Abstract
Humoral immunity to viruses and encapsulated bacteria is comprised of T cell–independent type 2 (TI-2) antibody responses that are characterized by rapid antibody production by marginal zone and B1 B cells. We demonstrate that toll-like receptor (TLR) ligands influence the TI-2 antibody response not only by enhancing the overall magnitude but also by skewing this response to one that is dominated by IgG isotypes. Importantly, TLR ligands facilitate this response by inducing type I interferon (IFN), which in turn elicits rapid and significant amounts of antigen-specific IgG2c predominantly from FO (follicular) B cells. Furthermore, we show that although the IgG2c antibody response requires B cell–autonomous IFN-α receptor signaling, it is independent of B cell–intrinsic TLR signaling. Thus, innate signals have the capacity to enhance TI-2 antibody responses by promoting participation of FO B cells, which then elaborate effective IgG anti-pathogen antibodies.
The peripheral pool of mature B cells in adults is composed of several subpopulations, each of which is generally thought to make a distinct contribution to humoral immunity. As an example, natural serum IgM functions as a first line of defense against pathogens and is produced primarily by B1a B cells before exposure (Baumgarth et al., 1999; Haas et al., 2005). Upon bacterial or viral infection, marginal zone (MZ) and B1 B cell subsets respond rapidly, constituting the immediate acquired antibody response (Martin et al., 2001). Finally, FO (follicular) B cells dominate the delayed highly specific antibody response comprised by somatically mutated higher affinity class-switched antibodies and memory B cells. These latter processes occur in germinal center reactions that occur after productive interactions between responding B cells and antigen-specific helper T cells (Martin and Kearney, 2002; McHeyzer-Williams, 2003).
The nature of the antigen itself can also dictate which B cell subset is recruited into an antibody response. Using model antigens in rodents, B cell antigens have been classified as either T cell independent (TI) or T cell dependent (TD). TI antigens promote B cell proliferation, differentiation, and antibody production in the absence of T cells and are further classified into two subgroups: type I (TI-1) or type 2 (TI-2). TI-1 antigens are mitogens that stimulate all B cells to produce antibody in a polyclonal manner and irrespective of antigen specificity. Physiological TI-1 antigens include Toll-like receptor (TLR) ligands, such as LPS which is expressed by gram negative bacteria (Coutinho et al., 1974), or certain viral coat proteins (Berberian et al., 1993; Blutt et al., 2004). TI-2 antigens are instead composed of repetitive epitopes displayed on a backbone that simultaneously engage multiple antigen receptors on the surface of antigen-specific B cells. TI-2 antigens elicit robust IgM and IgG3 antibody production in a TI fashion, although the presence of noncognate T cell help promotes production of other IgG isotypes (Mongini et al., 1984). These TI antigens include polysaccharides found on encapsulated bacteria and highly organized viral capsid proteins such as those found on vesicular stomatitis virus and poliovirus (Bachmann et al., 1995; Bachmann and Zinkernagel, 1997; Fehr et al., 1998). In contrast to TI antigens, TD antigens are generally monomeric soluble proteins that display single or few epitopes to antigen-specific B cells and require cognate T cell help for induction of highly specific antibody responses generated through germinal center reactions.
Although not absolute, a general division of labor is also acknowledged between B cell subsets and the response to TI-2 and TD antigens. B1 and MZ B cell subpopulations have been considered to be primarily responsible for the antibody response to TI-2 antigens (Fagarasan and Honjo, 2000; Martin et al., 2001; Balázs et al., 2002), whereas FO B cells dominate antibody responses to soluble protein TD antigens. In accord with generating rapid antibody responses, MZ and B1 B cells have lower thresholds for activation compared with FO B cells and are physically poised at sites either in tissues or at the blood–lymphoid interface that facilitates these early responses (Martin and Kearney, 2002). B1 and MZ B cells are described as innate B cells in that they express a restricted repertoire of germline-encoded BCRs with polyreactive specificities (Bendelac et al., 2001). Responding MZ B cells produce antigen-specific antibody at extrafollicular splenic sites early during the antibody response that is low affinity and predominantly IgM but also includes limited IgG subclasses. Although evidence exists that MZ B cells can also mount TD responses and initiate germinal center reactions (Song and Cerny, 2003; Phan et al., 2005), the ability of FO B cells to directly participate in rapid extrafollicular TI-2 antibody responses is minor (Ron and Sprent, 1985; Goud et al., 1988; Liu et al., 1988).
Characterization of the TI-2 antibody response has predominantly relied on hapten-coupled carbohydrates as model antigens. However, physiological TI-2 antigens would rarely be encountered in isolation but, rather, are typically associated with pathogen-associated molecular patterns (PAMPs) recognized by pattern recognition receptors (PRRs; Janeway, 1989; Snapper, 2006). TLRs are one family of innate immune PRRs that have been shown to enhance the antibody response (Coutinho et al., 1974; Seppälä and Mäkelä, 1984; Sen et al., 2005; Heer et al., 2007; Rubtsov et al., 2008; Eckl-Dorna and Batista, 2009). However, whether this regulation is physiologically relevant has been controversial (Pasare and Medzhitov, 2005; Gavin et al., 2006).
A major consequence of signaling by many PRRs is the rapid elaboration of inflammatory type I IFN cytokines (Baccala et al., 2007; McCartney and Colonna, 2009), and many viral and bacterial infections lead to IFN-α/β production (Bogdan, 2000; Mancuso et al., 2009). Type I IFN has been previously characterized to modulate antibody production both in vitro and in vivo, including promoting class-switch recombination and polarizing antibody responses toward IgG2a/c production (Finkelman et al., 1991; Le Bon et al., 2001, 2006; Coro et al., 2006; Heer et al., 2007). How IFN-α/β acts to effect these changes in humoral immunity is not well established, nor is whether the type I IFN produced as a result of innate immune receptor signaling contributes to regulating the antibody response.
It has been proposed that in addition to their antigen-specific BCR, B cells may also use innate mechanisms for pathogen recognition. However, it is still unclear which of the innate receptors are used by B cells or what the consequence of these signals is to the B cell antibody response. The rapid TI-2 antibody response provides a temporal bridge between the early innate and late adaptive immune responses. Thus, we were interested to investigate how innate signals would augment the TI-2 antibody response and influence the different B cell populations during this response.
In this paper, we show that TLR agonists significantly enhance the TI-2 antibody response through the elaboration of type I IFN that promotes production of IgG2c by FO B cells. This contribution elicited by FO B cells is shown to accelerate the kinetics and enhance the magnitude and quality of the TI-2 antibody response. Thus, these data not only provide a mechanistic example of how innate immunity influences adaptive immunity but also define how humoral immunity counters physiological pathogens that present TI-2 antigens in the presence of ligands for innate immune receptors.
RESULTS
Poly(I:C) enhances the magnitude and kinetics of the TI-2 antibody response
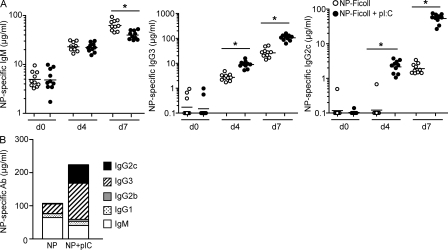
Poly(I:C) is a synthetic double-stranded RNA (dsRNA) that mimics viral nucleic acids and is recognized by several innate PRRs, including the TLR3 receptor. MZ B cells are considered to be largely responsible for the antibody response to TI-2 antigens (Martin et al., 2001) and express significantly higher levels of the TLR3 transcript than FO B cells (Genestier et al., 2007). Thus, we questioned how poly(I:C) might influence the TI-2 antibody response by immunizing mice with the model TI-2 antigen NP-Ficoll in the presence or absence of poly(I:C). These results demonstrated that poly(I:C) markedly enhanced the antigen-specific IgG3 and IgG2c antibody responses at both day 4 and day 7 after immunization (Fig. 1 A). In contrast, poly(I:C) did not alter the day-4 IgM response but instead promoted a significant decrease in the NP-specific IgM response of 1.6-fold at 7 d after immunization (Fig. 1 A).
Figure 1.
Poly(I:C) enhances the antibody response to a model TI-2 antigen. (A) C57BL/6 mice were immunized with 5 µg NP-Ficoll alone or 5 µg NP-Ficoll + 100 µg poly(I:C) and serum NP-specific IgM, IgG3, and IgG2c antibodies were measured in preimmune (day 0 [d0]), day 4, and day 7 after immunization sera. Symbols represent individual mice, and horizontal bars indicate geometric mean. Data were combined from two independent experiments with five mice per group per experiment. *, P ≤ 0.005. (B) Total serum NP-specific IgG2c, IgG3, IgG2b, IgG1, and IgM were measured 7 d after immunization with NP-Ficoll ± poly(I:C). Data represent geometric means calculated from data combined from two independent experiments, with five mice per group per experiment. Geometric means from NP-specific IgM, IgG3, and IgG2c responses were the same as those represented in A.
Overall, poly(I:C) led to a twofold increase in total antigen-specific Ig 7 d after immunization (Fig. 1 B). The composition of the TI-2 antigen-specific antibody response was skewed by poly(I:C) from an IgM-dominated (61.3%) to an IgG-dominated (81.6%) response (Fig. 1 B). However, poly(I:C) did not similarly enhance the magnitudes of all IgG subclasses but, rather, had the most striking effect on the NP-specific IgG2c response by accelerating the kinetics and increasing the magnitude of this isotype (Fig. 1, A and B). Relative to other IgG isotypes, IgG2c is the least abundant isotype produced during the NP-Ficoll response in the absence of poly(I:C) (Fig. 1 B; Mongini et al., 1981, 1984). Enhancement of the IgG2c response by poly(I:C) was dose dependent (Fig. S1 A) and, importantly, not a result of nonspecific antibody production by polyclonally activated B cells (Fig. S1, B and C). Poly(I:C) alone did not induce either NP-specific IgG2c or total antigen nonspecific IgG2c. These results were confirmed by measuring the total IgG2c levels after immunization with NP-Ficoll ± poly(I:C) (Fig. S1 C). Although poly(I:C) was found to increase total IgG2c serum levels on day 7, this increase was fully accounted for by the increase in NP-specific IgG2c at the same time point (Fig. 1 A). Together, these results negate a role for polyclonal IgG2c antibody production and verify that poly(I:C) enhances the TI-2 antibody response by an antigen-specific mechanism.
It is of note that IgG2c (IgG2a in Igha expressing mouse strains) is not only the predominant Ig isotype generated in antiviral antibody responses (Coutelier et al., 1987) but is also considered a highly effective antipathogen Ig isotype (Nimmerjahn and Ravetch, 2005, 2008). Thus, we restricted our subsequent focus to understanding the mechanism by which poly(I:C) altered the production of this IgG2c response.
Poly(I:C) augments the long-lived IgG2c TI-2 antibody response
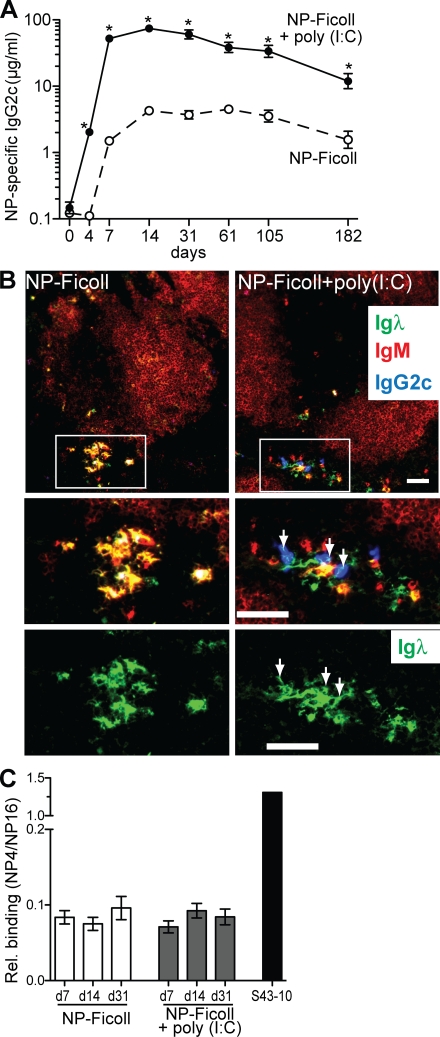
NP-Ficoll elicits relatively long-lived serum NP-specific antibody responses in the absence of adjuvants (García de Vinuesa et al., 1999; Obukhanych and Nussenzweig, 2006). We examined the adjuvant efficacy of poly(I:C) on this response and found that poly(I:C) significantly enhanced NP-specific IgG2c antibody levels at all time points evaluated (Fig. 2 A). However, although the levels of long-lived antigen-specific antibody were considerably increased, this did not result from an increased longevity of the antigen-specific IgG2c antibody response as the rate of decline of NP-specific IgG2c antibodies was similar regardless of poly(I:C) (Fig. 2 A).
Figure 2.
Poly(I:C) promotes a long-lived, extrafollicular, and low-affinity IgG2c antibody response to NP-Ficoll. (A) C57BL/6 mice were immunized with either NP-Ficoll alone or with poly(I:C) and serum NP-specific IgG2c antibodies measured before and at the indicated days after immunization. Data are expressed as the geometric mean ± SEM. Results shown are from one representative experiment of two independent experiments performed. *, P ≤ 0.002. (B) Histological spleen sections from C57BL/6 mice 7 d after immunization with NP-Ficoll or NP-Ficoll + poly(I:C). Top panels show sections stained for Igλ, IgM, and IgG2c. B cell follicles within the white pulp were revealed by IgM staining to provide orientation for the general lymphoid architecture. Top panels are 10× original magnification with enlarged areas within white outlined boxes represented in middle and bottom panels. Bottom panels depict only green Igλ+ ASCs and white arrows identify IgG2c ASCs. Bars, 50 µm. Data are representative of two independent experiments. (C) Relative affinity of NP-specific IgG2c antibodies was determined as the binding ratio of NP4/NP16 and was measured by ELISA. Sera samples were analyzed from mice at 7, 14, and 31 d after immunization with NP-Ficoll or NP-Ficoll + poly(I:C). Results were combined from two independent experiments with four to eight mice per immunization group. Data are expressed as the arithmetic mean ± SEM. The NP-specific IgG2c mAb S43-10 was used as a positive control for a high-affinity binding antibody.
Poly(I:C) facilitates an extrafollicular and low-affinity IgG2c antibody response to NP-Ficoll
TI-2 antigens typically elicit a rapid extrafollicular antibody response with limited isotype class switching and without somatic hypermutation (MacLennan et al., 2003). Given the rapid nature of the IgG2c antibody response induced by poly(I:C) to NP-Ficoll, we tested whether this would likewise be a low-affinity extrafollicular response.
The primary antibody response to NP-Ficoll is dominated by Igλ+ NP-specific antibodies (Tesch et al., 1984), thus we used Igλ expression as a surrogate for NP-specific ASCs (Claassen et al., 1986; Jacob et al., 1991; García de Vinuesa et al., 1999; Shih et al., 2002). NP-Ficoll alone elicited Igλ+ IgM–expressing ASCs localized to extrafollicular foci in the bridging channel of the red pulp (Fig. 2 B) as previously reported (Claassen et al., 1986; García de Vinuesa et al., 1999; Shih et al., 2002). NP-Ficoll + poly(I:C) induced not only extrafollicular Igλ+ IgM–expressing ASCs but also Igλ+ IgG2c–expressing ASCs within extrafollicular foci. Additionally, Igλ+ plasma cells that lacked IgM or IgG2c expression (Fig. 2 B) were also observed with poly(I:C) and are likely to be NP-specific IgG3-producing ASCs, as this isotype comprised a significant amount of the response (Fig. 1 B).
The relative affinity of poly(I:C)-induced NP-specific IgG2c antibodies was also measured and found to be comprised of relatively low-affinity antibodies, which is similar to that produced in response to NP-Ficoll alone (Fig. 2 C). In addition, this relative affinity did not increase with time indicating the absence of affinity maturation (Fig. 2 C). Thus, we show that poly(I:C) promotes a rapid IgG2c class-switched TI-2 antibody response characterized by both extrafollicular antibody secretion and low-affinity antibodies.
Both splenic and lymph node B cells participate in the poly(I:C)-elicited IgG2c antibody response to NP-Ficoll
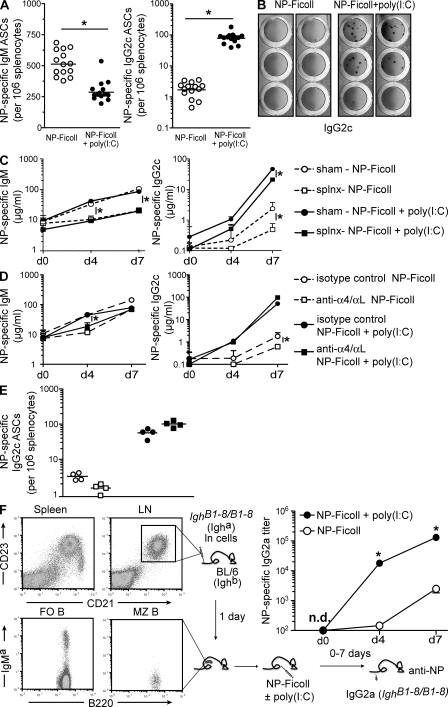
Measuring the frequency of splenic NP-specific IgG2c ASCs after immunization with poly(I:C) revealed a relative increase in the frequency of splenic NP-specific B cells that differentiated into IgG2c ASCs (Fig. 3 A). In accordance with antibody levels in serum (Fig. 1 A), poly(I:C) also promoted a concomitant decline in the frequency of splenic NP-specific IgM ASCs. Furthermore, no significant differences were measured in the frequency of either NP-specific IgG2c or IgM ASCs in the bone marrow of mice regardless of poly(I:C) immunization (unpublished data).
Figure 3.
Poly(I:C) elicits recruitment of FO B cells to participate in the enhanced IgG2c TI-2 antibody response. (A) Frequency of splenic NP-specific IgM and IgG2c ASCs as measured by ELISPOT 7 d after immunization with either NP-Ficoll alone or NP-Ficoll + poly(I:C). Each symbol represents an individual mouse and horizontal bars indicate either mean (IgM) or geometric mean (IgG2c). Data was combined from four independent experiments with three to five mice per group per experiment. *, P < 0.0001. (B) ELISPOT of NP-specific IgG2c ASCs in lymph nodes from C57BL/6 mice at 7 d after immunization with either NP-Ficoll alone or NP-Ficoll + poly(I:C). Lymph nodes were pooled from three immunized mice. Data was from two representative samples shown for each immunization condition. Data was from one experiment of two independent experiments performed. (C) Wild-type splenectomized or sham-operated mice were immunized with NP-Ficoll alone or NP-Ficoll + poly(I:C) and serum NP-specific IgM and IgG2c antibody measured at days 0, 4, and 7 after immunization. Data are expressed as the geometric mean + SEM. Results were combined from two independent experiments with four to six mice per group per experiment. *, P < 0.05. (D) C57BL/6 mice were treated with 100 µg each of anti-α4 + anti-αL antibodies or isotype control antibodies. 7 d after antibody injection, mice were immunized with either NP-Ficoll alone or NP-Ficoll + poly(I:C) and serum NP-specific IgM and IgG2c antibody responses measured at the indicated times. Data are expressed as the geometric mean + SEM. Results are from one experiment with four to five mice per group. *, P < 0.05. (E) Frequency of NP-specific IgG2c splenic ASCs measured from antibody-treated wild-type mice. Symbols are the same as those described in D and represent the frequency measured from individual animals. Horizontal bars indicate geometric mean. Results are from one experiment with four mice per group. (F) Representative flow cytometric analysis of CD21 and CD23 expression in spleen and lymph node of BL6-IghB1-8/B1-8 (Igha) mice. Total lymph node cells (5 × 107) were transferred to C57BL/6 (Ighb) mice and immunized 1 d later. The presence and phenotype of transferred Igha+ lymph node B cells within a splenic FO B cell gate (CD21int CD23high) and MZ B cell gate (CD21high CD23low) in one representative recipient 1 d after transfer is shown. Recipient mice were immunized with either NP-Ficoll alone or NP-Ficoll + poly(I:C) and NP-specific IgG2a antibodies measured in sera at the indicated time points. Antibody concentrations are shown as relative titers and data are expressed as the geometric mean ± SEM. Results shown from one representative experiment of two independent experiments performed, with four mice per group. n.d., not detected. *, P ≤ 0.0003.
Given the accelerated kinetics of the IgG2c response induced by poly(I:C), we initially considered that MZ B cells destined to produce IgM were subverted to class switch to IgG2c during this TI-2 response. However, antigen-specific IgG2c ASCs were also present in the lymph nodes of mice immunized with NP-Ficoll + poly(I:C) and were virtually absent in mice immunized with antigen alone (Fig. 3 B). As MZ B cells do not normally recirculate in rodents (Gray et al., 1982; Kumararatne et al., 1982), the presence of lymph node NP-specific IgG2c ASCs indicated that a non-MZ population contributed at least partially to the poly(I:C)-induced IgG2c response. Although the capacity of lymph node B cells to respond to TI-2 antigens has been shown (Ron and Sprent, 1985; Goud et al., 1988; Liu et al., 1988; Vinuesa et al., 2003), this response is limited with respect to other B cell subsets (Martin et al., 2001; Balázs et al., 2002). Thus, our data suggest that innate signals augment the TI-2 antibody response in part via enhanced production of antigen-specific IgG2c by lymph node B cells.
MZ B cells are not required for the IgG2c antibody responses to NP-Ficoll + poly(I:C)
B1 and MZ B cells are considered the major contributors to the TI-2 antibody response (Fagarasan and Honjo, 2000; Martin et al., 2001). However, with regards to NP-Ficoll in particular, MZ B cells have been shown to be predominantly responsible for the TI-2 antibody response (Otipoby et al., 1996; Guinamard et al., 2000; Samardzic et al., 2002; Shih et al., 2002). Because MZ B cells are restricted to the spleen in rodents, we addressed whether MZ B cells contributed to the poly(I:C)-induced IgG2c response by splenectomy and immunization with NP-Ficoll ± poly(I:C). As previously reported (Amlot et al., 1985), splenectomized mice mounted significantly reduced NP-specific IgM responses at days 4 and 7 after immunization with NP-Ficoll compared with sham-operated animals and irrespective of poly(I:C) (Fig. 3 C). Likewise, at day 7 the antigen-specific IgG2c response to NP-Ficoll alone was diminished approximately sevenfold in splenectomized mice (Fig. 3 C). However, in the presence of poly(I:C) there was only a twofold reduction in the NP-specific IgG2c response measured in asplenic mice as compared with controls (Fig. 3 C). These data confirm MZ B cells are largely responsible for the NP-Ficoll IgM response and further show this is true in the presence of poly(I:C). Conversely, the small IgM and IgG2c antibody responses generated in asplenic mice highlight a minor role for FO B cells in the NP-Ficoll TI-2 response (Ron and Sprent, 1985; Goud et al., 1988; Liu et al., 1988; Vinuesa et al., 2003). Collectively, our findings suggest that in the presence of poly(I:C), a B cell population distinct from MZ B cells is the predominant source of NP-specific IgG2c.
To provide further support for this notion, we used a second model in which the MZ B cell population was selectively depleted by in vivo treatment with anti-integrin (α4/αL) antibodies (Lu and Cyster, 2002) while FO B cells were left intact (Belperron et al., 2005, 2007). MZ B cells are retained within the MZ by integrin adhesion and are released into circulation by administration of anti-integrin mAbs (Fig. S2, A and B; Lu and Cyster, 2002). Consistent with the splenectomy data, depletion of MZ B cells led to a large reduction in the NP-specific IgM response to NP-Ficoll ± poly(I:C) at 4 d after immunization as compared with controls (Fig. 3 D). Although a similar reduction in NP-specific IgM was not observed at day 7 after immunization, this time point coincided with the return of MZ B cells to the spleen after integrin treatment and MZ B cell depletion (Fig. S2). In contrast, mice depleted of MZ B cells generated equivalent poly(I:C)-elicited NP-specific IgG2c responses at all time points evaluated (Fig. 3 D). Although these results do not formally eliminate a role for MZ B cells in the poly(I:C)-induced IgG2c response, they illustrate that MZ B cells do not make a significant contribution to this response.
It remained possible that MZ B cells relocated outside the spleen but were still responsive to poly(I:C) and able to generate NP-specific IgG2c from this extrasplenic site. However, this was excluded as the frequency of splenic NP-specific IgG2c ASCs generated upon immunization with NP-Ficoll + poly(I:C) was the same regardless of MZ B cell depletion (Fig. 3 E). These findings strongly suggest that FO B cells are the predominant source of the poly(I:C)-induced IgG2c antibody response to the TI-2 antigen NP-Ficoll.
Poly(I:C) promotes FO B cells to participate in the IgG2c class-switched TI-2 antibody response
Our findings indicated that FO B cells contributed significantly to the poly(I:C)-induced NP-Ficoll response, although the capacity of lymph node B cells to contribute to the TI-2 antibody response has been considered minimal (Ron and Sprent, 1985; Goud et al., 1988; Liu et al., 1988). To directly examine if FO B cells mounted poly(I:C)-induced IgG2c antibody responses to NP-Ficoll in vivo, we adoptively transferred lymph node cells from IghB1-8/B1-8 (Igha) mice (Sonoda et al., 1997) into recipients and immediately assessed their ability to respond to NP-Ficoll ± poly(I:C). The B cells within the lymph node were phenotypically homogenous as CD21int CD23high FO B cells and maintained this phenotype after adoptive transfer (Fig. 3 F). The B1-8 IgH chain confers NP-specificity when paired with an Igλ light chain (Jack et al., 1977; Reth et al., 1978; Bothwell et al., 1981), effectively increasing the NP-specific cells to ∼5% of the B cell population (unpublished data). Before immunization, the adoptively transferred FO B cells comprised ∼2.5% of the total splenic B cell compartment, and thus ∼0.1% of the B cells in the recipient were IgMa (B1-8) and NP specific (unpublished data).
After immunization with NP-Ficoll alone, adoptively transferred FO B cells generated a relatively weak and delayed NP-specific IgG2a response (Fig. 3 F). As predicted, poly(I:C) markedly enhanced both the magnitude and kinetics of the NP-specific IgG2a response produced from FO B cells in vivo (Fig. 3 F) and similar to that elicited by poly(I:C) in wild-type mice (Fig. 1 A). Similar findings were found when fivefold fewer (107) LN cells were transferred (unpublished data). These findings directly demonstrate that FO B cells are able to generate IgG2c/a antibody responses to a TI-2 antigen and, more importantly, show that this response is both accelerated and amplified by poly(I:C). Collectively, these results reveal that FO B cells provide a rapid and robust innate-like IgG2c antibody response to a TI-2 antigen elicited by poly(I:C).
Poly(I:C)-induced NP-specific IgG2c does not require TLR3 signaling by B cells
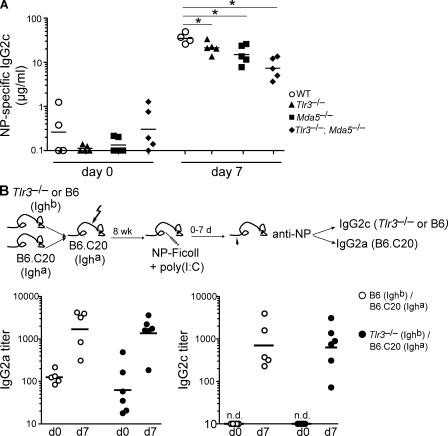
As a dsRNA analogue, poly(I:C) is recognized by both TLR3 and MDA5 PRRs in vivo (Alexopoulou et al., 2001; Gitlin et al., 2006; Kato et al., 2006). To assess the in vivo contribution of these PRRs in the poly(I:C)-induced IgG2c antibody response, Tlr3−/− and Mda5−/− single and compound mutants were immunized with NP-Ficoll + poly(I:C). These results revealed modest but significant reductions in NP-specific IgG2c in the absence of either TLR3 or MDA5 that were additive in Tlr3−/− Mda5−/− double mutants (Fig. 4 A). The combined reduction observed in the double-deficient mice implicates both TLR3 and MDA5 in the recognition of poly(I:C) and subsequent induction of a class-switched IgG2c B cell response.
Figure 4.
The TI-2 IgG2c response induced by poly(I:C) is not mediated by B cell–intrinsic TLR3 signaling. (A) NP-specific IgG2c responses in wild type, Tlr3−/−, Mda5−/−, and Tlr3−/− Mda5−/− mice measured from sera collected preimmunization (day 0) and at 7 d after NP-Ficoll + poly(I:C) immunization. Symbols represent individual mice, and horizontal bars indicate geometric mean. Shown is one representative of two independent experiments performed, with four to five mice per group. *, P < 0.05. (B) NP-specific IgG2c/a antibody responses in mixed bone marrow chimeras 7 d after immunization with NP-Ficoll + poly(I:C). A mixture of bone marrow cells from B6.C20 (Igha) and Tlr3−/− (Ighb; experimental chimeras; filled) or B6.C20 and wild-type C57BL/6 (Ighb; control chimeras; open) was adoptively transferred to lethally irradiated B6.C20 hosts. 8 wk after reconstitution, chimeras were immunized and NP-specific antibody measured in preimmune and day-7 sera. Reconstitution frequencies of IgMa- and IgMb-expressing B cells were measured as described in the Materials and methods and found to be equivalent between both experimental and control chimeras. Symbols represent individual chimeric mice, and horizontal bars indicate geometric mean. Data were combined from two independent experiments with five to six mice per group. n.d., not detected.
The requirement for direct TLR signaling by B cells during antibody responses has been the subject of recent debate (Pasare and Medzhitov, 2005; Gavin et al., 2006; Barr et al., 2007, 2009; Jegerlehner et al., 2007; Meyer-Bahlburg et al., 2007). Thus, we next questioned whether B cell–autonomous TLR3 signaling was required for the poly(I:C)-induced NP-Ficoll IgG2c response. To address this, we generated mixed bone marrow chimeras whereby wild-type (B6.C20-Igha) and either Tlr3−/− or Tlr3+/+ (Ighb) B cells were juxtaposed in direct competition with each other in recipient mice, and we measured the capacity of both populations to generate an IgG2a/c antibody response to NP-Ficoll + poly(I:C) (Fig. 4 B). In these chimeras, TLR3−/− (Ighb) B cells mounted equivalent NP-specific IgG2c responses upon immunization with NP-Ficoll + poly(I:C) as compared with wild-type (C57BL/6-Ighb) B cells in control chimeras (Fig. 4 B). These results demonstrate that direct TLR3 signaling by B cells is not required for poly(I:C)-induced TI-2 IgG2c responses. Furthermore, these findings suggest that downstream secondary innate cytokine signals induced by PRR signaling may suffice in promoting B cell IgG2c antibody responses.
Type I IFN mediates poly(I:C)-induced antigen-specific IgG2c during the TI-2 antibody response
Both TLR3 and MDA5 dsRNA-sensing receptors are capable of recognizing poly(I:C) to induce a rapid type I IFN response (Alexopoulou et al., 2001; Gitlin et al., 2006; Kato et al., 2006; Longhi et al., 2009). Given the accelerated IgG2c response to poly(I:C), we assessed the contribution of type I IFN to this response by immunizing Ifnar1−/− mice. These results showed that the NP-specific IgG2c response to poly(I:C) immunization was reduced ∼90% in Ifnar1−/− mice compared with wild type (Fig. 5 A) and inversely mirrored by a modest but significant increase in the NP-specific IgM response over wild-type controls (Fig. 5 A). These findings demonstrate that type I IFN signaling is necessary for efficient induction of the TI-2 IgG2c antibody response promoted by poly(I:C).
Figure 5.
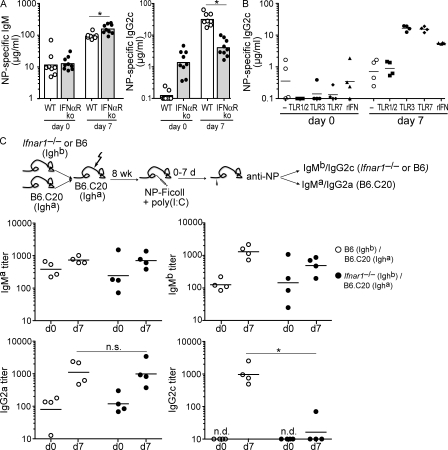
Poly(I:C) induction of IgG2c requires type I IFN receptor signaling by B cells. (A) NP-specific IgM and IgG2c responses in wild-type and Ifnar1−/− mice measured from sera collected at days 0 and 7 after immunization with NP-Ficoll + poly(I:C). Symbols represent individual mice, and bars represent geometric means. Data were combined from two independent experiments with three to five mice per group per experiment. *, P ≤ 0.005. (B) C57BL/6 mice were immunized with 5 µg NP-Ficoll alone or in the presence of 100 µg Pam3CSK4 (TLR1/2), 100 µg poly(I:C) (TLR3), 100 µg 3M-012 (TLR7), or 8 × 105 U rIFN-α11. NP-specific IgG2c responses were measured from sera collected preimmune (day 0) and at 7 d after immunization. Symbols represent individual mice, and horizontal bars indicate geometric mean. Shown is one representative of two independent experiments performed, with four mice per group. (C) Mixed bone marrow chimeras were generated as described in Fig. 2 B, except that experimental chimeras were generated from the transfer of Ifnar1−/− (Ighb) + B6.C20 (Igha) bone marrow. After reconstitution, chimeric mice were immunized with NP-Ficoll + poly(I:C) and NP-specific IgMa, IgMb, IgG2a, and IgG2c serum antibody responses measured preimmune (day 0) and at 7 d after immunization. Symbols represent individual mice, and horizontal bars indicate geometric mean. Representative data are from one experiment shown of two independent experiments performed, with four mice per group. n.d., not detected. *, P < 0.05.
Type I IFN is an early hallmark of the antiviral innate immune response initiated from the recognition of viral PAMPs. Thus, we predicted that the instruction to class switch to IgG2c during the TI-2 antibody response would be a general property of all PAMPs capable of stimulating endogenous type I IFN production. To test this, we immunized mice with a representative group of TLR agonists known to stimulate type I IFN (TLR7 agonist, 3M-012; Gorden et al., 2005) or not (TLR1/2 agonist, Pam3CSK4; Longhi et al., 2009) and measured the IgG2c antibody response elicited to NP-Ficoll. As predicted, mice immunized with a type I IFN-inducing TLR7 agonist produced NP-specific IgG2c antibody responses similar to that induced by poly(I:C) (Fig. 5 B). Conversely, the Pam3CSK4 TLR1/2 agonist did not promote NP-specific IgG2c above levels seen with antigen alone (Fig. 5 B). These findings indicate that type I IFN-inducing adjuvants promote class-switching to IgG2c during the TI-2 antibody response. Furthermore, these results are consistent with results showing that type I IFN augments particular isotypes during the B cell antibody response to soluble protein antigens and viruses (Finkelman et al., 1991; Le Bon et al., 2001; Coro et al., 2006; Heer et al., 2007).
We next considered whether we could bypass the requirement for signaling through PRRs altogether by directly providing exogenous type I IFN during immunization. Mice immunized with NP-Ficoll and a single administration of rIFN-α were found to mount significantly higher levels of NP-specific IgG2c than NP-Ficoll alone (Fig. 5 B). Although the magnitude of the IgG2c response elicited by rIFN-α immunization was reduced compared with poly(I:C) immunization, it was nevertheless notable that a single treatment induced a considerable IgG2c antibody response after 1 wk.
Type I IFN receptor expression by B cells is required for the IgG2c TI-2 antibody response
To address whether expression of the type I IFN receptor was required by B cells for the poly(I:C)-induced IgG2c TI-2 response antibody response, Ifnar1−/− B cells were evaluated in mixed bone marrow chimeras in direct competition with wild-type B cells. Antibody responses by Ifnar1−/− (Ighb) B cells or wild-type B6 (Ighb) B cells were measured as NP-specific IgMb and IgG2c, whereas competing wild-type (B6.C20-Igha) responses in both experimental and control chimeras were detected as NP-specific IgMa and IgG2a (Fig. 5 C).
Upon immunization with NP-Ficoll + poly(I:C), wild-type (B6-Ighb) B cells in control chimeras mounted significant NP-specific IgMb and IgG2c antibody responses (Fig. 5 C). As predicted from immunization of IFNαR1−/− mice (Fig. 5 A), Ifnar1−/− (Ighb) B cells also produced an NP-specific IgMb antibody response (Fig. 5 C). In contrast, Ifnar1−/− (Ighb) B cells did not generate any significant NP-specific IgG2c antibody (Fig. 5 C). Moreover, wild-type (B6.C20-Igha) B cells in competition with IgMb-expressing B cells in both sets of chimeras made comparable NP-specific IgMa and IgG2a antibody responses (Fig. 5 C), further eliminating a role for non–B cell hematopoietic populations and indicating a B cell–autonomous defect. This impaired ability of Ifnar1−/− B cells to class switch to IgG2c was not a result of abnormal B cell development, as Ifnar1−/− mice have normal compositions of mature splenic B cell compartments (Le Bon et al., 2006; Green et al., 2009).
These data show that Ifnar1−/− B cells contribute normally to TI-2 IgM responses but that in the presence of type I IFN–inducing PAMPs such as poly(I:C), they are unable to generate antigen-specific IgG2c TI-2 antibody response. This establishes an in vivo role for type I IFN receptor signaling by FO B cells in directly promoting the production of the antiviral IgG2c isotype and, thus, enhancing the B cell antibody response.
IFN-α acts selectively on BCR-activated FO B cells to increase CD69 expression
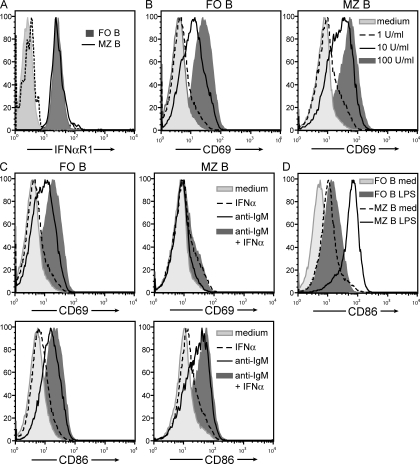
FO and MZ B cells express similar levels of the type I IFN receptor (Fig. 6 A), excluding differential receptor expression as an origin for the dominant FO B cell response to type I IFN-promoting adjuvants. Thus, we predicted that type I IFN differentially influenced FO B cells relative to MZ B cells.
Figure 6.
IFN-α induces BCR-stimulated FO B cells to up-regulate CD69 to a greater extent than MZ B cells. (A) Histograms represent flow cytometric analysis of IFNαR1 surface expression on CD21int CD23high FO B cells and CD21high CD23low MZ B cells isolated from naive wild-type mice. Isotype control antibody staining for both FO (shaded light gray) and MZ (dashed line) B cells is provided. Data are representative from two independent experiments. (B–D) FO and MZ B cells were sorted to ≥94% purity from naive C57BL/6 mice and separately incubated with the indicated stimuli in vitro for 6 or 10 h. After stimulation, surface expression of CD69 (6 h) or CD86 (10 h) was subsequently assessed in both B cell populations by flow cytometry and data depicted as histograms. The following stimuli and doses were used: (B) rIFN-α at 1, 10, or 100 U/ml; (C) rIFN-α at 1 U/ml and anti-IgM F(ab′)2 at 10 µg/ml; (D) LPS at 10 µg/ml. Med, medium. Data are representative from at least two independent experiments.
Type I IFN can directly activate B cells in vitro (Morikawa et al., 1987; Braun et al., 2002; Coro et al., 2006; Kamphuis et al., 2006; Shiow et al., 2006) and in vivo (Chang et al., 2007; Coro et al., 2006; Shiow et al., 2006). Thus, we assessed the ability of IFN-α to activate either FO or MZ B cells and found that both populations displayed similar sensitivity and kinetics to recombinant IFN as measured by induction of surface expression of CD69 and CD86 activation antigens (Fig. 6 B and not depicted).
We next questioned whether FO and MZ B cells might differ in their response to IFN-α stimulation in the context of BCR-mediated activation similar to the situation during an antibody response. Concomitant treatment of FO B cells with anti-IgM and suboptimal levels of IFN-α led to CD69 expression to levels greater than those detected with anti-IgM stimulation alone (Fig. 6 C). In contrast, anti-IgM treatment of purified MZ B cells failed to increase CD69 expression to any appreciable level and, importantly, this expression was not enhanced by concomitant IFN-α treatment (Fig. 6 C). The inability of anti-IgM to induce CD69 expression on MZ B cells was not true of all activation markers as anti-IgM ± IFN-α increased CD86 expression similar to FO B cells (Fig. 6 C). Furthermore, MZ B cells exhibited increased sensitivity to LPS activation as measured by CD86 expression (Fig. 6 D) as previously shown (Oliver et al., 1999).
Overall, these data demonstrate that IFN-α selectively acts on BCR-stimulated FO, but not MZ, B cells to elevate CD69 surface expression. Type I IFN-mediated surface expression of CD69 is inversely correlated with S1P1 chemoattractant receptor responsiveness (Shiow et al., 2006), suggesting that selective CD69 expression by antigen-experienced FO B cells could alter the trafficking of these cells during a response.
DISCUSSION
Study of B cell antibody response to hapten model antigens over the last several decades has provided considerable insight into how humoral immunity deals with different types of antigens. In particular, the reductionist nature of these model antigens facilitated our understanding of how different B cell subpopulations respond to distinct antigens. However, it is now evident that extrapolating from these studies is limited with respect to modeling humoral immunity to physiological pathogens. Pathogens such as viruses and bacteria not only present TI and TD antigens to B cells but also display a variety of PAMPs capable of alerting the innate immune system. This study addresses how these PAMPs influence humoral immunity to TI-2 antigens.
The most dramatic changes elicited by poly(I:C) to the TI-2 antibody response were measured in the acceleration of the kinetics and overall increase in the magnitude of the antigen-specific IgG2c response. Heightened production of IgG2c is significant, as this isotype is the most efficient IgG subclass for anti-pathogen FcR-mediated effector functions (Nimmerjahn and Ravetch, 2005) and has long been recognized as the predominant isotype elicited by viral infections (Coutelier et al., 1987). Thus, these findings support the notion that PAMPs polarize antibody responses to those isotypes best suited to deal with particular pathogens (Janeway, 1989). We further show that during the TI-2 antibody response, poly(I:C) promotes a persistence of elevated levels of antigen-specific IgG2c serum antibody, which are similar to those previously shown for TD antibody responses (Le Bon et al., 2001). The durability of this enhanced response has implications for vaccine design given the importance of neutralizing antibody concentration in providing efficacious antiviral protective immunity (Bachmann and Zinkernagel, 1997; Bachmann et al., 1997; Pulendran, 2009).
Generally, pathogen-specific antibody responses divert from characterized hapten-specific responses, as they are typically faster and elicit IgG isotypes earlier than anti-hapten responses (Bachmann and Zinkernagel, 1997). We show that including a type I IFN-inducing PRR agonist with the NP-Ficoll TI-2 antigen results in a rapid anti-hapten IgG2c response. This class-switched anti-hapten response more closely reflects previously characterized antiviral and antimicrobial antibody responses with regard to kinetics, isotype specificity, and extrafollicular antibody production, illustrating the applicability of these studies to model pathogens. For instance, among the many arms of the influenza antibody response, a localized IgG2c extrafollicular B cell antibody response is detectable within a few days in mediastinal lymph nodes after intranasal infection (Coro et al., 2006). Similarly, vesicular stomatitis virus has been demonstrated to have a highly organized antigenic capsid capable of eliciting TI antibody responses (Bachmann et al., 1995) while simultaneously inducing an early isotype-switched antibody response (Bachmann et al., 1996). These early class-switched responses are not exclusive to antiviral responses, as Salmonella typhimurium likewise promotes an extrafollicular IgG2c switched plasma cell response detectable 4 d after infection (Cunningham et al., 2007). Thus, the common feature of this rapid and highly efficient IgG subclass elicited to both major anti-pathogen responses further highlights the physiological and biological relevance of our findings.
Mature naive murine B cells constitutively express transcripts for the majority of TLRs (Barr et al., 2007; Genestier et al., 2007; Gururajan et al., 2007), and mature B cells can respond to TLR3 signaling in vitro (Alexopoulou et al., 2001). The requirement for direct TLR signaling by B cells during antibody responses has been controversial. In particular, previous studies have demonstrated a B cell–intrinsic requirement for TLR signaling in the generation of B cell antibody responses (Pasare and Medzhitov, 2005; Jegerlehner et al., 2007). Conversely, studies have also provided evidence that disregards TLR signaling for initiating antibody responses and supporting an instructive role for TLRs in augmenting antibody isotypes (Gavin et al., 2006). Our results demonstrate a minor role for TLR3 signaling in optimal induction of IgG2c by poly(I:C), supporting previous studies showing that TLR signaling is largely unnecessary for efficient antibody responses (Gavin et al., 2006). However, the ability of Tlr3−/− B cells to mount normal antibody responses in reconstituted hosts clearly negates a role for B cell–intrinsic TLR3 signaling in IgG2c induction by poly(I:C). Discrepancies observed in the role of B cell–intrinsic TLR signaling may be explained by a lack of direct conjugation of the TLR agonist with antigen, whereby conjugation would allow for the TLR agonist to be directed through BCR-mediated internalization to responding B cells for concomitant TLR signaling (Eckl-Dorna and Batista, 2009). Our data do not exclude whether autonomous B cell expression of other PRRs, such as MDA5, is required for the IgG2c TI-2 antibody response. Whether B cell–intrinsic RIG-I–like helicase signaling may affect antibody responses remains unclear but would be interesting to address with a TLR3−/− MDA5−/− B cell chimeric model similar to that used here.
Our findings indicate that poly(I:C) is recognized predominantly by TLR3 and MDA5 for subsequent indirect induction of the TI-2 IgG2c antibody response through type I IFN. However, we note that in Tlr3−/− Mda5−/− double knockout mice, poly(I:C) remains able to induce NP-specific IgG2c but is ∼80% reduced compared with wild type (Fig. 4 A), suggesting residual type I IFN induction. In accordance with this, it has recently been reported that poly(I:C) induction of CD4 T cell responses is incompletely abolished in TLR3−/− MDA5−/− double knockout chimeric mice (Longhi et al., 2009), which is similar to our findings with the B cell antibody response. As RIG-I is also a cytosolic PRR capable of recognizing low-molecular-wt poly(I:C) to signal type I IFN production (Kato et al., 2008), we envision that this dsRNA sensor is likely responsible for the type I IFN that promotes a modest increase in NP-specific IgG2c in the double mutants. Ultimately, our data demonstrate that non–TLR-innate signals, such as type I IFN, can substitute for TLR sensing of non-self to augment antibody responses. This redundancy in the recognition of poly(I:C) by multiple dsRNA-sensing receptors highlights the importance of IFN-α/β as a global non-self warning signal for enhancing the efficiency of anti-pathogen antibody responses. Indeed, it has been noted that all vertebrates examined have immune systems comprised by T and B lymphocytes and genes encoding IFN-α and IFN-β (Stetson and Medzhitov, 2006), suggesting that the type I IFN-mediated innate response is closely associated with adaptive immunity.
For humoral immunity, type I IFN elaborated during antiviral responses promotes IgG2a/c antibody production in vivo (Finkelman et al., 1991; Heer et al., 2007). However, the role for B cell–intrinsic signaling by type I IFN to directly instruct IgG2c class switching in vivo is unclear. Specifically, signaling through the IFN-αR expressed by B and T cells has been shown to enhance IFN-α–mediated IgM and IgG responses to soluble proteins (Le Bon et al., 2006). In opposition, direct IFN-αR signaling by B cells has been found to be unnecessary for the antiviral IgG2c response during acute influenza infection (Coro et al., 2006). Using a chimeric B cell competition model, we demonstrate that B cell–autonomous IFN-αR signaling does induce IgG2a/c switching in vivo during systemic type I IFN responses, such as that elicited by poly(I:C). The discordance between our findings and other studies likely illustrates the complexity of additional inflammatory cytokines, beyond type I IFN, that are elicited during viral infections and that also regulate the instruction to switch between Ig subclasses (Coro et al., 2006).
A potential mechanism to account for the type I IFN-induced response from FO B cells is suggested by the ability of type I IFN to modulate CD69 expression on FO B cells. It has been shown that IFN-induced CD69 surface expression inhibits lymphocyte egress from lymphoid organs by the concomitant loss of sphingosine-1-phosphate responsiveness (Shiow et al., 2006). In accord with this, virus-induced IFN-α/β also causes accumulation of circulating FO B cells to regional lymph nodes early during antiviral responses (Chang et al., 2007). The enhanced response by FO B cells to up-regulate CD69 in response to IFN-α and BCR-mediated activation would presumably ensure that these normally recirculating B cells would be retained within secondary lymphoid organs where they can receive further noncognate secondary signals necessary for TI-2 antibody responses (Chang et al., 2007). In addition, as TI-2 antigens would likely be associated with TD antigens within a pathogen, the loss of S1P1 responsiveness would also be expected to direct TI-2 antigen-activated B cells to the T–B cell border where associated TD antigens could be presented to antigen-specific T cells.
Protective humoral immunity to pathogens is contributed by distinct B cell subsets with unique activation requirements and response patterns. Studies from dissected model pathogens, such as S. pneumoniae and influenza, have taught us that multifaceted protective humoral immune responses are comprised by a collection of antibodies derived from distinct B cell subsets (Baumgarth et al., 1999, 2008; Snapper, 2006). Features that dictate which B cell subset responds have been characterized, including the intrinsic responsiveness of each B cell subset and the biochemical properties of the antigens. Yet, it is still not understood how innate signals encountered during these infections dictate which B cell subset participates in each facet of the antibody response. The TI-2 response to NP-Ficoll has been characterized by the domination of IgM and IgG3 isotypes derived from MZ B cells (Otipoby et al., 1996; Guinamard et al., 2000; Samardzic et al., 2002; Shih et al., 2002). We demonstrate in this paper that in the presence of type I IFN innate signals, FO B cells are rapidly promoted to participate in a switched TI-2 antibody response through B cell–intrinsic IFN-α/β signaling that leads to antigen-specific IgG2c antibody production. Whether type I IFN recruits additional FO B cells to respond to a TI-2 antigen or promotes enhanced clonal expansion of responding antigen-specific FO B cells remains to be established.
Rapid low-affinity extrafollicular innate-like antibody responses are crucial to providing early acquired immunity against pathogens. This study provides the first direct evidence for an innate-like switched FO B cell antibody response to a TI-2 antigen. The participation by FO B cells to this switched TI-2 response was unexpected given the long-standing model that FO B cells are a slower responding B cell subset and predominately produce higher affinity post-germinal center antibodies during TD responses (Martin and Kearney, 2002; McHeyzer-Williams, 2003). Economically, one rationale behind the enhanced contribution of FO B cells to providing the IFN-α/β–induced IgG2c response, as opposed to the subset which produces the TI-2 IgM response, is that this division of labor allows for IgG2c induction but not at the expense of the crucial initial IgM response. Furthermore, this division of labor illustrates the importance of both arms of the anti-pathogen humoral response: a rapid IgM response to provide antibody capable of neutralizing and controlling the infection and a robust and highly efficient IgG2c response effective at specialized FcR-mediated clearance (Nimmerjahn and Ravetch, 2005, 2008). These findings propose a novel role for FO B cells in responding to innate signals in vivo to participate in innate-like antibody responses. Future studies will be required to address whether a discreet subset of innate FO B cells exist that solely have the capacity to respond in this manner or whether this phenomenon is a general characteristic of all FO B cells recruited in response to particular innate signals.
MATERIALS AND METHODS
Mice.
C57BL/6 mice (The Jackson Laboratory) were used directly or bred and maintained within the Biological Resource Center at National Jewish Health (NJH; Denver, CO). C57BL/6-IghB1-8/B1-8;Igha mice (Sonoda et al., 1997; gift from K. Rajewsky, Harvard University, Boston, MA) and B6.C20 mice (C57BL/6 mice congenic for Igha; gift from L. Herzenberg, Stanford University, Palo Alto, CA) were both bred and maintained within the Biological Resource Center at NJH. Ifnar1−/− mice (Müller et al., 1994) on the C57BL/6 genetic background were provided by P. Marrack (NJH, Denver, CO). MDA5−/− (Gitlin et al., 2006), Tlr3−/−, and Mda5−/−, Tlr3−/− mice on C57BL/6 genetic backgrounds were maintained at Washington University (St. Louis, MO). Tlr3−/− mice (Alexopoulou et al., 2001) on the C57BL/6 background were provided by R. Flavell (Yale University, New Haven, CT) and also bred and housed within the NJH Biological Resource Center. Animals were used between 8 and 12 wk of age, unless otherwise specified. All experiments were performed in accordance with NJH and Washington University Institutional Animal Care and Use Committees.
Flow cytometric analyses and antibodies.
Spleen and lymph node cells were harvested and stained according to standard protocols. Fluorescently labeled mAbs against the following mouse surface antigens were used for staining and either purchased or isolated and conjugated in our laboratory: B220 (RA3-6B2; BD and eBioscience), IgM (R33-24.12; hybridoma), IgMb (AF6-78; hybridoma), CD21 (7G6; hybridoma), CD19 (1D3; hybridoma and BD), CD1d (1B1; eBioscience), CD69 (H1.2F3; eBioscience), CD86 (GL1; eBioscience), CD23 (B3B4; BD), TCR-β (H57-597; BD), IgHB1-8 (anti-idiotype; Ac146; hybridoma; Reth et al., 1979), IgMa (DS-1; BD), Igλ (JC5-1; SouthernBiotech), IgD (11-26c; BD), CD11a (2D7; BD), IFNαR1 (MAR1-5A3; BioLegend), and Mouse IgG1,κ isotype control (MOPC-21; BD). For detection of NP-specific B cells by flow cytometry, cells were stained with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated to Phycoerythrin (NP40–PE; Biosearch Technologies) after standard flow cytometric staining protocol. The secondary detection reagent for detecting biotin-conjugated antibodies by flow cytometry was streptavidin-PE (eBioscience). Flow cytometric analyses were performed by acquiring data on either a FACSCalibur (BD) or a BD LSRII (BD) and with FlowJo v8.8.6 software (Tree Star, Inc.).
Immunizations.
Mice were immunized i.p. with 5 µg NP (4-Hydroxy-3-nitrophenylacetic) hapten conjugated to NP–Ficoll (Amino-Ethyl-Carboxy-Methyl-FICOLL) at NP32–, NP41–, or NP50–Ficoll valencies (Biosearch Technologies). Unless otherwise specified, the following doses of adjuvants or cytokines were included with NP-Ficoll in immunizations as detailed: 100 µg poly(I:C) (polyinosine-polycytidylic acid; InvivoGen), 100 µg Pam3CSK4 (N-Palmitoyl-S-[2,3-bis(palmitoyloxy)-(2, RS)-propyl]-[R]-Cys-[S]-Ser-[S]-Lys4; InvivoGen), 100 µg 3M-012 (33080; TLR7 agonist; 3M Pharmaceuticals; gift from R. Kedl, NJH), or 8 × 105 U recombinant mouse IFN-α11 (Genbank accession no. AY225954; gift from R. Kedl, NJH). Antigens, adjuvants, and cytokines were either purchased as endotoxin free (≤0.125 EU/mg) or tested by an endpoint chromogenic Limulus Amebocyte Lysate (LAL) assay (Cambrex) and found to be at levels ≤0.1 EU per mouse for immunization.
NP-specific ELISAs.
To measure NP-specific serum antibody responses, 96-well flat-bottom MaxiSorp MicroWell plates (Thermo Fisher Scientific) were coated overnight with 2 µg/ml (4-Hydroxy-3-iodo-5-nitrophenylacetyl) NIP15–BSA (Biosearch Technologies) diluted in PBS at 4°C. Plates were washed once (PBS, 0.1% Tween 20; Thermo Fisher Scientific), blocked (PBS, 1% BSA, and 0.05% NaN3) for a minimum of 2 h at 37°C, and washed once again. For capture of antigen-specific antibodies, sera were initially diluted 1:20 and subsequent threefold serial dilutions were made into blocking buffer and incubated overnight at 4°C. Plates were washed three times before incubation with an alkaline phosphatase (AP)–conjugated goat anti–mouse isotype-specific detection antibody (SouthernBiotech) for 1 h at 37°C. After three washes, plates were developed by the addition of alkaline phosphatase substrate buffer consisting of 1 mg/ml 4-Nitrophenyl phosphate disodium salt hexahydrate (Alkaline Phosphatase Substrate; Sigma-Aldrich) diluted in 1 M diethanolamine, 8.4 mM MgCl2, and 0.02% NaN3, pH 9.8, and absorbance values read at 405 nm (VersaMax ELISA reader; MDS Analytical Technologies). The following NP-specific mouse mAbs were used as standards to quantify the absolute concentration of NP-specific antibody present in the sera of immunized mice: B1-8 μ (NP-specific IgM; Reth et al., 1978), S24/63/63 (NP-specific IgG3; Baumhäckel et al., 1982), N1G9 (NP-specific IgG1; Cumano and Rajewsky, 1985), S43-10 (NP-specific IgG2c; Reth et al., 1978), and D3-13F1 (NP-specific IgG2b; gift from K. Rajewsky). In the cases where a standard was unavailable, the titer at an OD50 was determined from the serial dilution curves for each sample.
For both adoptive transfer and mixed bone marrow chimera analyses, detection of NP-specific IgG2a or IgG2c serum antibodies by ELISA was accomplished with AP-conjugated AffiniPure goat anti–mouse IgG, Fcγ Subclass 2a–specific (Jackson ImmunoResearch Laboratories), or AP-conjugated AffiniPure goat anti–mouse IgG, Fcγ Subclass 2c–specific (Jackson ImmunoResearch) antibodies, respectively. The detection of NP-specific IgMa or IgMb serum antibody responses was accomplished as previously described (Rubtsov et al., 2005) with either biotin-conjugated mouse anti-IgMa (DS-1; BD) or biotin-conjugated mouse anti-IgMb (AF6-78; BD) antibodies, respectively. Plates were subsequently washed three times and bound antibody revealed by incubation with AP-conjugated Streptavidin (Southern Biotech).
For affinity maturation measurements, ELISAs were performed as in the previous paragraphs with the exception that plates were coated with either NP (4-Hydroxy-3-nitrophenylacetyl)4–BSA or NP16–BSA capture antigens (Biosearch Technologies) diluted at 2 µg/ml. The ratio of high-affinity antibodies bound to NP4–BSA relative to the total of both high- and low-affinity antibodies bound to NP16–BSA was calculated as previously described (Herzenberg et al., 1980; Cumano and Rajewsky, 1985). In brief, the relative NP4/NP16 binding ratio for a given sample was calculated as the OD50 titer of serum antibodies bound to NP4–BSA divided by the OD50 titer of antibodies bound to NP16–BSA. The NP-specific mAb S43-10 (Reth et al., 1978) was used as a positive control for a high-affinity NP-specific IgG2c antibody with an affinities of Kd = 2.4 × 10−8 M for NIP and Kd = 2.8 × 10−7 M for NP (Cumano and Rajewsky, 1985).
NP-specific ELISPOTS.
NP-specific ASCs were measured in 96-well flat-bottom EIA/RIA high-binding plates (Costar; Corning) coated overnight at 4°C with 2 µg/ml NIP15-BSA diluted in 0.05 M K2HPO4, pH 8.0. Plates were washed three times with PBS before blocking with warm PBS and 1% gelatin (Sigma-Aldrich) at 37°C for a minimum of 1 h. Plates were washed again three times with PBS before incubation with cells. Cells were isolated from spleen, bone marrow, or lymph nodes at the times indicated after immunization and seeded in duplicate at a starting density of 0.25–1 × 107 total viable cells per 100 µl in the first well, and threefold serial dilutions were performed down the plate. For those ELISPOTs performed on lymph node cells, lymph nodes harvested from three mice within the same treatment group were pooled together to constitute a single sample. Cultured cells were incubated at 37°C in 5% CO2 for 6 h in complete IMDM (Invitrogen) supplemented with 10% heat-inactivated FBS (Biosource, Invitrogen, or Gemini Bio-Products), 2 mM GlutaMAX-I (Invitrogen), 100 U/ml Penicillin (Invitrogen), 100 µg/ml Streptomycin (Invitrogen), and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich). Plates were then washed once with H2O and 0.05% Tween 20 for 10 min at room temperature and subsequently three times with PBS and 0.1% Tween 20. Secreted antibody was detected by incubating plates with an isotype-specific AP-conjugated goat anti–mouse Ig (SouthernBiotech) diluted in warm PBS, 1% gelatin, and 0.05% Tween 20 for 1 h at 37°C. After three washes (PBS, 0.1% Tween 20), plates were developed overnight at 4°C with 1 mg/ml 5-Bromo-4-chloro-3-indolyl phosphate p-toluidine (Sigma-Aldrich) salt substrate diluted in an alkaline buffer composed of 0.1 M 2-amino-2-methyl-1-propanol, 0.01% NaN3, 0.5 mM MgCl2, and 0.007% Triton X-405, pH 10.25. Plates were washed four times with deionized H2O, allowed to dry in the dark at room temperature, and scanned (Perfection 2450 Photo Scanner; Epson). Developed spots were counted visually from the scanned images and the frequency of NP-specific ASCs per total number of cells plated was enumerated.
Splenectomy operations.
For both vital splenectomy and sham operations, 6-wk-old C57BL/6 mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine, followed by administration of a subcutaneous injection of 0.5 mg/kg buprenorphine for pain. Once sedated, a 1–1.5-cm incision was made on the upper left abdomen through both the peritoneal wall and skin. For splenectomized animals, the spleen was exteriorized and the splenic artery and efferent venule were each ligated. For sham-operated mice, the spleen was gently replaced back into the abdomen. For all animals, the abdominal wall was closed by placing one to two sutures through the peritoneal membrane, and subsequently two wound clips were placed to adjoin the exterior skin of the incision. Postoperative care included administration of 1 ml of subcutaneous saline, rest near an indirect heat source, and monitoring until full motility was regained. Pain management was achieved by subcutaneous injections of 0.5 mg/kg buprenorphine every 12 h for 2–3 d after surgery. Mice were allowed to recover for 21–29 d after surgery, at which time the total serum IgM was determined to be equivalent between the sham-operated and splenectomized groups. At 23–32 d after operation, mice were immunized and serum NP-specific antibody responses measured by ELISA as described.
MZ B cell depletion.
For selective MZ B cell depletion in vivo, a protocol was used that was similar to a previously published protocol (Belperron et al., 2005, 2007). In brief, C57BL/6 mice were injected i.p. with 100 µg each of unconjugated purified rat IgG2a anti–mouse CD11a (Integrin αL, LFA-1α; clone M17/4; hybridoma) and rat IgG2b anti–mouse CD49d (Integrin α4; VLA-4; clone PS/2; hybridoma) mAbs diluted together in sterile PBS. Control mice were injected with 100 µg each of functional grade purified rat IgG2a (clone eBR2a; eBioscience) and rat IgG2b isotype control antibodies (clone eB149/10H5; eBioscience) also diluted in PBS. 3 h after injection of antibodies, mice were bled and PBLs stained for the mature B cell markers CD21 and CD1d to verify MZ B cell release into blood. Greater than 80% of MZ B cells are depleted entirely from the spleen up to 10 d after injection of anti-integrin antibodies (Fig. S2 A), and by 14 d after treatment >60% of normal frequencies of MZ B cells have returned to spleen (Fig. S2 A). Approximately 10% of the anti-integrin antibodies injected remain within circulation 4 d after injection and are essentially completely cleared by 7 d after injection (Fig. S2 C). However, surface integrin expression is also down-modulated by the anti-α4/αL treatment on remaining T and B lymphocytes up to 4 d after injection (Fig. S2D). Thus, for MZ B cell depletion experiments, mice were rested for 7 d after injection of anti-integrin antibodies and before immunization (Fig. S2 E) to allow both for clearance of rat anti-α4/αL antibodies (Fig. S2 C) and for remaining T and B lymphocytes to regain wild-type levels of surface integrin expression (Fig. S2 D). 7 d after injection of antibodies, mice were immunized with NP-Ficoll ± poly(I:C) as described in Fig. S2 E. Sera were collected either the day before immunization (preimmune) or at 4 and 7 d after immunization, and serum NP-specific antibody responses were measured as described. At 7 d after immunization, splenocytes were harvested and the frequency of NP-specific ASCs was evaluated by ELISPOT. Depleting anti-integrin antibodies were tested by the LAL assay and found to be at levels ≤0.1 EU/ml.
To determine the clearance of serum rat IgG antibodies over time in this model, serum was collected from mice injected with either rat IgG anti-integrin and isotype control antibodies and analyzed for the presence of rat IgG by ELISA. In brief, plates were coated with 10 µg/ml of unlabeled goat anti–rat IgM + IgG (H + L chain specific, absorbed against mouse Ig) antibody in PBS overnight at 4°C. After washing (PBS and 0.1% Tween 20), plates were blocked for 3 h at 37°C with preimmune C57BL/6 mouse serum diluted 1:200 in PBS, 1% BSA, and 0.05% NaN3 and subsequently washed twice. Sera and standards were diluted similarly as above and incubated overnight at 4°C. Plates were washed three times, and bound antibodies were detected by incubation with AP-conjugated goat anti–rat IgG (H + L chain specific, absorbed against mouse Ig; SouthernBiotech) diluted in PBS, 1% BSA, and 0.05% NaN3 buffer for 1 h at 37°C. After three washes, plates were developed and absorbances read according to that described for the NP-specific ELISA protocol. Purified unlabeled rat IgG antibody (SouthernBiotech) was used as a standard to quantify the concentration of total serum rat IgG for these ELISAs.
Adoptive cell transfers.
Lymph node cells were harvested and purified from BL6-IghB1-8/B1-8;Igha mice in IMDM supplemented with 5% FBS, washed twice (PBS), and resuspended in sterile PBS. Approximately 5 × 107 total lymph node cells were injected intravenously into each sex-matched C57BL/6-Ighb recipient mouse. 1 d after cell transfer, recipient mice were immunized with NP-Ficoll ± poly(I:C) as described. Serum was collected before adoptive cell transfer (preimmune) and at 4 and 7 d after immunization, and NP-specific antibody responses were subsequently measured by ELISA. For phenotypic confirmation, mice were left unimmunized and verification of transferred IgMa -expressing B cells within the splenic FO B cell compartment of C57BL/6-Ighb recipient mice 1 d after transfer was validated by flow cytometry.
Mixed bone marrow chimeras.
Bone marrow cells were isolated from 5–7-wk-old C57BL/6 (Ighb), B6.C20 (Igha), Tlr3−/− (Ighb), or Ifnar1−/− (Ighb) donor mice in IMDM supplemented with 5% FBS under sterile conditions. Donor bone marrow cells were washed once with sterile PBS (Cellgro; Mediatech, Inc.), resuspended at 1.33–2.67 × 107 cells/ml in PBS, and mixed (Ighb + Igha) to yield a final concentration of 2 × 107 cells/ml for reconstitution. A total of 4 × 106 bone marrow cells were injected intravenously into each sex-matched lethally irradiated (1,000 rad) 6–8-wk-old B6.C20 recipients. Recipient mice were maintained on Septra food for 7–8 wk after reconstitution. At 8 wk after reconstitution, PBLs from chimeric mice were analyzed by flow cytometric analysis to ensure no significant differences were observed between the ratio of IgMb:IgMa B cell reconstitution frequencies in the experimental chimeras as compared with the control chimeras. Chimeric mice were immunized at 9 wk after reconstitution and serum NP-specific antibody responses were measured by ELISA as described. At 14 d after immunization, the ratio of IgMb:IgMa B cell reconstitution frequencies was determined within the chimeric splenic mature B cell compartments and found to be identical to those previously measured in the circulating B cell population.
In vitro stimulation.
B cells were negatively enriched from naive C57BL/6 mice by depletion of CD43+ cells with magnetic cell sorting on LS columns (Miltenyi Biotec) with MACS buffer (PBS, 2 mM EDTA, and 0.5% BSA) according to the manufacturer’s protocol. MZ (CD21high CD1dhigh) and FO (CD21int CD1dint) B cells were subsequently sorted based on surface expression of CD21 and CD1d to ≥94% purity using a MoFlo XDP Cell Sorter (Beckman Coulter) with Summit Software version 5.1.3.6886. Sorted MZ and FO B cells were cultured separately at 2.5–5 × 105 cells/ml in 1 ml of complete IMDM in 24-well flat bottom cell culture plates (CELLSTAR; Greiner Bio-One) with the indicated stimuli at 37°C in 5% CO2. Stimuli and their concentrations cultured with cells included the following: 1–100 U/ml recombinant mouse IFN-α11 (PBL InterferonSource), 1–10 µg/ml AffiniPure F(ab′)2 goat anti-mouse IgM, μ chain specific (anti-IgM; Jackson ImmunoResearch Laboratories), and 0.1–10 µg/ml LPS (Escherichia coli O26:B6; Sigma-Aldrich). B cell activation was subsequently assessed as CD69 and CD86 cell surface marker up-regulation measured by flow cytometry after stimulation for 6 or 10 h, respectively. Endotoxin levels of stimuli used for in vitro cultures were either measured by the LAL assay or purchased as <1 EU/µg.
Immunofluorescence histology.
Spleens were harvested from mice 7 d after immunization and frozen in OCT compound (EM Sciences) at −80°C. Tissues were cut into 5–7-µm sections and allowed to dry at room temperature. Unfixed sections were rehydrated with PBS for 20 min and blocked for 15–30 min with rat anti–mouse FcγR blocking antibody (2.4G2) diluted in PBS, 2% BSA, and 0.05% Tween 20 at room temperature. Sections were stained with antibody mixtures diluted in PBS for 30–60 min at room temperature in the dark, followed by three 5-min washes with shaking with PBS. Incubation and washing steps were repeated in the cases where a secondary detection reagent was used. Sections were dried, mounted with Bion IFA mounting medium (Bion Enterprises, Ltd.), visualized at room temperature with a microscope (Axiovert 200M; Carl Zeiss, Inc.) using a 3i Marianis System, and analyzed with Slidebook 4.0 software (Intelligent Imaging Innovations). Antibodies and secondary reagents used for immunofluorescence histological detection included the following: FITC-conjugated rat anti–mouse CD169 (MOMA-1; AbD Serotec), Alexa Fluor 546–conjugated rat anti–mouse IgM (R33-24.12; our laboratory), Alexa Fluor 647–conjugated mouse anti–mouse IgD (1.3–5; our laboratory), biotin-conjugated rat anti–mouse Lambda (JC5-1; SouthernBiotech), streptavidin–FITC (BD), and Cy5-conjugated goat anti–mouse IgG, Fcγ Subclass 2c specific (Jackson ImmunoResearch Laboratories). All Alexa Fluor–conjugated antibodies were purified and conjugated in our laboratory using Alexa Fluor Protein Labeling kits (Invitrogen).
Statistical and data analyses.
Data were graphed and analyzed using Prism version 5.0a (GraphPad Software). Statistical significance was assessed with a Student’s t test with unequal variance, and the appropriateness of one-tailed or two-tailed significance was determined on an individual experiment basis. P-values ≤0.05 were considered significant. Where appropriate, data were expressed as the geometric mean ± SEM, unless otherwise stated.
Online supplemental material.
Fig. S1 shows dose response of poly(I:C) on the production of NP-specific IgG2c and lack of polyclonal IgG2c production. Data presented in Fig. S2 characterizes the kinetics of MZ B cell depletion by anti-integrin treatment. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092695/DC1.
Acknowledgments
The authors wish to thank Paul Waterman, Ginger Woznicki, and Catherine Haluszczak for technical assistance, the NJH flow cytometry facility for cell sorting, and Philippa Marrack and Ross Kedl for reagents. Additionally, we thank Klaus Rajewsky for generosity with reagents and mice. We also appreciate the advice and thoughtful comments from Ross Kedl, John Kappler, and members of the laboratory during these studies.
This work was supported by the National Institutes of Health (AI052310 to R. Pelanda and AI052157 and AI078468 to R.M. Torres).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- dsRNA
- double-stranded RNA
- MZ
- marginal zone
- PAMP
- pathogen-associated molecular pattern
- PRR
- pattern recognition receptor
- TD
- T cell dependent
- TI
- T cell independent
- TLR
- Toll-like receptor
References
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738 10.1038/35099560 [DOI] [PubMed] [Google Scholar]
- Amlot P.L., Grennan D., Humphrey J.H. 1985. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur. J. Immunol. 15:508–512 10.1002/eji.1830150516 [DOI] [PubMed] [Google Scholar]
- Baccala R., Hoebe K., Kono D.H., Beutler B., Theofilopoulos A.N. 2007. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 13:543–551 10.1038/nm1590 [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Zinkernagel R.M. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15:235–270 10.1146/annurev.immunol.15.1.235 [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Hengartner H., Zinkernagel R.M. 1995. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 25:3445–3451 10.1002/eji.1830251236 [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Odermatt B., Hengartner H., Zinkernagel R.M. 1996. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 183:2259–2269 10.1084/jem.183.5.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Kalinke U., Althage A., Freer G., Burkhart C., Roost H., Aguet M., Hengartner H., Zinkernagel R.M. 1997. The role of antibody concentration and avidity in antiviral protection. Science. 276:2024–2027 10.1126/science.276.5321.2024 [DOI] [PubMed] [Google Scholar]
- Balázs M., Martin F., Zhou T., Kearney J. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 17:341–352 10.1016/S1074-7613(02)00389-8 [DOI] [PubMed] [Google Scholar]
- Barr T.A., Brown S., Ryan G., Zhao J., Gray D. 2007. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol. 37:3040–3053 10.1002/eji.200636483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr T.A., Brown S., Mastroeni P., Gray D. 2009. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J. Immunol. 183:1005–1012 10.4049/jimmunol.0803706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Herman O.C., Jager G.C., Brown L., Herzenberg L.A., Herzenberg L.A. 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA. 96:2250–2255 10.1073/pnas.96.5.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Choi Y.S., Rothaeusler K., Yang Y., Herzenberg L.A. 2008. B cell lineage contributions to antiviral host responses. Curr. Top. Microbiol. Immunol. 319:41–61 10.1007/978-3-540-73900-5_3 [DOI] [PubMed] [Google Scholar]
- Baumhäckel H., Liesegang B., Radbruch A., Rajewsky K., Sablitzky F. 1982. Switch from NP-specific IgG3 to IgG1 in the mouse hybridoma cell line S24/63/63. J. Immunol. 128:1217–1220 [PubMed] [Google Scholar]
- Belperron A.A., Dailey C.M., Bockenstedt L.K. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681–5686 [DOI] [PubMed] [Google Scholar]
- Belperron A.A., Dailey C.M., Booth C.J., Bockenstedt L.K. 2007. Marginal zone B-cell depletion impairs murine host defense against Borrelia burgdorferi infection. Infect. Immun. 75:3354–3360 10.1128/IAI.00422-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Bonneville M., Kearney J.F. 2001. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 1:177–186 10.1038/35105052 [DOI] [PubMed] [Google Scholar]
- Berberian L., Goodglick L., Kipps T.J., Braun J. 1993. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 261:1588–1591 10.1126/science.7690497 [DOI] [PubMed] [Google Scholar]
- Blutt S.E., Crawford S.E., Warfield K.L., Lewis D.E., Estes M.K., Conner M.E. 2004. The VP7 outer capsid protein of rotavirus induces polyclonal B-cell activation. J. Virol. 78:6974–6981 10.1128/JVI.78.13.6974-6981.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419–424 10.1016/S0952-7915(00)00111-4 [DOI] [PubMed] [Google Scholar]
- Bothwell A.L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. 1981. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 24:625–637 10.1016/0092-8674(81)90089-1 [DOI] [PubMed] [Google Scholar]
- Braun D., Caramalho I., Demengeot J. 2002. IFN-alpha/beta enhances BCR-dependent B cell responses. Int. Immunol. 14:411–419 10.1093/intimm/14.4.411 [DOI] [PubMed] [Google Scholar]
- Chang W.L., Coro E.S., Rau F.C., Xiao Y., Erle D.J., Baumgarth N. 2007. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178:1457–1467 [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., Dijkstra C.D., Van Rooijen N. 1986. Marginal zone of the spleen and the development and localization of specific antibody-forming cells against thymus-dependent and thymus-independent type-2 antigens. Immunology. 57:399–403 [PMC free article] [PubMed] [Google Scholar]
- Coro E.S., Chang W.L., Baumgarth N. 2006. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176:4343–4351 [DOI] [PubMed] [Google Scholar]
- Coutelier J.P., van der Logt J.T., Heessen F.W., Warnier G., Van Snick J. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165:64–69 10.1084/jem.165.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E., Bullock W.W., Möller G. 1974. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J. Exp. Med. 139:74–92 10.1084/jem.139.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. 1985. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur. J. Immunol. 15:512–520 10.1002/eji.1830150517 [DOI] [PubMed] [Google Scholar]
- Cunningham A.F., Gaspal F., Serre K., Mohr E., Henderson I.R., Scott-Tucker A., Kenny S.M., Khan M., Toellner K.M., Lane P.J., MacLennan I.C. 2007. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 178:6200–6207 [DOI] [PubMed] [Google Scholar]
- Eckl-Dorna J., Batista F.D. 2009. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 113:3969–3977 10.1182/blood-2008-10-185421 [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Honjo T. 2000. T-Independent immune response: new aspects of B cell biology. Science. 290:89–92 10.1126/science.290.5489.89 [DOI] [PubMed] [Google Scholar]
- Fehr T., Naim H.Y., Bachmann M.F., Ochsenbein A.F., Spielhofer P., Bucher E., Hengartner H., Billeter M.A., Zinkernagel R.M. 1998. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat. Med. 4:945–948 10.1038/nm0898-945 [DOI] [PubMed] [Google Scholar]
- Finkelman F.D., Svetic A., Gresser I., Snapper C., Holmes J., Trotta P.P., Katona I.M., Gause W.C. 1991. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J. Exp. Med. 174:1179–1188 10.1084/jem.174.5.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García de Vinuesa C., O’Leary P., Sze D.M., Toellner K.M., MacLennan I.C. 1999. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 29:1314–1323 [DOI] [PubMed] [Google Scholar]
- Gavin A.L., Hoebe K., Duong B., Ota T., Martin C., Beutler B., Nemazee D. 2006. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 314:1936–1938 10.1126/science.1135299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestier L., Taillardet M., Mondiere P., Gheit H., Bella C., Defrance T. 2007. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J. Immunol. 178:7779–7786 [DOI] [PubMed] [Google Scholar]
- Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R.A., Diamond M.S., Colonna M. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA. 103:8459–8464 10.1073/pnas.0603082103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden K.B., Gorski K.S., Gibson S.J., Kedl R.M., Kieper W.C., Qiu X., Tomai M.A., Alkan S.S., Vasilakos J.P. 2005. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 174:1259–1268 [DOI] [PubMed] [Google Scholar]
- Goud S.N., Muthusamy N., Subbarao B. 1988. Differential responses of B cells from the spleen and lymph node to TNP-Ficoll. J. Immunol. 140:2925–2930 [PubMed] [Google Scholar]
- Gray D., MacLennan I.C., Bazin H., Khan M. 1982. Migrant mu+ delta+ and static mu+ delta- B lymphocyte subsets. Eur. J. Immunol. 12:564–569 10.1002/eji.1830120707 [DOI] [PubMed] [Google Scholar]
- Green N.M., Laws A., Kiefer K., Busconi L., Kim Y.M., Brinkmann M.M., Trail E.H., Yasuda K., Christensen S.R., Shlomchik M.J., et al. 2009. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J. Immunol. 183:1569–1576 10.4049/jimmunol.0803899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R., Okigaki M., Schlessinger J., Ravetch J.V. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31–36 10.1038/76882 [DOI] [PubMed] [Google Scholar]
- Gururajan M., Jacob J., Pulendran B. 2007. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2:e863 10.1371/journal.pone.0000863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K.M., Poe J.C., Steeber D.A., Tedder T.F. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 23:7–18 10.1016/j.immuni.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Heer A.K., Shamshiev A., Donda A., Uematsu S., Akira S., Kopf M., Marsland B.J. 2007. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178:2182–2191 [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A., Black S.J., Tokuhisa T., Herzenberg L.A. 1980. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J. Exp. Med. 151:1071–1087 10.1084/jem.151.5.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack R.S., Imanishi-Kari T., Rajewsky K. 1977. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur. J. Immunol. 7:559–565 10.1002/eji.1830070813 [DOI] [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165–1175 10.1084/jem.173.5.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54:1–13 [DOI] [PubMed] [Google Scholar]
- Jegerlehner A., Maurer P., Bessa J., Hinton H.J., Kopf M., Bachmann M.F. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a. J. Immunol. 178:2415–2420 [DOI] [PubMed] [Google Scholar]
- Kamphuis E., Junt T., Waibler Z., Forster R., Kalinke U. 2006. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 108:3253–3261 10.1182/blood-2006-06-027599 [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., et al. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 441:101–105 10.1038/nature04734 [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T.S., Fujita T., Akira S. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid–inducible gene-I and melanoma differentiation–associated gene 5. J. Exp. Med. 205:1601–1610 10.1084/jem.20080091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumararatne D.S., MacLennan I.C., Bazin H., Gray D. 1982. Marginal zones: the largest B cell compartment of the rat spleen. Adv. Exp. Med. Biol. 149:67–73 [DOI] [PubMed] [Google Scholar]
- Le Bon A., Schiavoni G., D’Agostino G., Gresser I., Belardelli F., Tough D.F. 2001. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461–470 10.1016/S1074-7613(01)00126-1 [DOI] [PubMed] [Google Scholar]
- Le Bon A., Thompson C., Kamphuis E., Durand V., Rossmann C., Kalinke U., Tough D.F. 2006. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 176:2074–2078 [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Oldfield S., MacLennan I.C. 1988. Thymus-independent type 2 responses in lymph nodes. Adv. Exp. Med. Biol. 237:113–117 [DOI] [PubMed] [Google Scholar]
- Longhi M.P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A.M., Colonna M., Steinman R.M. 2009. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206:1589–1602 10.1084/jem.20090247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T.T., Cyster J.G. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 297:409–412 10.1126/science.1071632 [DOI] [PubMed] [Google Scholar]
- MacLennan I.C., Toellner K.M., Cunningham A.F., Serre K., Sze D.M., Zúñiga E., Cook M.C., Vinuesa C.G. 2003. Extrafollicular antibody responses. Immunol. Rev. 194:8–18 10.1034/j.1600-065X.2003.00058.x [DOI] [PubMed] [Google Scholar]
- Mancuso G., Gambuzza M., Midiri A., Biondo C., Papasergi S., Akira S., Teti G., Beninati C. 2009. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 10:587–594 10.1038/ni.1733 [DOI] [PubMed] [Google Scholar]
- Martin F., Kearney J.F. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335 10.1038/nri799 [DOI] [PubMed] [Google Scholar]
- Martin F., Oliver A.M., Kearney J.F. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 14:617–629 10.1016/S1074-7613(01)00129-7 [DOI] [PubMed] [Google Scholar]
- McCartney S.A., Colonna M. 2009. Viral sensors: diversity in pathogen recognition. Immunol. Rev. 227:87–94 10.1111/j.1600-065X.2008.00726.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams M.G. 2003. B cells as effectors. Curr. Opin. Immunol. 15:354–361 10.1016/S0952-7915(03)00046-3 [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A., Khim S., Rawlings D.J. 2007. B cell–intrinsic TLR signals amplify but are not required for humoral immunity. J. Exp. Med. 204:3095–3101 10.1084/jem.20071250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P.K., Stein K.E., Paul W.E. 1981. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J. Exp. Med. 153:1–12 10.1084/jem.153.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P.K., Longo D.L., Paul W.E. 1984. T cell regulation of immunoglobulin class expression in the B cell response to TNP-Ficoll: characterization of the T cell responsible for preferential enhancement of the IgG2a response. J. Immunol. 132:1647–1653 [PubMed] [Google Scholar]
- Morikawa K., Kubagawa H., Suzuki T., Cooper M.D. 1987. Recombinant interferon-alpha, -beta, and -gamma enhance the proliferative response of human B cells. J. Immunol. 139:761–766 [PubMed] [Google Scholar]
- Müller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2005. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 310:1510–1512 10.1126/science.1118948 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- Obukhanych T.V., Nussenzweig M.C. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203:305–310 10.1084/jem.20052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Kearney J.F. 1999. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 162:7198–7207 [PubMed] [Google Scholar]
- Otipoby K.L., Andersson K.B., Draves K.E., Klaus S.J., Farr A.G., Kerner J.D., Perlmutter R.M., Law C.L., Clark E.A. 1996. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 384:634–637 10.1038/384634a0 [DOI] [PubMed] [Google Scholar]
- Pasare C., Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature. 438:364–368 10.1038/nature04267 [DOI] [PubMed] [Google Scholar]
- Phan T.G., Gardam S., Basten A., Brink R. 2005. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. J. Immunol. 174:4567–4578 [DOI] [PubMed] [Google Scholar]
- Pulendran B. 2009. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat. Rev. Immunol. 9:741–747 [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G.J., Rajewsky K. 1978. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur. J. Immunol. 8:393–400 10.1002/eji.1830080605 [DOI] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. 1979. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur. J. Immunol. 9:1004–1013 10.1002/eji.1830091216 [DOI] [PubMed] [Google Scholar]
- Ron Y., Sprent J. 1985. Prolonged survival in vivo of unprimed B cells responsive to a T-independent antigen. J. Exp. Med. 161:1581–1586 10.1084/jem.161.6.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov A., Strauch P., Digiacomo A., Hu J., Pelanda R., Torres R.M. 2005. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 23:527–538 10.1016/j.immuni.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Rubtsov A.V., Swanson C.L., Troy S., Strauch P., Pelanda R., Torres R.M. 2008. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J. Immunol. 180:3882–3888 [DOI] [PubMed] [Google Scholar]
- Samardzic T., Marinkovic D., Danzer C.P., Gerlach J., Nitschke L., Wirth T. 2002. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 32:561–567 [DOI] [PubMed] [Google Scholar]
- Sen G., Khan A.Q., Chen Q., Snapper C.M. 2005. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J. Immunol. 175:3084–3091 [DOI] [PubMed] [Google Scholar]
- Seppälä I.J., Mäkelä O. 1984. Adjuvant effect of bacterial LPS and/or alum precipitation in responses to polysaccharide and protein antigens. Immunology. 53:827–836 [PMC free article] [PubMed] [Google Scholar]
- Shih T.A., Roederer M., Nussenzweig M.C. 2002. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat. Immunol. 3:399–406 10.1038/ni776 [DOI] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdicková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 440:540–544 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Snapper C.M. 2006. Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae. Curr. Protein Pept. Sci. 7:295–305 10.2174/138920306778017972 [DOI] [PubMed] [Google Scholar]
- Song H., Cerny J. 2003. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 198:1923–1935 10.1084/jem.20031498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Pewzner-Jung Y., Schwers S., Taki S., Jung S., Eilat D., Rajewsky K. 1997. B cell development under the condition of allelic inclusion. Immunity. 6:225–233 10.1016/S1074-7613(00)80325-8 [DOI] [PubMed] [Google Scholar]
- Stetson D.B., Medzhitov R. 2006. Type I interferons in host defense. Immunity. 25:373–381 10.1016/j.immuni.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Tesch H., Smith F.I., Müller-Hermes W.J., Rajewsky K. 1984. Heterogeneous and monoclonal helper T cells induce similar anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibody populations in the primary adoptive response. I. Isotype distribution. Eur. J. Immunol. 14:188–194 10.1002/eji.1830140215 [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Sze D.M., Cook M.C., Toellner K.M., Klaus G.G., Ball J., MacLennan I.C. 2003. Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur. J. Immunol. 33:297–305 10.1002/immu.200310003 [DOI] [PubMed] [Google Scholar]