homologous recombination. Positive ES cell clones were used to derive chimeric mice. Genotyping analysis by PCR and gene sequencing confirmed that the mutant S1p1r gene was linked to the loxP site 290 bp downstream of exon 2. To identify the S1P1 WT (S1p1WT/WT) and mutant S1p1S5A/S5A by PCR, the following primers were used: P1, 5′-AAGTGCAGTGAGGCCAAACA-3′; and P2, 5′-GCAAGCATGAGGCAAGTCTA-3′. P1 and P2 amplify a 282-bp fragment for the WT allele and a 376-bp fragment for the S5A allele. S1p1WT/WT and S1p1S5A/S5A mice were backcrossed onto the C57BL/6 background for at least five generations. Protocols were approved by the Institutional Animal Care and Use Committees of the University of Connecticut Health Sciences Center and Weill Cornell Medical College.
Agonist, antagonist, and inhibitor treatments.
6–8-wk-old WT and S5A-S1P1 animals were treated by oral gavage with 0.5 mg/kg FTY720 or 1 mg/kg AUY954 in 2% 2-hydoxypropyl-β-cyclodextrin (Sigma-Aldrich). Control animals received 2% 2-hydoxypropyl-β-cyclodextrin in water. For S1P lyase inhibition, animals were treated by oral gavage with 10 mg/kg THI dissolved in water, and peripheral blood was collected at the time points after treatment indicated in the figures. Control animals were gavaged with water alone.
Lymphocyte chemotaxis assay.
The chemotactic response of lymph node cells isolated from S1p1WT/WT and S1p1S5A/S5A mice to S1P (Enzo Life Sciences, Inc.), SDF-1 (R&D Systems), CCL21 (R&D Systems), and FTY720-P (provided by Novartis, Basel, Switzerland) were studied using a Boyden chamber with 5-µm polycarbonate filters (Neuro Probe). Lymph node cell suspensions (500,000 cells/300 µl/well) in RPMI 1640 medium supplemented with 0.1% fatty acid–free BSA (Sigma-Aldrich) were added to the top chamber. The bottom chambers contained different concentrations of the chemoattractants prepared in RPMI/fatty acid–free BSA. Wells containing medium without chemoattractants were used as controls. After 3 h at 37°C, cells in the bottom chamber were enumerated in a cell and particle counter (Beckman Coulter).
Flow cytometry.
At the time points after treatment indicated in the figures, thymus, spleen, lung, or peripheral lymph nodes were dissected and mechanically disaggregated in FACS buffer (PBS with 0.1% FBS and 0.1% sodium azide), and single-cell suspensions were obtained using a 40-µm cell strainer. Peripheral blood was treated with NH4Cl for 5 min to lyse erythrocytes. Single-cell suspensions were then incubated on ice for 30 min with the indicated antibodies, washed, and analyzed by flow cytometry. The antibodies used were Pacific blue–labeled anti-CD4 (BioLegend), PE-Cy7 anti-CD8 (eBioscience), and allophycocyanin–Alexa Fluor 750 anti-CD62L (eBioscience). S1P1 surface expression was determined using a rabbit polyclonal anti-S1P1 antibody, as previously described (Pham et al., 2008), and gating on CD4+ or CD8+ and CD62Lhi cells. For S1P1 staining of lymph node endothelial cells, single-cell suspensions were obtained as described and fixed in 2% buffered formalin for 30 min. Cells were washed thoroughly and incubated at 4°C overnight in FACS buffer with PE anti-S1P1 (R&D Systems), PE-Cy7 anti-CD31 (eBioscience), and Pacific blue anti-CD45. Stained cells were subsequently washed and processed using an LSR II (BD). For all studies, live cells were gated by forward versus side scatter. Data were analyzed using FlowJo software (version 8; Tree Star, Inc.).
Immunohistochemistry.
10-µm frozen sections of lymph nodes and thymi were fixed in cold acetone and stained with fluorescently conjugated CD8, B220, and CD11c antibodies (all from BD). For PECAM (BD) and LYVE-1 (Abcam) staining, sections were incubated with specific antibodies detected by fluorescently labeled secondary antibodies.
cAMP measurement.
Thymocytes were isolated from S1p1WT/WT and S1p1S5A/S5A mice treated with 0.5 mg/kg FTY720 for 2 h; suspended in buffered medium containing 20 mM Hepes, pH 7.4, 100 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 0.5 mM CaCl2, 25 mM NaHCO3, 15 mM glucose, and 0.1% fatty acid–free BSA; and incubated for 15 min before treatment with 200 mM IBMX, 50 µM forskolin, and the concentrations of AUY954 indicated in the figures for 15 min. Intracellular cAMP was extracted with HCl and measured using a cAMP Biotrak EIA system (GE Healthcare) according to the manufacturer’s instructions.
Adoptive transfer.
Single-cell lymphocyte suspensions were isolated from the spleen and lymph nodes and labeled with the fluorescent dye CFSE (Invitrogen; Khanna et al., 2008). After washing the cells with PBS, each animal was injected via the tail vein with 107 labeled cells. Beginning 24 h after cell injection, mice were treated with 0.5 mg/kg FTY720 by oral gavage, and 2 h later peripheral blood cells were analyzed by flow cytometry.
Online supplemental material.
Fig. S1 shows that HEK293 cells expressing S5A-S1P1 demonstrate mitogen-activated protein kinase activation kinetics similar to WT transfectants. Fig. S2 depicts the immunofluorescence of lymph nodes and the thymus from WT and S5A mice. Fig. S3 shows a FACS analysis of CD4 and CD8 expression on cells isolated from the thymus, lymph node, blood, spleen, and lung of WT and S5A mice. Figs. S4, S5, S6, S7, and S8 show a FACS analysis of T cell activation markers on CD8+ and CD4+ cells collected from the thymus, lymph node, spleens, lung, or blood, respectively, of WT and S5A mice. Fig. S9 shows that homeostatic recirculation of S5A CD8+ and CD4+ cells is not impaired, as determined by integrin blockade. Fig. S10 shows decreased down-regulation of S5A-S1P1 on CD45−CD31+ endothelial cells versus WT S1P1 in response to FTY720 administration. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091343/DC1.
Acknowledgments
We thank T. Arnon and J. Cyster (University of California, San Francisco, San Francisco, CA) for conducting the FACS experiment (Fig. 2), and critical comments on the manuscript. We are also grateful to B. Jung and C. Stoddard of the University of Connecticut Health Center for technical assistance.
This work is supported by National Institutes of Health grants HL67330 and HL89934 to T. Hla, AI076457 and AI056172 to L. Lefrancois, and F32CA142117 to V.A. Blaho.
The authors declare that no competing financial interests or conflicts exist.
Footnotes
Abbreviations used:
- ERK
- extracellular signal-regulated kinase
- ES
- embryonic stem
- GPCR
- G protein–coupled receptor
- IBMX
- 3-isobutyl-1-methylxanthine
- PECAM
- platelet/endothelial cell adhesion molecule
- S1P
- sphingosine 1-phosphate
- S1P1
- S1P receptor 1
- SDF-1
- stromal cell–derived factor–1
- SP
- single positive
- THI
- 2-acetyl-4-tetrahydroxybutylimidazole
References
- Allende M.L., Proia R.L. 2002. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim. Biophys. Acta. 1582:222–227 [DOI] [PubMed] [Google Scholar]
- Allende M.L., Dreier J.L., Mandala S., Proia R.L. 2004. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279:15396–15401 10.1074/jbc.M314291200 [DOI] [PubMed] [Google Scholar]
- Ammit A.J., Hastie A.T., Edsall L.C., Hoffman R.K., Amrani Y., Krymskaya V.P., Kane S.A., Peters S.P., Penn R.B., Spiegel S., Panettieri R.A., Jr 2001. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 15:1212–1214 [DOI] [PubMed] [Google Scholar]
- Bandhuvula P., Saba J.D. 2007. Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends Mol. Med. 13:210–217 10.1016/j.molmed.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Bandhuvula P., Tam Y.Y., Oskouian B., Saba J.D. 2005. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J. Biol. Chem. 280:33697–33700 10.1074/jbc.C500294200 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Davis M.D., Heise C.E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277:21453–21457 10.1074/jbc.C200176200 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Cyster J.G., Hla T. 2004. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 4:1019–1025 10.1111/j.1600-6143.2004.00476.x [DOI] [PubMed] [Google Scholar]
- Chiba K. 2005. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol. Ther. 108:308–319 10.1016/j.pharmthera.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Cohen J.A., Barkhof F., Comi G., Hartung H.P., Khatri B.O., Montalban X., Pelletier J., Capra R., Gallo P., Izquierdo G., et al. ; TRANSFORMS Study Group 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362:402–415 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- Cyster J.G. 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23:127–159 10.1146/annurev.immunol.23.021704.115628 [DOI] [PubMed] [Google Scholar]
- Deutschman D.H., Carstens J.S., Klepper R.L., Smith W.S., Page M.T., Young T.R., Gleason L.A., Nakajima N., Sabbadini R.A. 2003. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am. Heart J. 146:62–68 10.1016/S0002-8703(03)00118-2 [DOI] [PubMed] [Google Scholar]
- Gräler M.H., Goetzl E.J. 2004. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 18:551–553 [DOI] [PubMed] [Google Scholar]
- Grigorova I.L., Schwab S.R., Phan T.G., Pham T.H., Okada T., Cyster J.G. 2009. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 10:58–65 10.1038/ni.1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu A.C., von Zastrow M. 2008. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48:537–568 10.1146/annurev.pharmtox.48.113006.094830 [DOI] [PubMed] [Google Scholar]
- Hla T. 2001. Sphingosine 1-phosphate receptors. Prostaglandins Other Lipid Mediat. 64:135–142 10.1016/S0090-6980(01)00109-5 [DOI] [PubMed] [Google Scholar]
- Hla T. 2004. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 15:513–520 10.1016/j.semcdb.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Ishii M., Egen J.G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J., Proia R.L., Germain R.N. 2009. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 458:524–528 10.1038/nature07713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E., Sanna M.G., Gonzalez-Cabrera P.J., Thangada S., Tigyi G., Osborne D.A., Hla T., Parrill A.L., Rosen H. 2005. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem. Biol. 12:703–715 10.1016/j.chembiol.2005.04.019 [DOI] [PubMed] [Google Scholar]
- Kabashima K., Haynes N.M., Xu Y., Nutt S.L., Allende M.L., Proia R.L., Cyster J.G. 2006. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 203:2683–2690 10.1084/jem.20061289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna K.M., Aguila C.C., Redman J.M., Suarez-Ramirez J.E., Lefrançois L., Cauley L.S. 2008. In situ imaging reveals different responses by naïve and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur. J. Immunol. 38:3304–3315 10.1002/eji.200838602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M., Hla T., Sekiguchi M., Kawahito Y., Yoshimura R., Miyazawa K., Iwasaki T., Sano H., Saba J.D., Tam Y.Y. 2006. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 54:742–753 10.1002/art.21668 [DOI] [PubMed] [Google Scholar]
- Ledgerwood L.G., Lal G., Zhang N., Garin A., Esses S.J., Ginhoux F., Merad M., Peche H., Lira S.A., Ding Y., et al. 2008. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 9:42–53 10.1038/ni1534 [DOI] [PubMed] [Google Scholar]
- Lee M.J., Thangada S., Paik J.H., Sapkota G.P., Ancellin N., Chae S.S., Wu M., Morales-Ruiz M., Sessa W.C., Alessi D.R., Hla T. 2001. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell. 8:693–704 10.1016/S1097-2765(01)00324-0 [DOI] [PubMed] [Google Scholar]
- Liu C.H., Thangada S., Lee M.J., Van Brocklyn J.R., Spiegel S., Hla T. 1999. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 10:1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jenkins N.A., Copeland N.G. 2003. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13:476–484 10.1101/gr.749203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.G., Lu T.T., Cyster J.G. 2003. Integrin-dependence of lymphocyte entry into the splenic white pulp. J. Exp. Med. 197:353–361 10.1084/jem.20021569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdouh Z., Mikhailov A., Muller W.A. 2009. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J. Exp. Med. 206:2795–2808 10.1084/jem.20082745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G.J., Card D., Keohane C., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296:346–349 10.1126/science.1070238 [DOI] [PubMed] [Google Scholar]
- Marchese A., Paing M.M., Temple B.R.S., Trejo J. 2008. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 48:601–629 10.1146/annurev.pharmtox.48.113006.094646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E.A., Huff I.V., Junt T., Wagers A.J., Mazo I.B., von Andrian U.H. 2007. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 131:994–1008 10.1016/j.cell.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- Mechtcheriakova D., Wlachos A., Sobanov J., Bornancin F., Zlabinger G., Baumruker T., Billich A. 2007. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 581:3063–3068 10.1016/j.febslet.2007.05.069 [DOI] [PubMed] [Google Scholar]
- Muller W.A. 2009. Mechanisms of transendothelial migration of leukocytes. Circ. Res. 105:223–230 10.1161/CIRCRESAHA.109.200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo M.L., Thangada S., Wu M.T., Liu C.H., Macdonald T.L., Lynch K.R., Lin C.Y., Hla T. 2007. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282:9082–9089 10.1074/jbc.M610318200 [DOI] [PubMed] [Google Scholar]
- Pan S., Mi Y., Pally C., Beerli C., Chen A., Guerini D., Hinterding K., Nuesslein-Hildesheim B., Tuntland T., Lefebvre S., et al. 2006. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 13:1227–1234 10.1016/j.chembiol.2006.09.017 [DOI] [PubMed] [Google Scholar]
- Pham T.H., Okada T., Matloubian M., Lo C.G., Cyster J.G. 2008. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 28:122–133 10.1016/j.immuni.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Proia R.L., Olivera A. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8:753–763 10.1038/nri2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gonzalez-Cabrera P., Marsolais D., Cahalan S., Don A.S., Sanna M.G. 2008. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol. Rev. 223:221–235 10.1111/j.1600-065X.2008.00645.x [DOI] [PubMed] [Google Scholar]
- Sachpatzidis A., Benton B.K., Manfredi J.P., Wang H., Hamilton A., Dohlman H.G., Lolis E. 2003. Identification of allosteric peptide agonists of CXCR4. J. Biol. Chem. 278:896–907 10.1074/jbc.M204667200 [DOI] [PubMed] [Google Scholar]
- Sanchez T., Estrada-Hernandez T., Paik J.H., Wu M.T., Venkataraman K., Brinkmann V., Claffey K., Hla T. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278:47281–47290 10.1074/jbc.M306896200 [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Cyster J.G. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8:1295–1301 10.1038/ni1545 [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 309:1735–1739 10.1126/science.1113640 [DOI] [PubMed] [Google Scholar]
- Sebzda E., Zou Z., Lee J.S., Wang T., Kahn M.L. 2008. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat. Immunol. 9:292–300 10.1038/ni1565 [DOI] [PubMed] [Google Scholar]
- Singer I.I., Tian M., Wickham L.A., Lin J., Matheravidathu S.S., Forrest M.J., Mandala S., Quackenbush E.J. 2005. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J. Immunol. 175:7151–7161 [DOI] [PubMed] [Google Scholar]
- Sinha R.K., Park C., Hwang I.Y., Davis M.D., Kehrl J.H. 2009. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 30:434–446 10.1016/j.immuni.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroon A., Heijnen C.J., Kavelaars A. 2006. GRKs and arrestins: regulators of migration and inflammation. J. Leukoc. Biol. 80:1214–1221 10.1189/jlb.0606373 [DOI] [PubMed] [Google Scholar]
- Walzer T., Chiossone L., Chaix J., Calver A., Carozzo C., Garrigue-Antar L., Jacques Y., Baratin M., Tomasello E., Vivier E. 2007. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 8:1337–1344 10.1038/ni1523 [DOI] [PubMed] [Google Scholar]
- Watterson K.R., Johnston E., Chalmers C., Pronin A., Cook S.J., Benovic J.L., Palmer T.M. 2002. Dual regulation of EDG1/S1P(1) receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J. Biol. Chem. 277:5767–5777 10.1074/jbc.M110647200 [DOI] [PubMed] [Google Scholar]
- Wei S.H., Rosen H., Matheu M.P., Sanna M.G., Wang S.K., Jo E., Wong C.H., Parker I., Cahalan M.D. 2005. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat. Immunol. 6:1228–1235 10.1038/ni1269 [DOI] [PubMed] [Google Scholar]
- Weninger W., von Andrian U.H. 2003. Chemokine regulation of naïve T cell traffic in health and disease. Semin. Immunol. 15:257–270 10.1016/j.smim.2003.08.007 [DOI] [PubMed] [Google Scholar]
- Yamanaka M., Anada Y., Igarashi Y., Kihara A. 2008. A splicing isoform of LPP1, LPP1a, exhibits high phosphatase activity toward FTY720 phosphate. Biochem. Biophys. Res. Commun. 375:675–679 10.1016/j.bbrc.2008.07.165 [DOI] [PubMed] [Google Scholar]
- Zemann B., Kinzel B., Müller M., Reuschel R., Mechtcheriakova D., Urtz N., Bornancin F., Baumruker T., Billich A. 2006. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 107:1454–1458 10.1182/blood-2005-07-2628 [DOI] [PubMed] [Google Scholar]