Abstract
Purpose
With their prolonged survival and malnutrition, cancer patients, and especially gastrointestinal (GI) tract cancer patients, can develop Wernicke's encephalopathy (WE). The aim of this study is to remind physicians of the importance of WE and prompt management in patients with GI tract cancer.
Materials and Methods
This study is a retrospective review of 2 cases of WE in advanced gastric cancer (AGC) patients, and we review the literature for cases of GI tract cancer related to WE.
Results
A 48-year-old female with AGC presented dizziness and diplopia for 5 days and a 20 kg weight loss. Neurologic exam showed nystagmus and gaze disturbance. Her symptoms improved after daily parenteral injection of thiamine 100 mg for 17 days. A 58-year-old female with AGC presented with sudden disorientation, confusion and 15 kg weight loss. Neurologic exam showed gaze limitation and mild ataxia. Despite daily parenteral injection of thiamine 100 mg for 4 days, she died 5 days after the onset of neurologic symptoms. Combining the cases noted in the literature review with our 2 cases, the 7 gastric cancer cases and 2 colorectal cancer cases related to WE showed similar clinical characteristics; 1) a history of long-period malnutrition and weight loss, 2) relatively typical neurologic signs and symptoms and 3) specific magnetic resonance image findings. Except for 2 patients who had irreversible neurologic symptoms, the other 7 patients were improved with prompt thiamine treatment.
Conclusion
It is important to consider WE in GI tract cancer patients with acute neurologic symptoms and who are in a state of malnutrition. Thiamine should be given as soon as possible when WE is suspected.
Keywords: Wernicke's encephalopathy, Advanced gastric cancer, Thiamine
Introduction
Wernicke's encephalopathy (WE) is a neurologic syndrome that is caused by thiamine deficiency, and this is clinically characterized by ophthalmoplegia, ataxia and acute confusion (1). It has been underdiagnosed because WE does not always present with all these typical symptoms. Although WE usually results from chronic alcohol dependency, non-alcoholic causes are reported in 20% to 50% of the cases, such as gastrointestinal (GI) tract surgery, hyperemesis gravidarum, AIDS, chronic malnutrition, long term parenteral nutrition and uncommonly in cancer patients (2-5). There are some case reports of WE in malignant disease such as malignant lymphoma, acute leukemia and breast cancer. As the exact incidence is not known due to the lack of concern about WE, the pathophysiology of WE in cancer patients has not been determined as well. Increased thiamine consumption due to the rapid growth of cancer cells and inadequate nutrition due to the nausea and anorexia from chemotherapy or malabsorption syndrome have been suggested as possible reasons for cancer-associated WE (6).
Patients with advanced gastric cancer (AGC) have tradionally had a poor prognosis and short life expectancy despite aggressive chemotherapy. Through the development of new chemotherapeutic agents/regimens and the introduction of the best supportive care, including total parenteral nutrition (TPN), there has been an increased number of AGC patients who achieve long term survival. However, there are still a significant number of AGC patients who suffer from peritoneal carcinomatosis with or without intestinal obstruction. Therefore, AGC patients have higher risk of chronic malnutrition combined with cancer-specific cachexia, suggesting they have a high risk of developing WE. Yet the cases of AGC combined with WE are not as frequently reported as we expected and this probably due to a lack of awareness of this combined condition.
We describe here 2 patients with WE combined with AGC and we also review the medical literature about the clinicopathologic features of WE in advanced GI tract cancer patients.
Results
1. Case 1
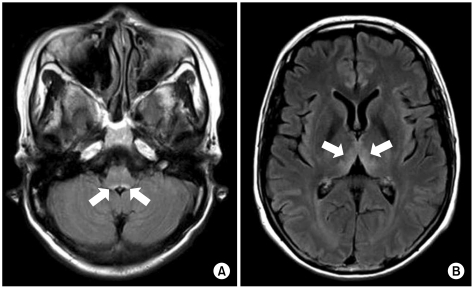
A 48-year-old woman with AGC (signet ring cell carcinoma) and left ovarian metastasis (cTxNxM1, Stage IV) received 16 cycles of paclitaxel/S-1 chemotherapy [paclitaxel 70 mg/m2 i.v. infusion day 1, 8; S-1 70 mg/m2 p.o. for 14 days, every 3 weeks] as the palliative chemotherapy. One week after the last chemotherapy, she was admitted for nausea, vomiting and dysphagia. Obstruction of the esophagogastric junction was observed on the abdomen-pelvis computed tomography, and esophagogastric junction stent insertion was performed. After stent insertion, she could tolerate a liquid diet, and she was discharged. Two months later, however, she relapsed with dysphagia, and so she received intermittent parenteral nutrition at home. Her weight loss was about 20 kg during the next 2 months. She was readmitted because of dizziness and diplopia for 5 days. She also had nystagmus and ataxia. The laboratory findings on admission were serum total protein 7.0 g/dL, albumin 3.5 g/dL, BUN 22.3 mg/dL, creatinine 0.7 mg/dL, AST 18 IU/L, ALT 16 IU/L, Na 137 mmol/L, K 3.8 mmol/L, Cl 97 mmol/L, tCO2 25 mmol/L, Ca++ 4.49 mg/dL, Mg++ 1.41 mg/dL, ammonia 94 µg/dL, PT 97% (INR 1.02) and aPTT 27.1 sec. There was no evidence of central nervous system infection or leptomeningeal seeding. Magnetic resonance imaging (MRI) of the brain showed high signal lesion on the bilateral thalami, the midbrain, and the ventral side of the pons on the flare images, and all this was compatible with WE (Fig. 1). After 2 days of thiamine 100 mg i.v. administration, which started on hospital day 2, her nystagmus and diplopia were improved. Seventeen days after thiamine injection, she was discharged from the hospital with only mild nystagmus.
Fig. 1.
(A) Bilateral symmetric signal increase at the midbrain. (B) Bilateral symmetric signal increase at the medial part of the thalami (Flair MRI).
2. Case 2
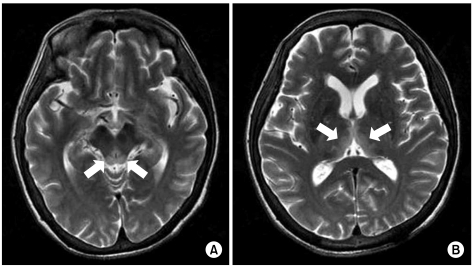
A 58-year-old woman with AGC (signet ring cell carcinoma) received subtotal gastrectomy with lymph node dissection and gastroduodenostomy (pT3N3M0, Stage IV). She did not receive postoperative chemotherapy because of her poor general condition. About 6 months later, she developed metastasis to the rectus abdominis muscle, omentum and liver, which was seen on abdomenpelvis computed tomography. After a 2nd round of palliative FOLFOX-4 chemotherapy [oxaliplatin 100 mg/m2 i.v. infusion day 1; leucovorin 200 mg/m2 day 1~2; 5-fluorouracil (5-FU) 400 mg/m2 i.v. bolus day 1~2; 5-FU 600 mg/m2 i.v. infusion day 1~2, every 2 weeks], she could not continue chemotherapy due to her general weakness, anorexia, nausea, vomiting and weight loss (15 kg for 2 months). Forty-five days after the last chemotherapy, she developed confusion and disorientation for 2 days, and this was followed by ataxia and gaze palsy. Her laboratory findings on admission were serum total protein 5.3 g/dL, albumin 2.8 g/dL, BUN 20.0 mg/dL, creatinine 0.7 mg/dL, AST 16 IU/L, ALT 14 IU/L, total bilirubin 3.6 mg/dL, Na 134 mmol/L, K 4.6 mmol/L, Cl 95 mmol/L, tCO2 24 mmol/L, Ca++ 0.42 mg/dL, Mg++ 0.18 mg/dL, ammonia 53 µg/dL, PT 81% (INR 1.15) and aPTT 40.8 sec. There was no evidence of central nervous system infection or leptomeningeal seeding. MRI of the brain showed high signal lesion on the bilateral thalami, the hypothalamus and the mammillary bodies on the T2 weighted images (Fig. 2). On hospital day 2, she also had generalized clonic seizure. The electroencephalogram (EEG) that was taken on hospital day 4 showed low amplitude delta background activity and no posterior dominant rhythm. With the impression of WE, thiamine was injected 100 mg per day on hospital day 2. She then had recurrent seizure attack and aggravation of her general condition, which resulted in death on hospital day 6.
Fig. 2.
(A) High signal lesion around the hypothalamus. (B) High signal lesion at the medial part of the thalami (T2WI MRI).
Discussion
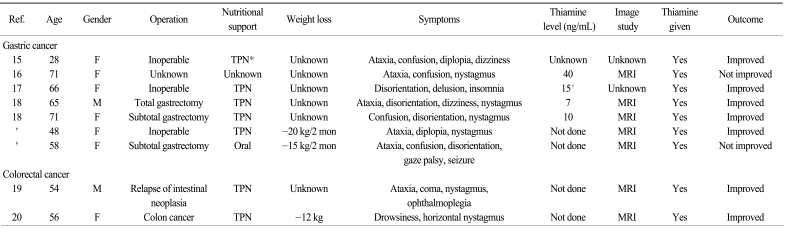
The clinical characteristics of WE in patients with GI tract cancer, based on the literature review and our 2 patients, are presented in Table 1. Among the 7 gastric cancer patients, the females were more common (n=6). The age at diagnosis ranged from 28 to 71 years old, while more than half (n=4) were older than 65 years. WE developed in patients with or without primary gastric surgery. Almost all the patients experienced deterioration of their general performance and they needed parenteral nutrition. More than half of the patients showed ataxia (n=5), nystagmus (n=4) and disorientation (n=4). Five patients showed all the classic triad of WE: ophthalmoplegia, ataxia and encephalopathy. Only one patient showed seizure, which represented the disease severity from long-term thiamine deficiency. Brain MRI was performed in five patients, and all the MRIs showed the characteristic findings of WE. The interval from symptom development to the start of thiamine infusion ranged from the 0 to 12 days. However, in the second case of our report, thiamine replacement started 3 days after the neurologic symptoms and it was ineffective, suggesting the importance of prompt thiamine administration. In addition to the gastric cancer cases, 2 cases of WE were reported in patients with colorectal malignancy and they had clinical characteristics that were similar to those of the AGC patients (Table 1).
Table 1.
Characteristics of Wernicke's encephalopathy in gastrointestinal tract cancer patients
*total parenteral nutrition, †4 days later after thiamine i.v., ‡cases of our reports.
WE develops when thiamine (vitamin B1) is deficient, which is necessary for the metabolism of carbohydrate. Thiamine is activated as thiamine pyrophosphate and it converts pyruvate into acetyl-CoA, which works as one of the key molecules in the tricarboxylic acid (TCA) cycle. In WE, the thiamine deficiency results in decreased thiamine pyrophosphate, and this deteriorates the carbohydrate metabolism in the brain and the synaptic transmission and it triggers damage of DNA synthesis (7). WE is clinically diagnosed by the classic triad: ophthalmoplegia, ataxia and encephalopathy (1). Yet WE is underdiagnosed because not all of the classic triad is presented in many cases (8). WE is partially or fully reversible with thiamine administration (1), but in case of delayed treatment, there is the possibility of death within two weeks after the development of neurologic symptoms (9). In our 2 cases and the 7 cases from the literature, 7 of 9 patients had improvement of their neurologic signs and symptoms. Therefore, we should pay attention to WE in the GI tract cancer patient with chronic malnutrition. As the proper treatment with thiamine can easily improve the symptoms, an early diagnosis and the early treatment of WE are important.
Both our reported cases showed common clinical features as follows: 1) nutritional deficiency due to poor nutritional supplement after chemotherapy for AGC and peritoneal carcinomatosis, 2) deterioration of their general condition with a poor performance status, and 3) weight loss over 12~15 kg during 2 months. Also, the imaging studies as well as the neurologic symptoms and signs were appropriate for WE. Including the other gastric cancer cases from the literature review, the brain MRI findings were compatible for WE. If WE is suspected clinically, not only a neurologic examination, but also brain MRI are useful for making the diagnosis of WE in GI tract cancer patients.
It is not easy to diagnose WE at the early stage in gastric cancer patients. As the symptoms of WE are nonspecific, when AGC patients develop these symptoms, metastasis to the central nervous system or other metabolic conditions such as drug, electrolyte imbalance, hypoxia, uremia, sepsis, liver failure and encephalopathy due to hypoglycemia are usually suspected first, while it is difficult to suspect the metabolic encephalopathy from a relative nutritional deficiency (10). Delayed encephalopathy due to 5-FU can be considered for those patients who have received 5-FU containing chemotherapy, although it is rare (11,12), and especially in our second case with seizure. Yet neurologic disorders such as ophthalmoplegia are rare, and high dose 5-FU infusion over 2,200 mg/m2 per week is usually required to develop encephalopathy, so the possibility of developing delayed encephalopathy due to 5-FU is thought to be low (13).
Gastric cancer is the most prevalent cancer and the second highest cause of cancer death in East Asia (14). The median overall survival of advanced gastric cancer was 3~4 months in the past without chemotherapy, while the median survival has recently increased to 12~13 months in several randomized clinical studies with the newly developed chemotherapeutic agents and the best supportive care. There is the risk of chronic weight loss and nutritional deficiency associated with long term chemotherapy or longer survival. There have been few studies concerned with or guidelines suggested for the proper replacement of trace elements following surgery or chemotherapy for gastric cancer. Therefore, the possibility of metabolic encephalopathy, including WE, should be considered whenever acute neurologic symptoms are developed by AGC patients who have a poor nutritional status. It is necessary to make the early diagnosis with imaging studies, as well as according to a neurologic examination. Parenteral thiamine administration should not be delayed in clinically suspected WE patients when we consider the cost-effectiveness and few side effects such as pruritus and sweating. While there is no consensus about intermittent prophylactic thiamine administration for high risk patients, further studies are required to establish the guidelines for replacement of the trace elements in these patients with GI tract malignancy.
Conclusion
As the median survival of AGC patients has been increased, many gastric cancer patients have a poor nutritional status because of GI tract obstruction, anorexia after chemotherapy and their poor general condition. It is important to consider WE in AGC patients who have acute neurologic symptoms such as ataxia, nystagmus or disorientation. Thiamine replacement should be done as soon as possible if a patient is diagnosed with WE.
Footnotes
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2007.
References
- 1.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. 1971;7:1–206. [PubMed] [Google Scholar]
- 2.Homewood J, Bond NW. Thiamin deficiency and Korsakoff's syndrome: failure to find memory impairments following nonalcoholic Wernicke's encephalopathy. Alcohol. 1999;19:75–84. doi: 10.1016/s0741-8329(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 3.Lindboe CF, Loberg EM. Wernicke's encephalopathy in non-alcoholics. An autopsy study. J Neurol Sci. 1989;90:125–129. doi: 10.1016/0022-510x(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 4.Ogershok PR, Rahman A, Nestor S, Brick J. Wernicke encephalopathy in nonalcoholic patients. Am J Med Sci. 2002;323:107–111. doi: 10.1097/00000441-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- 6.Merkin-Zaborsky H, Ifergane G, Frisher S, Valdman S, Herishanu Y, Wirguin I. Thiamine-responsive acute neurological disorders in nonalcoholic patients. Eur Neurol. 2001;45:34–37. doi: 10.1159/000052086. [DOI] [PubMed] [Google Scholar]
- 7.Henderson GI, Schenker S. Reversible impairment of cerebral DNA synthesis in thiamine deficiency. J Lab Clin Med. 1975;86:77–90. [PubMed] [Google Scholar]
- 8.Harper C. Wernicke's encephalopathy: a more common disease than realised. A neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry. 1979;42:226–231. doi: 10.1136/jnnp.42.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wernicke C. Lehrbuch der gehirnkrankheiten für aerzte und studirende. Kassel Theodor Fischer. 1881;2:229–242. [Google Scholar]
- 10.Ropper A, Brown R. Adams and Victor's Principles of Neurology. 8th ed. New York: McGraw-Hill; 2005. [Google Scholar]
- 11.Pirzada NA, Ali II, Dafer RM. Fluorouracil-induced neurotoxicity. Ann Pharmacother. 2000;34:35–38. doi: 10.1345/aph.18425. [DOI] [PubMed] [Google Scholar]
- 12.Langer CJ, Hageboutros A, Kloth DD, Roby D, Shaer AH. Acute encephalopathy attributed to 5-FU. Pharmacotherapy. 1996;16:311–313. [PubMed] [Google Scholar]
- 13.Kim YA, Chung HC, Choi HJ, Rha SY, Seong JS, Jeung HC. Intermediate dose 5-fluorouracil-induced encephalopathy. Jpn J Clin Oncol. 2006;36:55–59. doi: 10.1093/jjco/hyi214. [DOI] [PubMed] [Google Scholar]
- 14.Korea National Statistical Office. National database of cancer (1999-2002): Annual report of statistisc of death causes. Seoul: Korea National Statistical Office; 2005. [Google Scholar]
- 15.Kondo K, Fujiwara M, Murase M, Kodera Y, Akiyama S, Ito K, et al. Severe acute metabolic acidosis and Wernicke's encephalopathy following chemotherapy with 5-fluorouracil and cisplatin: case report and review of the literature. Jpn J Clin Oncol. 1996;26:234–236. doi: 10.1093/oxfordjournals.jjco.a023220. [DOI] [PubMed] [Google Scholar]
- 16.Weidauer S, Rosler A, Zanella FE, Lanfermann H. Diffusion-weighted imaging in Wernicke encephalopathy associated with stomach cancer: case report and review of the literature. Eur Neurol. 2004;51:55–57. doi: 10.1159/000075093. [DOI] [PubMed] [Google Scholar]
- 17.Onishi H, Kawanishi C, Onose M, Yamada T, Saito H, Yoshida A, et al. Successful treatment of Wernicke encephalopathy in terminally ill cancer patients: report of 3 cases and review of the literature. Support Care Cancer. 2004;12:604–608. doi: 10.1007/s00520-004-0637-y. [DOI] [PubMed] [Google Scholar]
- 18.Kim MH, Baek JM, Sung GY, Lee S, You WJ, Choi YB, et al. Wernicke's encephalopathy following gastrectomy in patients with gastric cancer. J Korean Surg Soc. 2006;70:218–222. [Google Scholar]
- 19.Nolli M, Barbieri A, Pinna C, Pasetto A, Nicosia F. Wernicke's encephalopathy in a malnourished surgical patient: clinical features and magnetic resonance imaging. Acta Anaesthesiol Scand. 2005;49:1566–1570. doi: 10.1111/j.1399-6576.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 20.Pagnan L, Berlot G, Pozzi-Mucelli RS. Magnetic resonance imaging in a case of Wernicke's encephalopathy. Eur Radiol. 1998;8:977–980. doi: 10.1007/s003300050499. [DOI] [PubMed] [Google Scholar]