Abstract
The quality of a Th1 response can be a prospective correlate of vaccine-mediated protection against certain intracellular pathogens. Using two distinct vaccine platforms, we evaluate the influence of interleukin (IL) 10 production on the magnitude, quality, and protective capacity of CD4+ T cell responses in the mouse model of Leishmania major infection. Multiparameter flow cytometry was used to delineate the CD4+ T cell production of interferon (IFN) γ, IL-2, tumor necrosis factor (TNF), and IL-10 (or combinations thereof) after vaccination. Immunization with a high dose of adenovirus (ADV) expressing leishmanial proteins (MML-ADV) elicited a limited proportion of multifunctional IFN-γ+IL-2+TNF+ Th1 cells, a high frequency of IL-10–producing CD4+ T cells, and did not protect against subsequent challenge. Surprisingly, in the absence of IL-10, there was no change in the magnitude, quality, or protective capacity of the Th1 response elicited by high-dose MML-ADV. In contrast, after immunization with MML protein and CpG (MML + CpG), IL-10 limited the production of IL-12 by DCs in vivo, thereby decreasing the generation of multifunctional Th1 cells. Consequently, three immunizations with MML + CpG were required for full protection. However, inhibiting IL-10 at the time of immunization enhanced the magnitude and quality of the Th1 response sufficiently to mediate protection after only a single immunization. Overall, we delineate distinct mechanisms by which vaccines elicit protective Th1 responses and underscore the importance of multifunctional CD4+ T cells.
CD4+ T cell responses after vaccination or infection show substantial heterogeneity in terms of their phenotype and functional capacity. Inducing protective responses without the appropriate means to assess such heterogeneity has limited the ability to define correlates of protection after vaccination. Thus, understanding the type of CD4+ T cell response required to mediate protection is critical for rational design of vaccines against infections requiring Th1 immunity. Historically, T cell responses have been characterized by magnitude (frequency), proliferative capacity, or the mean TCR avidity. Recently, multicolor flow cytometry has broadened the spectrum of parameters that can be measured simultaneously at the single-cell level to include phenotypic markers and/or specific combinations of functional responses (e.g., cytokines). This more extensive characterization, termed quality, is defined by the pattern of cytokine production at the single-cell level (Seder et al., 2008). The quality can be related to the spectrum of Th1 differentiation from IFN-γ–negative, IL-2–producing, and/or TNF-producing central memory cells to multifunctional IFN-γ+IL-2+TNF+ or IFN-γ+IL-2−TNF+ effector memory cells to terminally differentiated IFN-γ single-positive cells (Seder et al., 2008; Wu et al., 2002). Furthermore, when quantified on a per-cell basis, Th1 cells secreting all three cytokines (IFN-γ+IL-2+TNF+) produce considerably more IFN-γ than double- or single-producing IFN-γ cells (Darrah et al., 2007). In addition, the ability of multifunctional Th1 cells to also secrete TNF and IL-2 provides additional effector function and enhanced proliferative capacity, respectively, making these cells optimized for durable effector function. The quality of a vaccine-elicited response was first shown to be predictive of disease protection against Leishmania major in as much as a CD4+ T cell quality comprising a high frequency of multifunctional IFN-γ+IL-2+TNF+ Th1 cells correlated with protection (Darrah et al., 2007). Moreover, this Th1-based metric has since been correlated with a favorable outcome to a variety of other infections including tuberculosis (Forbes et al., 2008; Lindenstrøm et al., 2009), malaria (Roestenberg et al., 2009), and vaccinia (Trumpfheller et al., 2008).
As the quality of a Th1 response may predict outcome against infection, understanding the mechanisms that influence the generation of multifunctional Th1 cells can be used to improve vaccine design. Using various vaccine formulations or by altering the dose of a specific vaccine, we were able to elicit qualitatively distinct Th1 responses that confer varying levels of protection. Indeed, vaccination with a single low dose of MML-adenovirus (ADV) elicited a Th1 response comprising a high frequency of multifunctional cells and protection against L. major challenge, whereas a single high dose of MML-ADV elicited fewer multifunctional cells, a high proportion of IFN-γ single-positive cells, and no protection (Darrah et al., 2007). Although the lack of protection after high-dose MML-ADV immunization was consistent with a poor quality Th1 response, it remained possible that inhibitory cytokines, which have a well established role in limiting protection against L. major (Sacks and Anderson, 2004), were influencing outcome. In this regard, IL-10 produced by CD4+ T cells promotes susceptibility and prevents healing in mice and humans infected with L. major (Sacks and Anderson, 2004). In the context of a self-healing L. major infection, IL-10 from natural regulatory T (T reg) cells prevents the eradication of parasites, allowing a persistence of low-level antigen that sustains T cell memory (Belkaid et al., 2001, 2002). Additionally, immune suppression during chronic L. major infection is mediated by IL-10 production from IFN-γ–producing Th1 cells (Anderson et al., 2007). Thus, in the setting of L. major infection in vivo, CD4+ T cell–derived IL-10 prevents clearance of parasites. Alternatively, IL-10 produced by Th1 cells can have an important regulatory effect by limiting excess inflammation during Toxoplasma gondii or Flu infection (Jankovic et al., 2002; Sun et al., 2009). Although these studies show that CD4+ T cell–derived IL-10 can have distinct regulatory effects on an ongoing infection in vivo, it has not been shown whether CD4+IFN-γ+IL-10+ Th1 cells can be elicited by vaccination with clinically based vectors or how they would influence the quality of the Th1 response or protection. Thus, a major focus of this study was to investigate a role for CD4+ T cell–derived (adaptive) IL-10 after immunization with high-dose MML-ADV vaccination and to determine its effect on Th1 quality and protection.
IL-10 can also limit Th1 responses indirectly by decreasing APC function or APC production of the canonical polarizing cytokine IL-12 (Moore et al., 2001; Trinchieri, 2001). Indeed, APC-derived (innate) IL-10 induced by vaccination could profoundly impact the efficiency and extent of Th1 differentiation by regulating production of IL-12 by DCs. In this regard, we previously showed three immunizations with MML protein and the toll-like receptor (TLR) 9 ligand CpG (MML + CpG) are required to elicit a high frequency of multifunctional Th1 cells and protective immunity against L. major (Darrah et al., 2007). As CpG is a potent inducer of IL-10 and IL-12 from DCs (Chu et al., 1997; Roman et al., 1997; Boonstra et al., 2006; Samarasinghe et al., 2006), we hypothesized that this innate cross-regulation after immunization might influence the number of immunizations required to achieve protection and the type of Th1 response induced. Thus, the other major focus of the study was to examine the role IL-10 and IL-12 production from innate immune cells on Th1 immunity and protection after MML + CpG immunization.
In this study, we investigate how IL-10 production by CD4+ T cells or APCs might influence the magnitude, quality, and protective capacity of Th1 responses elicited by MML-ADV or MML + CpG immunization. Thus, a novel multiparameter flow cytometry panel was developed to define the quality of the MML-specific response after vaccination, measured by the production of IL-10, IFN-γ, IL-2, and TNF. In addition, IL-10−/− mice and WT mice treated with anti–IL-10 receptor (α-IL-10R) were used to define the role of innate and adaptive IL-10 on vaccine-induced immunity and Th1-mediated protection. Overall, this study delineates the mechanisms of how two distinct vaccine formulations generate protective immunity and elucidates the role that IL-10 has on this process.
RESULTS
Characterization of distinct cytokine-producing CD4+ T cell populations using multiparameter flow cytometry
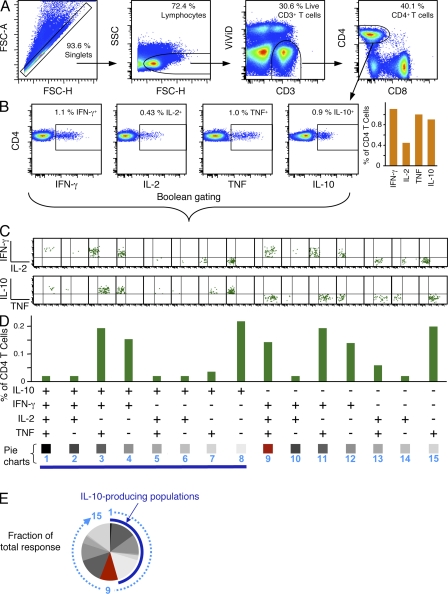
Immunization with high-dose (1010 viral particles [vp]) and low-dose (107 vp) MML-ADV results in a similar magnitude of IFN-γ–producing CD4+ T cells but only low-dose MML-ADV confers durable protection (Darrah et al., 2007). One explanation for such findings is that low-dose MML-ADV elicits a Th1 response of better quality than high-dose MML-ADV. However, it was recently shown that IL-10 from CD4+ T cells contributes to susceptibility to L. major infection even in the presence of a strong Th1 response (Anderson et al., 2005, 2007). Thus, we developed a multiparameter flow cytometry panel to investigate a role for CD4+ T cell–derived IL-10 in vaccine-mediated protection of mice after MML-ADV. Fig. 1 outlines the gating strategy, cytokine analysis, and data presentation used in this study. Shown is the gating of CD4+ T cells (Fig. 1 A) used to quantify the total frequency of IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells (Fig. 1 B), as well as the Boolean analysis (Fig. 1 C) and graphical representation (Fig. 1, D and E) of the 15 individual cytokine combinations of IFN-γ, IL-2, TNF, or IL-10 produced by individual CD4+ T cells. The proportion (of the total response) of each cytokine alone or in any combination produced at the single-cell level reflects the quality of the response and is illustrated using pie charts (Fig. 1 E).
Figure 1.
Characterization of 15 distinct cytokine-producing CD4+ T cell populations using multiparameter flow cytometry. Typical gating tree and analysis for an eight-color flow cytometry panel used to simultaneously analyze IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells from a mouse spleen after in vitro stimulation with antigen, 10 d after vaccination with high-dose MML-ADV. (A) Initial gating of total events included a singlet cell gate, followed by selection for lymphocytes, live T cells (ViViD−CD3+), and CD4+CD8− T cells. (B) The total frequency of antigen-specific IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells is gated and graphed (background corrected) as shown. In this analysis, individual cells produce multiple cytokines; therefore, the frequency of cells producing any cytokine is less than the sum of the individual cytokine gates. (C) Boolean gating analysis of cytokine-positive populations separately categorizes each cell based on its functionality or quality with respect to cytokine production. Each responding cell is assigned to 1 of 15 possible combinations of IFN-γ, IL-2, TNF, or IL-10. (D and E) Boolean-gated data in C are corrected for background and then presented either as a bar chart showing the frequency of each cytokine subset (D) or as a pie chart illustrating the fraction of the total response (E). In the pie chart shown, the numbers represent the order of cytokine subsets within the pie, the proportion of multifunctional IFN-γ+IL-2+TNF+ Th1 cells is depicted in red and the dark blue arc highlights the total proportion of IL-10–producing cells. Note that for vaccinations that do not elicit IL-10, Boolean analysis of IFN-γ–, IL-2–, and TNF-producing cells yields seven distinct populations (not depicted).
High-dose MML-ADV immunization elicits a poor quality Th1 response and a high frequency of IL-10-producing CD4+ T cells
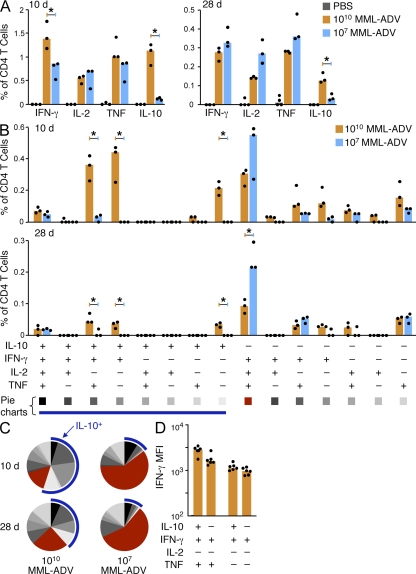
Consistent with our previous studies, the total frequency of IFN-γ–producing CD4+ splenocytes after i.m. vaccination of mice with high-dose MML-ADV (1010 vp) was greater than with low-dose MML-ADV (107 vp) at the peak of the immune response (10 d after vaccination; Fig. 2 A, left) but equivalent by the time of challenge (28 d after vaccination; Fig. 2 A [right]). Strikingly, a large frequency of IL-10–producing CD4+ T cells was detectable 10 and 28 d after vaccination with high- but not low-dose MML-ADV (Fig. 2 A). The IL-10 produced was antigen specific and not derived from natural T reg cells because all IL-10+ cells were confined to the CD4+CD25–Foxp3− population (Fig. S1). In assessing the quality of the response 10 and 28 d after vaccination, high-dose MML-ADV elicited CD4+ T cells that produced antigen-specific IL-10 alone or in combination with IFN-γ or IFN-γ and TNF (Fig. 2 B). It is of note that there were low to undetectable populations of CD4+ T cells that produced both IL-10 and IL-2 (Fig. 2 B). With respect to IL-10− populations, high-dose MML-ADV elicited a lesser frequency of IFN-γ+IL2+TNF+ multifunctional Th1 cells compared with low-dose MML-ADV (Fig. 2 B), which is consistent with our previous findings (Darrah et al., 2007). Thus, in comparing the overall quality of the Th1 response, high-dose MML-ADV elicits a heterogeneous CD4+ T cell cytokine response comprising a greater proportion of IL-10–producing cells (Fig. 2 C, blue arc) and a lesser proportion of IFN-γ+IL2+TNF+ multifunctional Th1 cells (Fig. 2 C, red pie slice) compared with low-dose MML-ADV. Additionally, in separate experiments, similar results were observed using the s.c. route of immunization (Fig. S2).
Figure 2.
High-dose MML-ADV immunization elicits IL-10–producing CD4+ T cells. MML-specific cytokine production by splenic CD4+ T cells 10 or 28 d after i.m. vaccination of mice with high- (1010 vp) or low- (107 vp) dose MML-ADV. (A–C) Multiparameter flow cytometry was used to quantify the total frequency of IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells (A), the frequency of cells expressing each of the fifteen possible combinations of IFN-γ, IL-2, TNF, or IL-10 (B) and the fraction of the total response represented by each of the fifteen individual cytokine populations (C). For pie charts, multifunctional Th1 cells are depicted in red and the blue arc highlights IL-10–producing cells. (D) The IFN-γ MFI of CD4+ T cells from mice vaccinated with high-dose MML-ADV (i.m. or s.c.) producing combinations of IFN-γ, TNF, or IL-10 10 d after vaccination. Bars show the median response from individual (n = 3–6) mice (dots; *, P < 0.05) for one of at least three experiments.
The production of IL-10 by CD4+ T cells might counteract APC-activating signals to weaken pathogen-killing mechanisms, accounting for the limited capacity of high-dose MML-ADV to protect. Alternatively, IL-10 might cause an intrinsic defect in Th1 function such as reduced potency of IFN-γ–producing effector populations that simultaneously secrete IL-10. In this regard, the IFN-γ median fluorescent intensity (MFI) of CD4+IFN-γ+IL-10+ T cells was not decreased compared with that of CD4+IFN-γ+IL-10− cells (Fig. 2 D), which is consistent with recently published data describing CD4+IFN-γ+IL-10+ T cell populations during infection with T. gondii (Jankovic et al., 2007).
IL-10 signaling does not influence the generation of Th1 or IL-10–producing CD4+ T cells after immunization with high-dose MML-ADV
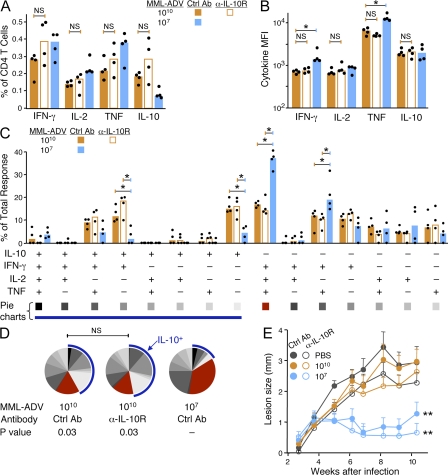
The next series of experiments focused on how IL-10 was elicited from CD4+ T cells after high-dose MML-ADV immunization and its effect, if any, on the quality of the immune response and protection. It is of note that IL-10 itself has been shown to give rise to antigen-specific IL-10 production from immunosuppressive CD4+ Tr1 cells (Groux et al., 1997). Thus, mice were treated with α-IL-10R at the time of priming to determine whether inhibition of IL-10 signaling would influence the magnitude and quality of Th1 cells or IL-10–producing CD4+ T cells. Treatment with α-IL-10R at the time of i.m. (Fig. 3) or s.c. (Fig. S3) immunization with high-dose MML-ADV did not significantly alter the total frequency (Fig. 3 A and Fig. S3 A) or the MFI (Fig. 3 B and Fig. S3 B) of IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells from the spleen 21 d later. Thus, IL-10 signaling appeared to have no role in priming for IL-10 production by CD4+ T cells. Furthermore, the quality of the response after high-dose MML-ADV was comparable in all mice independent of α-IL-10R treatment (Fig. 3, C and D; and Fig. S3, C and D). Accordingly, protection after high-dose MML-ADV vaccination was not enhanced in animals that received α-IL-10R treatment at the time of immunization, as determined by lesion progression (Fig. 3 E) or parasite burdens (not depicted).
Figure 3.
IL-10 signaling does not affect the generation of Th1 or IL-10–producing CD4+ T cells after high-dose MML-ADV immunization. (A and B) MML-specific cytokine production by splenic CD4+ T cells from WT mice treated with α-IL-10R or Ctrl Ab at the time of immunization, 21 d after high- or low-dose MML-ADV vaccination (i.m.). Shown are the total frequency (A) and MFI (B; note log scale) of IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells. (C and D) The percentage of the total CD4+ T cell response producing each of the 15 possible combinations of IFN-γ, IL-2, TNF, or IL-10 is illustrated in bar charts (C) showing the median response from individual (n = 4) mice (dots; *, P < 0.05) and pie charts (D) showing the mean. For pie charts, multifunctional Th1 cells are depicted in red and the blue arc highlights the total proportion of IL-10–producing cells. Significant differences compared with low-dose MML-ADV are noted with p-values. NS, not significant. Data are representative of three experiments. (E) In a separate experiment, mice treated with α-IL-10R (open circles) or Ctrl Ab (closed circles) at the time of immunization with high- or low-dose MML-ADV (i.m.) were challenged intradermally (i.d.) in the ear with live L. major 28 d after vaccination. Shown is the mean lesion size ± SEM over time after infection from at least 12 ears per group. **, difference (6–10 wk; P < 0.05) from mice vaccinated by the same route with high-dose MML-ADV. Data are representative of two experiments.
Immunization with high-dose MML-ADV is not protective, even in the absence of IL-10
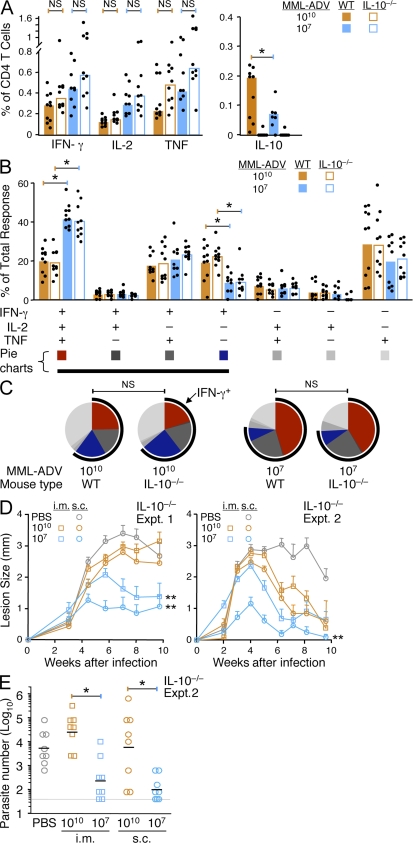
Because IL-10 from IFN-γ–producing CD4+ T cells has been shown to suppress parasite killing during chronic L. major infection (Anderson et al., 2007), we next determined whether the limited protection after high-dose MML-ADV immunization was a result of vaccine-induced IL-10 production by CD4+ T cells, poor Th1 quality, or both. Thus, we vaccinated WT or IL-10−/− mice with high- or low-dose MML-ADV. In data from three combined experiments, the total frequency of IFN-γ–, IL-2–, or TNF-producing CD4+ T cells from the spleen 21 d after i.m. (Fig. 4 A) or s.c. (Fig. S4 A) immunization with high-dose MML-ADV was not significantly enhanced in IL-10−/− mice compared with WT mice, which is consistent with the data in the previous section using α-IL-10R treatment. Moreover, the quality of the Th1 response generated in WT and IL-10−/− mice was identical when comparing the same dose and route of MML-ADV vaccination (Fig. 4, B and C; and Fig. S4, B and C). Thus, in both WT and IL-10−/− mice, high-dose MML-ADV vaccination elicits a qualitatively poor Th1 response, comprising a significantly smaller proportion of IFN-γ+IL2+TNF+ multifunctional Th1 cells and a significantly larger proportion of IFN-γ single-positive cells (Fig. 4, B and C; and Fig. S4, B and C) compared with low-dose MML-ADV vaccination. Finally, in two separate experiments the Th1 response elicited after i.m. or s.c. immunization with high-dose MML-ADV in the complete absence of IL-10 did not confer protection compared with low-dose MML-ADV, as determined by lesion progression (Fig. 4 D) or parasite burdens (Fig. 4 E). It is of note that lesion progression after challenge of IL-10−/− mice was similar to what we have previously reported in WT C57BL/6 mice (Darrah et al., 2007); however, IL-10−/− mice displayed lower parasite burdens than WT mice irrespective of vaccination (unpublished data), which is consistent with previous results (Belkaid et al., 2001). Collectively, these data show no significant effect of IL-10 by T cells or on T cells after high-dose MML-ADV immunization. Hence, the quality of the Th1 response is a critical determinant of protection after high-dose MML-ADV vaccination, independent of IL-10 production.
Figure 4.
The magnitude and quality of the Th1 response to high-dose MML-ADV immunization mice is not protective in IL-10−/− mice. (A–C) MML-specific cytokine production by splenic CD4+ T cells from WT or IL-10−/− mice 21 d after i.m vaccination with high- or low-dose MML-ADV. (A) The total frequency of IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells. (B and C) The percentage of the total CD4+ T cell response producing each of the seven possible combinations of IFN-γ, IL-2, or TNF is illustrated in bar charts (B) to show the median response from individual (n = 9–10) mice (dots; *, P < 0.05) and pie charts (C) to show the mean. IL-10 is excluded from the Boolean analysis because the relevant comparison is to IL-10−/− mice. For pie charts, multifunctional Th1 cells and IFN-γ single-positive cells are depicted in red and blue, respectively, and the black arc highlights the total proportion of IFN-γ–producing cells. Data are combined from three individual experiments. (D and E) 28 d after immunization, IL-10−/− mice that had been vaccinated with high- or low-dose MML-ADV either i.m. or s.c. were challenged in the ear (i.d.) with live L. major. (D) Shown is the mean lesion size ± SEM over time from infected ears (n = 12 per group) for two separate experiments. **, area under the curve is different (P < 0.05) from mice vaccinated by the same route with high-dose MML-ADV. (E) Shown is the number of parasites quantified from individual draining LN at 5 wk after infection for one experiment. Error bars indicate the geometric mean of individual (n = 8) LN (*, P < 0.01). The gray line indicates the level of detection.
MML + CpG, but not MML-ADV, immunization elicits IL-10 and IL-12 production from innate immune cells
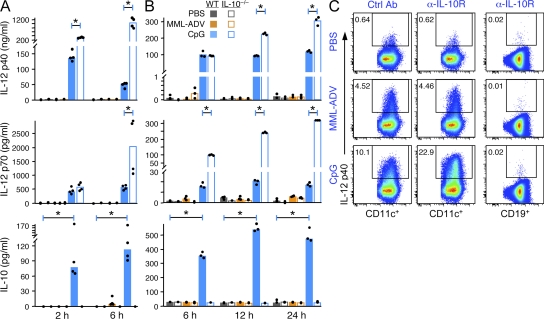
The prior data show that CD4+ T cell–derived IL-10 after high-dose MML-ADV immunization did not influence protection. Moreover, inhibiting IL-10 at the time of MML-ADV immunization had no effect on Th1 immunity. Although these data suggest that IL-10 is not involved in MML-ADV–induced immunity, we directly assessed production of IL-10 by innate immune cells in vitro and in vivo after stimulation with MML-ADV. To extend the innate profile, IL-12 production was also examined. As shown in Fig. 5, there was no detectable IL-10 or IL-12 (p40 or p70) in the serum after vaccination with MML-ADV (Fig. 5 A) or from BMDC stimulated with MML-ADV in vitro (Fig. 5 B), although IL-12 p40 was detected in LN DC immediately ex vivo after immunization compared with control mice (Fig. 5 C). However, there was no increase in IL-12 production in response to MML-ADV when IL-10 was absent or inhibited (Fig. 5, A-C). Thus, innate IL-10 production has no influence on the magnitude, quality, or IL-10–producing capacity of Th1 cells after high-dose MML-ADV immunization.
Figure 5.
CpG, but not MML-ADV, elicits innate IL-12 and IL-10. (A and B) Levels of IL-12 p40, IL-12 p70, or IL-10 protein in the serum (A) from WT and IL-10−/− mice 2 and 6 h after injection (i.v.) of PBS, 50 µg CpG, or 1010 vp MML-ADV, or in the culture supernatant (B) from WT and IL-10−/− BMDC after 6-, 12-, or 24-h stimulation with PBS, 1 µg/ml CpG, or 103 vp MML-ADV per BMDC measured by ELISA or cytometric bead array (CBA). Bars show the median response from individual mice (n = 4) or BMDC samples (n = 3). (C) Detection of IL-12 p40 (by intracellular staining [ICS]) from CD11c+ DCs or CD19+ B cells from pooled (n = 20) LN of Ctrl Ab– or α-IL-10R–treated mice that were injected (s.c.) 6 h earlier with PBS, 50 µg CpG, or 1010 vp MML-ADV. Each dataset (A–C) is representative of two experiments (*, P < 0.05).
The other vaccine formulation that we have previously studied for generating protective multifunctional Th1 responses against L. major in mice is MML protein with CpG (MML + CpG). With this vaccine approach, CpG promotes Th1 immunity through production of IL-12 from DCs (Chu et al., 1997; Roman et al., 1997; Fig. 5). CpG also induces production of IL-10 from innate immune cells (Boonstra et al., 2006; Samarasinghe et al., 2006; Fig. 5, A and B). Importantly, inhibition of such innate IL-10, through use of either IL-10−/− mice or α-IL-10R treatment, increases the amount of CpG-induced IL-12 p40 and IL-12 p70 in the serum (Fig. 5 A) from BMDC stimulated in vitro (Fig. 5 B) and from LN DC ex vivo (Fig. 5 C).
The magnitude and quality of the Th1 response after MML + CpG immunization is enhanced in the absence of IL-10
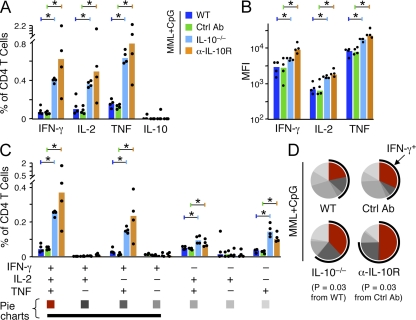
Fig. 5 shows that IL-12 production by APCs can be significantly increased by the inhibition of IL-10 during immunization with CpG; thus, we hypothesized that IL-10 may limit Th1 differentiation when using a CpG vaccine regimen. In this regard, three immunizations with MML + CpG are required to elicit a high frequency of multifunctional Th1 cells and protection after vaccination (Darrah et al., 2007). To assess whether manipulating IL-10 could enhance the efficiency of the MML + CpG vaccine regimen, mice were immunized once with MML + CpG in the presence (WT) or absence (IL-10−/−) of IL-10 or IL-10 signaling (α-IL-10R treatment). As shown in Fig. 6 A, there was a dramatic increase in the total magnitude of IFN-γ–, IL-2–, and TNF-producing CD4+ T cells 23 d after a single vaccination with MML + CpG in the absence of IL-10 or IL-10 signaling compared with WT mice. It is of note that IL-10–producing CD4+ T cells were not detected after a single vaccination of MML + CpG. In addition, there was a significant increase in the IFN-γ, IL-2, and TNF MFI (note log scale; Fig. 6 B), suggesting an underlying difference in the quality of the response. Indeed, of the four cytokine-producing populations that were significantly increased in the absence of IL-10 or IL-10 signaling, the most striking increase was in the multifunctional (IFN-γ+IL-2+TNF+) CD4+ T cells (Fig. 6 C) that produce significantly more IFN-γ (and therefore have a higher IFN-γ MFI) than double or single IFN-γ–producing CD4+ T cells and correlate with protection against L. major (Darrah et al., 2007). Thus, whereas WT animals generated low frequencies of predominantly TNF- or TNF- and IL-2–producing cells, animals vaccinated once with MML + CpG in the absence or blocking of IL-10 had a higher response with increased differentiation toward IFN-γ–producing cells (Fig. 6 D). It is of note that we consistently noted a greater effect when IL-10 was blocked using α-IL-10R rather than using IL-10−/− mice (Fig. 6, A–D), suggesting that compensatory mechanisms might exist in IL-10−/− mice.
Figure 6.
Th1 responses to a single MML + CpG immunization are enhanced in the absence of IL-10. (A and B) MML-specific cytokine production by splenic CD4+ T cells from WT, IL-10−/−, or Ctrl Ab– or α-IL-10R–treated WT mice 23 d after a single immunization of with MML + CpG. Shown is the total frequency (A) and MFI (B) of IFN-γ–, IL-2–, TNF-, or IL-10–producing CD4+ T cells. (C and D) Multifunctional cytokine analysis. Shown is the absolute frequency (C) and relative proportion (D) of each individual cytokine-producing population. For pie charts, multifunctional Th1 cells are depicted in red and a black arc highlights the total fraction of IFN-γ–producing cells. Significant differences in quality are indicated by p-values. Bars show the median response from individual (n = 4) mice (dots; *, P < 0.05). Data are representative of at least four experiments.
The effect of IL-10 on enhancing the Th1 response to MML + CpG is IL-12 dependent
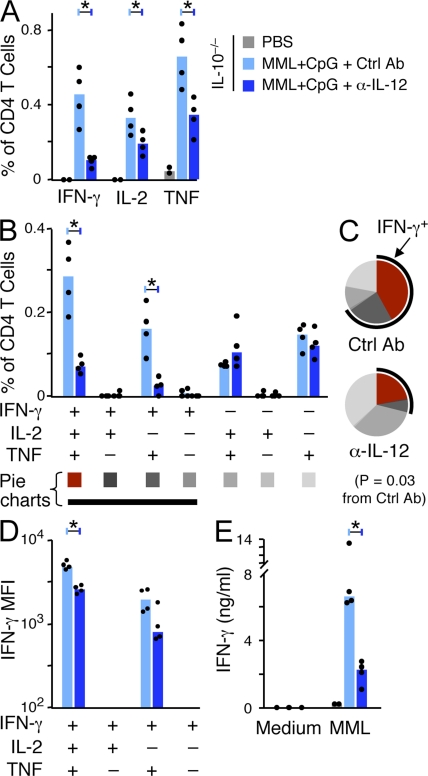
The dramatic change in magnitude and quality of the Th1 response after a single vaccination of MML + CpG in the absence of IL-10 or IL-10 signaling (Fig. 6) was likely driven by an increase in IL-12 because CpG-induced IL-12 was significantly enhanced in vitro and in vivo in the absence of IL-10 (Fig. 5). Accordingly, when IL-10−/− mice were treated with a neutralizing antibody to IL-12 (α-IL-12) at the time of a single immunization with MML + CpG, the median frequencies of IFN-γ, IL-2, and TNF compared with control antibody (Ctrl Ab)–treated IL-10−/− mice were decreased by ∼4.3-, 1.7-, and 1.9-fold, respectively (Fig. 7 A). Notably, the frequency of IFN-γ+IL-2+TNF+ multifunctional cells that produce high levels of IFN-γ and correlate with protection (Darrah et al., 2007), as well as IFN-γ+IL-2−TNF+ T cells, was significantly decreased (4.1- and 6.6-fold, respectively) by α-IL-12 treatment (Fig. 7 B). Furthermore, the quality of the Th1 response in α-IL-12–treated MML + CpG–immunized IL-10−/− mice (Fig. 7 C) appeared less differentiated than Ctrl Ab–treated IL-10−/− mice, as the proportion of cells producing only TNF or TNF and IL-2 (IFN-γ negative) was greater (69 vs. 32%). This less differentiated quality resembled the response in untreated WT mice receiving the same vaccine (Fig. 6 D, top). These data suggest that the neutralization of IL-12 effectively limited the extent of vaccine-induced Th1 differentiation in the absence of IL-10. Furthermore, immunization of IL-12 p40−/− mice confirmed the role of IL-12 in driving the magnitude and differentiation of the Th1 response after MML + CpG vaccination (Fig. S5). Lastly, α-IL-12 treatment of IL-10−/− mice at the time of vaccination resulted not only in lower frequencies of IFN-γ–producing Th1 populations but in IFN-γ+IL-2+TNF+ and IFN-γ+IL-2−TNF+ cells that secreted less IFN-γ on a per cell basis (note log scale; Fig. 7 D) and less antigen-specific IFN-γ production overall (Fig. 7 E) compared with Ctrl Ab–treated vaccinated IL-10−/− mice.
Figure 7.
Enhanced Th1 responses to MML + CpG immunization in IL-10−/− mice are IL-12–dependent. IL-10−/− mice were treated with α-IL-12 or Ctrl Ab at the time of a single immunization of MML + CpG. MML-specific cytokine responses in the spleen were determined 21 d later. (A) The total frequency of IFN-γ–, IL-2–, or TNF-producing CD4+ T cells. (B–D) Multifunctional cytokine analysis. Shown is the absolute frequency (B) and relative proportion (C) of each cytokine-producing population. For pie charts, multifunctional Th1 cells are depicted in red and a black arc highlights the total fraction of IFN-γ–producing cells. Significant differences are indicated by p-values. (D) The MFI of IFN-γ–producing CD4+ T cell populations. (E) IFN-γ secretion from total splenocytes measured by ELISA. Bars show the median responses from individual (n = 4) mice (dots; *, P < 0.05). Data are representative of two experiments.
The effect of IL-10 on enhancing the Th1 response to MML + CpG is indirect
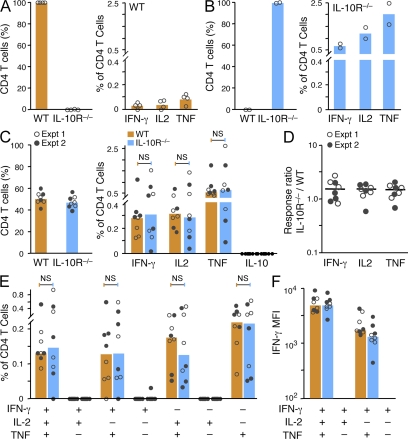
The previous data suggest that CpG-induced IL-10 limits the Th1 response to MML + CpG by restricting IL-12 production by APCs; however, a direct role for IL-10 on CD4+ T cells could not be excluded. To assess the in vivo mechanism for how vaccine-induced IL-10 influences Th1 immunity, BM chimera mice were generated by reconstituting irradiated Rag-1−/− mice with equal numbers of CD45-disparate WT and IL-10R−/− BM cells. Such mice provided a model to assess whether WT (IL-10R+/+) and IL-10R−/− Th1 cells generated within the same animal and under identical innate conditions would differ in the magnitude or quality after MML + CpG vaccination. Rag-1−/− control mice reconstituted with only WT cells had low but detectable frequencies of IFN-γ–, IL-2–, or TNF-producing CD4+ T cells after only a single vaccination of MML + CpG (Fig. 8 A). In contrast, much higher Th1 responses were measured in Rag-1−/− control mice reconstituted with only IL-10R−/− BM, in which all cells (including T cells and APCs) are refractory to IL-10 (Fig. 8 B). In mixed chimeric mice immunized with MML + CpG (Fig. 8, C–E), paired comparisons of WT and IL-10R−/− cytokine frequencies within the same mouse failed to demonstrate significant differences (Fig. 8 C) in two separate experiments (note symbols). Despite variation in the magnitude of cytokine production between mice, the ratio of IL-10R−/− to WT cytokine frequencies within the same mouse (Fig. 8 D) was consistent and was not significantly different than 1.0 (equal response ratio). Furthermore, there were no significant differences (by paired analysis) in the frequency of multifunctional cells between WT and IL-10R−/− Th1 cells (Fig. 8 E) or in the capacity of these populations to produce IFN-γ (Fig. 8 F). These data show that the restrictive effect of IL-10 on Th1 generation and differentiation after MML + CpG vaccination is not directly on T cells but on APCs to limit IL-12 production.
Figure 8.
The influence of IL-10 on Th1 responses elicited by MML + CpG immunization is indirect. MML-specific cytokine production by splenic CD4+ T cells from BM-reconstituted irradiated Rag-1−/− mice 21 d after a single immunization with MML + CpG. (A–C) The frequency of WT or IL-10R−/− CD4+ T cells in blood at time of vaccination (left) and the total frequency of IFN-γ–, IL-2–, TNF-, or IL-10–producing WT or IL-10R−/− CD4+ splenocytes (right) from vaccinated Rag-1−/− mice that had been reconstituted 5 mo earlier with WT BM only (A; n = 4), IL-10R−/− BM only (B; n = 2), or mixed BM (C; n = 8). (D) The cytokine response ratio for IFN-γ, IL-2, and TNF, calculated as the IL-10R−/− response (percentage of IL-10R−/− CD4+ T cells) divided by the WT response (percentage of WT CD4+ T cells) within the same chimeric mouse. The median ratio from individual chimeric mice (n = 8) is indicated by horizontal bars. (E and F) Multifunctional analysis of WT or IL-10R−/− CD4+ T cell responses from mixed BM chimera mice after vaccination. Shown are the absolute frequency (E) of each cytokine-producing population and the MFI (F) of IFN-γ–producing populations. Bars show the median response from individual mice (dots) combined from two separate experiments (indicated by open or closed symbols).
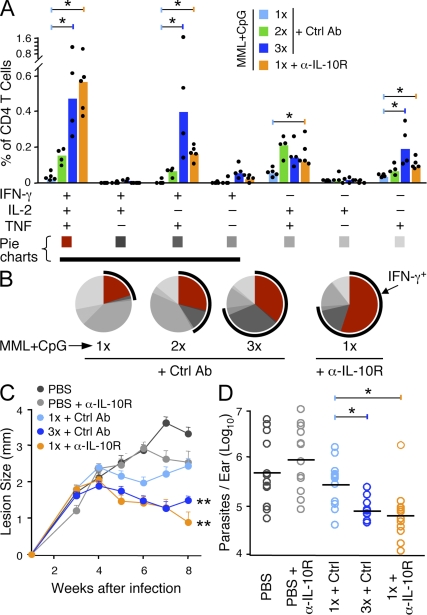
A single immunization with MML + CpG in the absence of IL-10 confers protection
We previously reported that three immunizations with MML + CpG elicits a high frequency of multifunctional IFN-γ+IL-2+TNF+ Th1 cells and confers protection (Darrah et al., 2007). In this paper, we have demonstrated that α-IL-10R treatment at the time of immunization dramatically enhances the magnitude and quality of the Th1 response to a single vaccination with MML + CpG. Thus, we sought to determine how Th1 responses elicited by a single immunization of MML + CpG in the presence of α-IL-10R compared with those elicited by three consecutive vaccinations in WT mice. When Th1 responses were analyzed 21 d after one (1×), two (2×), or three (3×) MML + CpG vaccinations with Ctrl Ab in WT mice, there was a clear hierarchy in the frequency (Fig. 9 A) and proportion (Fig. 9 B) of IFN-γ+IL-2+TNF+ and IFN-γ+IL2−TNF+ Th1 cells that correlated with the number of vaccinations. These data indicate that repetitive vaccination drives Th1 differentiation and are consistent with a linear differentiation model (Seder et al., 2008). Notably, the response elicited by a single vaccination of MML + CpG in WT mice treated with α-IL-10R (1× + α-IL-10R) appeared remarkably similar to the response observed in mice vaccinated 3× + Ctrl Ab, with respect to the frequency of multifunctional cells (Fig. 9 A) as well as the overall proportion of IFN-γ–producing cells (Fig. 9 B, black arc). To further assess whether these populations of CD4+ T cells were functionally equivalent, we compared their proliferative capacity, avidity for peptide, and ability to confer protection. Compared with 3×-vaccinated mice, CD4+ T cells from 1× + α-IL-10R–vaccinated mice proliferated to the same extent (Fig. S6 A) and, in preliminary studies, had comparable functional avidity (EC50: 1× + α-IL-10R, 70 ng/ml; 3×, 80 ng/ml; Fig. S6 B). The only evidence for a functional difference between mice receiving these vaccine regimens was in the trafficking of antigen-specific cells into the site of infection after L. major challenge. At 10 d after challenge, a significantly larger frequency of Th1 cells had migrated into the ears of mice vaccinated 3× with MML + CpG compared with mice vaccinated 1× + α-IL-10R (Fig. S7). However, despite the difference in frequency of antigen-specific Th1 cells in the ear shortly after infection, mice vaccinated 1× + α-IL10R were protected as well as those vaccinated 3× + Ctrl Ab as determined by ear lesion progression over time (Fig. 9 C) or parasite burdens (Fig. 9 D). Thus, blocking the effects of IL-10 only at the time of immunization converted the low magnitude and less differentiated quality elicited by a single MML + CpG vaccination into a robust vaccine that elicits a high frequency of multifunctional Th1 cells and protects as well as three vaccinations in WT mice.
Figure 9.
A single immunization with MML + CpG in the absence of IL-10 signaling elicits protection. Comparison of the immune response and subsequent protection in mice that received one (1×), two (2×), or three (3×) immunizations of MML + CpG (+ Ctrl Ab) to α-IL-10R–treated mice that received a single immunization of MML + CpG (1× + α-IL-10R). (A and B) Multifunctional analysis of the MML-specific CD4+ T cell response from the spleen 21 d after immunization. Shown is the absolute frequency (A) and the relative proportion (B) of each individual combination of IFN-γ–, IL-2–, or TNF-producing cells. Bars show the median response from individual (n = 4–5) mice (dots; *, P < 0.05). For pie charts, multifunctional Th1 cells are depicted in red and a black arc highlights the total fraction of IFN-γ–producing cells. Data are representative of three experiments. (C and D) Vaccine-elicited protection in mice challenged with live L. major 35 d after immunization. (C) Mean lesion size ± SEM over time from infected ears (n = 12 per group). **, different (5–8 wk; P < 0.05) from unvaccinated and 1×-vaccinated (+ Ctrl Ab) mice. (D) Parasite burdens were determined 7 wk after infection using real-time PCR. Horizontal bars show the geometric means of individual (n = 12) ears (*, P < 0.05). Data comparing 1× MML + CpG vaccination with and without α-IL-10R treatment are representative of three experiments.
DISCUSSION
In this paper, we extend our previous findings that a Th1 response of a particular quality is necessary to mediate protection against an intracellular infection, using two different vaccine modalities. Our study focused on the influence of vaccine-induced IL-10 from CD4+ T cells or APCs on the magnitude, quality, and protective capacity of the Th1 response. The data show a dose-dependent increase in IL-10 production by CD4+ T cells after immunization with MML-ADV; however, such CD4+ T cell-derived adaptive IL-10 did not impact Th1 immunity or protection. In contrast, inhibition of innate IL-10 produced by APCs at the time of immunization with MML + CpG significantly enhanced the magnitude of the response and the extent of Th1 differentiation and, importantly, increased protection. Together, these data demonstrate how IL-10 can be manipulated to achieve favorable Th1 quality and protection, depending on the vaccine platform. The mechanisms underlying these findings should inform vaccine design against infections requiring Th1 responses for protection.
In choosing specific vaccine platforms, recombinant ADV vectors are of broad interest based on their potential use as vaccines to prevent HIV, malaria, and tuberculosis infection. Among the various ADV serotypes, serotype 5 is the most potent inducer of T cell responses (Thorner et al., 2006). In this paper, we show that low-dose MML-ADV elicits a favorable Th1 quality and very few IL-10–producing CD4+ T cells, and it protects mice from L. major challenge. In contrast, high-dose MML-ADV elicits a poor quality Th1 response and a high frequency of IL-10–producing CD4+ T cells, and it does not protect. Further characterization of the Th1 response to high-dose MML-ADV at the single-cell level revealed that a majority of the IL-10 produced was from CD4+CD25−Foxp3− T cells that also produced IFN-γ with or without TNF, which is indicative of a Th1 lineage. Several recent studies have demonstrated dramatic effects of CD4+ T cell–derived IL-10 in vivo in the context of ongoing infection. In this regard, IL-10 production by CD4+IFN-γ+ Th1 cells limits an excessive proinflammatory response during the course of T. gondii (Jankovic et al., 2007) and Flu infections (Sun et al., 2009). Furthermore, in the setting of chronic L. major infection, production of IL-10 by CD4+IFN-γ+ Th1 cells limits parasite killing and protection (Anderson et al., 2007). With regard to vaccines, α-IL-10R treatment increased vaccine-elicited protection in the BALB/c model of L. major infection (Stober et al., 2005). In the latter study, it was speculated that blocking IL-10 derived from CD4+CD25+ Tr1-like cells during the course of infection enhanced protection (Stober et al., 2005). Given the findings in the L. major model, we hypothesized that IL-10–producing CD4+ T cells elicited by high-dose MML-ADV immunization limited control of infection. Surprisingly, in the absence of IL-10 or IL-10 signaling at the time of vaccination or throughout the infection, the magnitude or the quality of the Th1 response after high-dose MML-ADV immunization was not enhanced nor was the protection improved. As we were unable to demonstrate production of other inhibitory cytokines (IL-4, IL-13, and TGF-β) from CD4+ T cells after high-dose MML-ADV immunization (unpublished data), these data underscore that the quality of the Th1 response is a necessary determinant of protection against L. major infection. Because a single immunization with MML-ADV can confer protection when given at a lower dose, it is likely that the quality of the response can be negatively impacted by the vector providing more antigen, altered innate immunity, or both. Studies to analyze the effect of each of these variables on the quality of the Th1 response are underway.
The other vaccine formulation analyzed in this study was MML + CpG. Protein vaccines formulated with TLR ligands, such as monophosphoryl lipid A, are currently being tested in humans for prevention of leishmaniasis, tuberculosis, and malaria (Coler and Reed, 2005; Skeiky and Sadoff, 2006; Coler et al., 2009). They offer an advantage over certain viral vaccines in that they potently elicit both antibody and Th1 responses and can be used repetitively. Paradoxically, some TLR ligands, such as CpG or LPS, can prime Th1 responses through induction of IL-12 but also elicit the inhibitory cytokine IL-10 (Chu et al., 1997; Roman et al., 1997; Boonstra et al., 2006; Samarasinghe et al., 2006). Thus, we hypothesized that Th1 responses would be significantly altered by inhibiting IL-10 after vaccination with MML + CpG. Indeed, α-IL-10R treatment has been shown to enhance the Th1 recall response to soluble ovalbumin protein when primed in the presence of the LPS (Castro et al., 2000). In this paper, we extended these findings by defining the role that IL-10 has in the magnitude and quality of the Th1 response at the single-cell level in a model that allowed us to assess vaccine-elicited protection. CpG elicited high amounts of innate IL-12 (p40 and p70) and IL-10 in the serum after injection or from BMDCs stimulated in vitro; moreover, IL-12 levels were enhanced in IL-10−/− mice. In addition, the amount of IL-12 expressed ex vivo by LN DCs isolated after CpG immunization was higher when IL-10 signaling was inhibited. Such perturbations in IL-10 production also had a striking effect on vaccine-elicited Th1 responses. Vaccination of IL-10−/− or α-IL-10R–treated mice with a single injection of MML + CpG dramatically increased the magnitude of the Th1 response, altered the Th1 quality, and enhanced protection compared with a single vaccination of WT mice in an IL-12–dependent manner. Last, MML + CpG vaccination of BM chimera mice revealed no difference in a WT or IL-10R−/− Th1 response generated under identical innate conditions within the same mouse, ruling out a direct role of IL-10 on CD4+ T cells. Thus, innate IL-10 inhibits IL-12 production from DCs and limits the extent of Th1 differentiation in response to a transient amount of protein antigen when given with CpG.
The data from the MML + CpG vaccinations provide a mechanism for why multiple immunizations are required to elicit a frequency of multifunctional Th1 cells sufficient for protection using a protein vaccine with a TLR ligand that induces both IL-10 and IL-12. IL-10 can protect the host by down-regulating IL-12–driven proinflammatory Th1 responses during infection with T. gondii or influenza or exposure to LPS (Gérard et al., 1993; Howard et al., 1993; Jankovic et al., 2007; Sun et al., 2009). However, the same negative feedback limits Th1 generation and differentiation when adapting the constituents of such pathogens as vaccine adjuvants. The finding that limiting IL-10 can dramatically enhance Th1 responses generated by protein and TLR ligand-based vaccine formulations has implications for improving vaccine design in humans. Most protein-based vaccines must be given several times over a prolonged period of time to induce a protective response. If transient inhibition of IL-10 improves the efficiency of vaccination so that fewer immunizations are required, it might increase compliance and save cost in resource-poor areas. In this regard, inclusion of p38 MAPK inhibitor increased the protection of a low-dose CpG-based pertussis vaccine regimen through inhibition of T reg cell–derived IL-10, although a specific effect on the Th1 response was not demonstrated (Jarnicki et al., 2008).
This paper shows the effects of IL-10 by CD4+ T cells and APCs in the context of protection using different vaccine regimens. Indeed, we demonstrate that three vaccines (low-dose MML-ADV, 3× MML + CpG, and 1× MML + CpG + α-IL-10R) elicited Th1 responses of comparable frequency, quality, and level of protection. Importantly, the mechanisms by which these vaccines elicit such responses appear distinct vis-à-vis the role of innate immunity, antigen dose, persistence, and repetitive immunization. We have incorporated these variables in our model of Th1 differentiation (Fig. S8). In conclusion, balancing the extent of Th1 differentiation to achieve protective and durable Th1 immunity remains a hurdle for developing vaccines for infections such as leishmaniasis and tuberculosis. This study provides insight into how vaccines can be manipulated to achieve protective immunity.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (B6), B6.IL-10−/−, B6.IL-10R−/−, B6.IL-12 p40−/−, B6.CD45.1, and B6.RAG-1−/− were obtained from The Jackson Laboratory. BM chimeras were generated by injecting 2.5 × 106 BM cells from B6.IL-10R−/− (CD45.2) and WT B6.CD45.1 mice into irradiated (650 rad) B6.Rag-1−/− mice. All mice were maintained in animal care facilities at the National Institutes of Health under pathogen-free conditions and all experiments were approved by the Vaccine Research Center animal care and use committee.
Immunizations and in vivo mAb treatment.
MML (also known as Leish-111f) is a recombinant polyprotein derived from Leishmania species, shown to be protective in vivo, and comprising three proteins: TSA (also known as MAPS), LmSTI1 (also known as M15), and LeIF (Skeiky et al., 2002). Mice were immunized once with 1010 or 107 vp (∼16 vp per infectious unit) of replication-defective ADV serotype 5 expressing MML (MML-ADV; provided by S.G. Reed, Infectious Disease Research Institute, Seattle, WA) either i.m. (leg) or s.c. (foot). 25 µg MML protein (SAIC-Frederick, Inc.) mixed with 50 µg CpG 1826 (Pfizer) was administered s.c. once, twice, or three times, 2–3 wk apart, with the final vaccination for each group on the same day. In some experiments, 0.5 mg α-IL-10R (1B1.3) or GL113 (Ctl Ab) or 1 mg α-IL-12 (C17.8) was given i.p. once, 2–4 h before vaccination. 1B1.3, GL113, and C17.8 were provided by F.D. Finkelman (University of Cincinnati, Cincinnati, OH).
Multiparameter flow cytometry.
1.5 × 106 splenocytes or cells harvested from infected ears, as previously described (Belkaid et al., 2000), were cultured with 2 µg/ml α-CD28 (37.51) and 20 µg/ml MML or up to 2 µg/ml (each) MML peptides (Mimotopes; 15-mers overlapping by 11 corresponding to TSA and LmSTI1) for 2 h before addition of 10 µg/ml brefeldin A (BFA; Sigma-Aldrich) for an additional 4 h. For ICS, cells were washed and stained with the LIVE/DEAD Fixable Violet Dead Cell Stain (ViViD; Invitrogen) as previously described (Perfetto et al., 2006), followed by staining with panels of antibodies against the surface markers CD3 (145-2C11), CD4 (RM4-5), CD8 (53–6.7; BioLegend), CD45.1 (A20), CD45.2 (104), and CD25 (PC61) and the intracellular markers IFN-γ (XMG1.2), IL-2 (JES6-5H4), TNF (MP6-XT22), IL-10 (JES5-16E3; eBioscience), or Foxp3 (FJK-16s; eBioscience), using the Cytofix/Cytoperm kit or Foxp3 kit (for panels including Foxp3; eBioscience) according to the manufacturer’s instructions. All ICS reagents were purchased from BD except where noted. 250,000 live lymphocytes per sample were acquired using a modified LSR II (BD) and analyzed using FlowJo software (version 8.8.6; Tree Star, Inc.) and Pestle (version 1.6.2)/SPICE (version 4.2.3; M. Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD).
DCs and DC staining.
BMDCs used for innate cytokine screening by ELISA were obtained by culturing mouse BM for 9 d in mouse GM-CSF and collecting nonadherent cells. For IL-12 p40 ICS, popliteal LN were harvested from Ctrl Ab– or α-IL-10R–treated (250 µg, 1 d prior) mice 6 h after s.c. injection with 25 µg or 5 × 109 MML-ADV vp (per footpad) in medium containing 10 µg/ml BFA. Pooled LN from 10 mice were minced and incubated with 1 mg/ml collagenase D (Roche) and 10 µg/ml DNase I (Roche Applied Science) with 10 µg/ml BFA for 30 min at 37°C. LN homogenate was enriched for CD11c+ DCs by positive selection using MACS MicroBead (Miltenyi Biotec) cell separation according to the manufacturer’s instructions. Both column-enriched and column flow-through cells were surface stained for ViViD, CD11c (HL3; BD), NK1.1 (PK136; BioLegend), CD8 (53–6.7; BioLegend), CD19 (6D5; BioLegend), and B220 (RA3-6B2; BD), followed by ICS for CD3 (145-2C11) and IL-12 p40 (C15.6; BioLegend) as described in Multiparameter flow cytometry. Cells were collected and analyzed, as described in Multiparameter flow cytometry, using a DC gating strategy to exclude dead, NK, T, and B cells.
Infectious challenge and parasite quantitation.
Mice were challenged i.d. in the ear with 500–1,000 metacyclic L. major promastigotes (V1, MHOM/IL/80/Friedlin) 28–35 d after vaccination, as previously described (Belkaid et al., 2000). The diameter of dermal lesions (at least 12 ears per time point) was measured weekly using calipers. The number of parasites in ear or draining LN at 5–7 wk after infection was determined either by scoring the highest dilution of LN homogenate containing viable parasites after incubation for 5–6 d at 26°C or by extracting DNA from 6-mm punch biopsies from the ear and performing real-time PCR for leishmanial ribosomal DNA as previously described (Kimblin et al., 2008). PCR samples were run in duplicate on a Prism 7700 sequence detection system (Applied Biosystems) and compared with a standard curve of L. major DNA.
ELISA and CBA.
4 × 105 splenocytes from immunized mice or 7.5 × 105 BMDC were cultured with 10–20 µg/ml MML protein, 0.1–100 µg/ml CpG, or 102:1-104:1 (vp/BMDC) MML-ADV in a total volume of 200–250 µl for 6–48 h. For serum cytokine analysis, serum was collected from mice 2–6 h after i.v. injection with 50 µg CpG or 1010 vp MML-ADV. Cytokines in cell supernatants or serum were measured using Quantikine kits (R&D Systems) for IFN-γ, IL-10, IL-12/IL-23 p40, and IL-12 p70 (BMDC supernatant). CBA Inflammation kit (BD) was used for IL-12 p70 (serum).
Proliferation assay.
Splenocytes harvested from mice 17 d after vaccination were labeled with 0.25 µM CFSE (Invitrogen) at a concentration of 1.0 × 106 cells/ml in PBS for 7 min at 37°C. Cells were washed and cultured in duplicate at a concentration of 3.0 × 106 cells/ml/tube in the presence of 5 µg/ml MML for 5–6 d, protected from light. For the final 7 h, 1 µg/ml (each) MML peptides and 10 µg/ml BFA were added, followed by ICS as described in Multiparameter flow cytometry. A mean of 105 live T cells were analyzed per sample.
Statistics.
All cytokine frequencies are reported after background subtraction of the frequency of the identically gated population of cells from the same sample stimulated without antigen. Bars in figures show the median of individual mice (dots in figures). Total spleen cell counts and total numbers of viable CD4+ T cells were not significantly different between vaccine groups within the same experiment. All comparisons (bar and pie charts) of cytokine production between vaccine groups were done in SPICE v4.2.3 using a two-tailed Student’s t test assuming unequal variances or Wilcoxin-rank test (for n ≥ 4 mice). For BM chimeras, a paired analysis (t test and Wilcoxin) was used to compare responses of BM subsets within the same mouse. For challenge data, area under the curve or lesion sizes at individual time points were compared by Student’s t test using SigmaPlot (version 8; Systat Software Inc.) or JMP v5.1 (SAS Institute Inc.). Avidity was determined by a nonlinear least-squares fit of a standard Michaelis-Menten binding curve using JMP (version 5.1). Significance was determined by comparing the EC50 (effective concentration that elicits half of the maximum response) for titrations from each individual mouse. For all comparisons, p-values <0.05 are noted.
Online supplemental material.
Fig. S1 shows that high-dose MML-ADV does not induce T reg cells and elicits IL-10 from CD4+CD25−Foxp3− T cells. Fig. S2 shows that s.c. immunization with high-dose MML-ADV vaccination elicits IL-10–producing CD4+ T cells. Fig. S3 shows that blocking IL-10 signaling at the time of s.c. immunization with high-dose MML-ADV does not influence the generation of Th1 or IL-10–producing CD4+ T cells. Fig. S4 demonstrates that IL-10 does not impact the magnitude, quality, or protective capacity of the Th1 response after s.c. immunization with high-dose MML-ADV. Fig. S5 confirms the role of IL-12 p40 in driving the magnitude and differentiation of the Th1 response after MML + CpG vaccination. Fig. S6 demonstrates that antigen-specific CD4+ T cells generated after vaccination with 3× MML + CpG or 1× MML + CpG + α-IL-10R display comparable proliferation and functional avidity in vitro. Fig. S7 shows antigen-specific CD4+ T cells in the ear after L. major challenge of mice vaccinated with MML + CpG. Fig. S8 illustrates a model for linear differentiation of Th1 and IL-10–producing CD4+ T cells.
Acknowledgments
MML-ADV and MML DNA was provided by S.G. Reed (Infectious Disease Research Institute, Seattle, WA). Antibodies to IL-10R and IL-12 were provided by F.D. Finkelman (University of Cincinnati). Assistance in generation of BM chimera mice was provided by K.E. Foulds (National Institutes of Health [NIH]).
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ADV
- adenovirus
- α-IL-10R
- anti–IL-10 receptor
- CBA
- cytometric bead array
- Ctrl Ab
- control antibody
- ICS
- intracellular staining
- i.d.
- intradermally
- MFI
- median fluorescent intensity
- TLR
- toll-like receptor
- vp
- viral particle
- T reg
- regulatory T
References
- Anderson C.F., Mendez S., Sacks D.L. 2005. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J. Immunol. 174:2934–2941 [DOI] [PubMed] [Google Scholar]
- Anderson C.F., Oukka M., Kuchroo V.J., Sacks D. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297 10.1084/jem.20061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Mendez S., Lira R., Kadambi N., Milon G., Sacks D. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969–977 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Hoffmann K.F., Mendez S., Kamhawi S., Udey M.C., Wynn T.A., Sacks D.L. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti–IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497–1506 10.1084/jem.194.10.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507 10.1038/nature01152 [DOI] [PubMed] [Google Scholar]
- Boonstra A., Rajsbaum R., Holman M., Marques R., Asselin-Paturel C., Pereira J.P., Bates E.E., Akira S., Vieira P., Liu Y.J., et al. 2006. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J. Immunol. 177:7551–7558 [DOI] [PubMed] [Google Scholar]
- Castro A.G., Neighbors M., Hurst S.D., Zonin F., Silva R.A., Murphy E., Liu Y.J., O’Garra A. 2000. Anti–interleukin 10 receptor monoclonal antibody is an adjuvant for T helper cell type 1 responses to soluble antigen only in the presence of lipopolysaccharide. J. Exp. Med. 192:1529–1534 10.1084/jem.192.10.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu R.S., Targoni O.S., Krieg A.M., Lehmann P.V., Harding C.V. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623–1631 10.1084/jem.186.10.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler R.N., Reed S.G. 2005. Second-generation vaccines against leishmaniasis. Trends Parasitol. 21:244–249 10.1016/j.pt.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Coler R.N., Carter D., Friede M., Reed S.G. 2009. Adjuvants for malaria vaccines. Parasite Immunol. 31:520–528 10.1111/j.1365-3024.2009.01142.x [DOI] [PubMed] [Google Scholar]
- Darrah P.A., Patel D.T., De Luca P.M., Lindsay R.W., Davey D.F., Flynn B.J., Hoff S.T., Andersen P., Reed S.G., Morris S.L., et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 10.1038/nm1592 [DOI] [PubMed] [Google Scholar]
- Forbes E.K., Sander C., Ronan E.O., McShane H., Hill A.V., Beverley P.C., Tchilian E.Z. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. 1993. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J. Exp. Med. 177:547–550 10.1084/jem.177.2.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742 10.1038/39614 [DOI] [PubMed] [Google Scholar]
- Howard M., Muchamuel T., Andrade S., Menon S. 1993. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 177:1205–1208 10.1084/jem.177.4.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Hieny S., Caspar P., Collazo C.M., Sher A. 2002. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-) setting. Immunity. 16:429–439 10.1016/S1074-7613(02)00278-9 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Kullberg M.C., Feng C.G., Goldszmid R.S., Collazo C.M., Wilson M., Wynn T.A., Kamanaka M., Flavell R.A., Sher A. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnicki A.G., Conroy H., Brereton C., Donnelly G., Toomey D., Walsh K., Sweeney C., Leavy O., Fletcher J., Lavelle E.C., et al. 2008. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J. Immunol. 180:3797–3806 [DOI] [PubMed] [Google Scholar]
- Kimblin N., Peters N., Debrabant A., Secundino N., Egen J., Lawyer P., Fay M.P., Kamhawi S., Sacks D. 2008. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc. Natl. Acad. Sci. USA. 105:10125–10130 10.1073/pnas.0802331105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenstrøm T., Agger E.M., Korsholm K.S., Darrah P.A., Aagaard C., Seder R.A., Rosenkrands I., Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047–8055 10.4049/jimmunol.0801592 [DOI] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- Perfetto S.P., Chattopadhyay P.K., Lamoreaux L., Nguyen R., Ambrozak D., Koup R.A., Roederer M. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods. 313:199–208 10.1016/j.jim.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Roestenberg M., McCall M., Hopman J., Wiersma J., Luty A.J., van Gemert G.J., van de Vegte-Bolmer M., van Schaijk B., Teelen K., Arens T., et al. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361:468–477 10.1056/NEJMoa0805832 [DOI] [PubMed] [Google Scholar]
- Roman M., Martin-Orozco E., Goodman J.S., Nguyen M.D., Sato Y., Ronaghy A., Kornbluth R.S., Richman D.D., Carson D.A., Raz E. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849–854 10.1038/nm0897-849 [DOI] [PubMed] [Google Scholar]
- Sacks D., Anderson C. 2004. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol. Rev. 201:225–238 10.1111/j.0105-2896.2004.00185.x [DOI] [PubMed] [Google Scholar]
- Samarasinghe R., Tailor P., Tamura T., Kaisho T., Akira S., Ozato K. 2006. Induction of an anti-inflammatory cytokine, IL-10, in dendritic cells after toll-like receptor signaling. J. Interferon Cytokine Res. 26:893–900 10.1089/jir.2006.26.893 [DOI] [PubMed] [Google Scholar]
- Seder R.A., Darrah P.A., Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- Skeiky Y.A., Sadoff J.C. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469–476 10.1038/nrmicro1419 [DOI] [PubMed] [Google Scholar]
- Skeiky Y.A., Coler R.N., Brannon M., Stromberg E., Greeson K., Crane R.T., Webb J.R., Campos-Neto A., Reed S.G. 2002. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 20:3292–3303 10.1016/S0264-410X(02)00302-X [DOI] [PubMed] [Google Scholar]
- Stober C.B., Lange U.G., Roberts M.T., Alcami A., Blackwell J.M. 2005. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J. Immunol. 175:2517–2524 [DOI] [PubMed] [Google Scholar]
- Sun J., Madan R., Karp C.L., Braciale T.J. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15:277–284 10.1038/nm.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner A.R., Lemckert A.A., Goudsmit J., Lynch D.M., Ewald B.A., Denholtz M., Havenga M.J., Barouch D.H. 2006. Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J. Virol. 80:12009–12016 10.1128/JVI.01749-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. 2001. Regulatory role of T cells producing both interferon γ and interleukin 10 in persistent infection. J. Exp. Med. 194:F53–F57 10.1084/jem.194.10.f53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C., Caskey M., Nchinda G., Longhi M.P., Mizenina O., Huang Y., Schlesinger S.J., Colonna M., Steinman R.M. 2008. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. USA. 105:2574–2579 10.1073/pnas.0711976105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.Y., Kirman J.R., Rotte M.J., Davey D.F., Perfetto S.P., Rhee E.G., Freidag B.L., Hill B.J., Douek D.C., Seder R.A. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852–858 10.1038/ni832 [DOI] [PubMed] [Google Scholar]