Abstract
Purpose
Actinic keratosis (AK) is an incipient form of cutaneous squamous cell carcinoma (SCC). We determined if the pattern of expression of keratin-14 (K14) is a factor for tumor progression in AK and SCC.
Materials and Methods
Eighteen sections from the tissues of 16 patients were stained with anti-K14 antibody and p16INK4a. Among the 16 patients, 4 were diagnosed with both SCC and AK at the same site, but AK developed first and SCC developed subsequently. Thus, SCC may have evolved from AK. The other 12 patients were only diagnosed with AK.
Results
In all of the AK and SCC tissues, basement membranes showed positive staining for K14. However, strong reactivities were shown in the spinous and granular layers and focuses of dermal invasion in the SCC tissues developed from AK. Two and 3 of the 12 AK cases had moderately positive reactions for K14 in the spinous and granular layers, respectively. Also, all SCC tissues except one had moderate-to-strong reactions in the basal, spinous, and granular layers for p16INK4a. Two of the 12 AK cases had weak-to-moderate positive reactions in the basal, spinous, and horny layers for p16INK4a.
Conclusion
The results of our study advance our understanding of the pathogenesis of SCC developing from AK. The results also indicate a differential role in the control of K14 in normal epithelia, AK, and SCC. K14 expression in the spinous and granular layers may be a prognostic factor for tumor progression of AK.
Keywords: Keratosis, Actinic, Keratin-14, p16INK4a, Carcinoma, Squamous cell
Introduction
Actinic keratosis (AK) was first described over 100 years ago; AK is recognized by some researchers as being the most common pre-cancerous skin lesion. These lesions are caused by chronic exposure to ultraviolet rays in the form of sunlight (1). Squamous cell carcinomas (SCC) have been described as the most common form of cancer that affects some populations (2). Chronic exposure to ultraviolet rays is considered to be a major cause in the development of SCC.
Owing to a shared etiology, AK and SCC have a similar incidence profile. Indeed, SCC may be derived from AK, SCC in situ, or normal skin. It has traditionally been thought that SCC derived from AK is less aggressive than other types of SCC and is less likely to metastasize. However, subsequent studies have suggested that this theory might be incorrect (3). As expected, SCC derived from AK has the same incidence of metastasis as other types of SCC (3).
Until now, the established prognostic factors for the progression of AK to SCC include individual susceptibility and the cumulative UV radiation exposure. However, even though several studies have attempted to determine critical biomarkers, most of these studies have been inadequate (4).
All mammalian cells contain a complex intracytoplasmic cytoskeleton (5) that is composed of three principal structural units and associated proteins (microfilaments, microtubules, and intermediate filaments). Keratin belongs to the intermediate filaments. In 1982, Moll et al. (6) demonstrated a classification and numbering system for keratin according to molecular weight and acidity.
Among the keratins, keratin-14 (K14) is the most prominent basal cell marker of keratinocytes. The mRNA of K14 is synthesized only in basal keratinocytes. In a study by Chu et al. (7), K14 was shown to be a useful marker for making the differential diagnosis of SCC. Thus, we surveyed the degree of expression of K14 in cases of AK and AK-derived SCC with special attention to the possible role of the expression of K14 as a factor for tumor progression.
p16 protein is a tumor suppressor protein. In a previous report by Salama et al. (8), p16INK4a was shown to be a sensitive and specific marker for distinguishing Bowen's disease (BD) from AK and benign squamous cutaneous lesions. Therefore, we evaluated p16INK4a immunohistochemical staining as an adjunctive study for the utility of K14.
Materials and Methods
1. The cases of SCC which developed from AK
Four cases of SCC were collected from the pathologic data of the Department of Dermatology of St. Vincent Hospital. Four cases of SCC were selected because the patients were previously diagnosed with AK at the same site as the site that later developed SCC. Patients 1 and 2 had been diagnosed with AK on the left cheek 6 and 3 years previously, respectively. However, patients 1 and 2 patients were lost to follow-up, so no treatments were given to these patients. After several years, both patients presented again with a protruding mass at the AK site. SCCs were diagnosed by skin biopsy. Patients 3 and 4 were diagnosed with AK on the right cheek and buttock 2 and 5 years previously, respectively. Surgical excision and cryotherapy were performed by a dermatologist. The enlarging masses re-emerged at the same sites, both of which were shown to be SCC by pathologic findings.
2. Cases with AK and normal skin
Twelve cases with AK were collected from the same database and tissue archives. The ages and sites of the lesions were anticipated to show a similar distribution with the SCC cases of this study. The histologic types of the cases were classified as acantholytic (2 cases), atrophic (2 cases), bowenoid (4 cases), hypertrophic (3 cases), and pigmented (1 case). The profiles of these patients are displayed in Table 1. Also, normal skin samples were collected from healthy persons for the comparative analysis.
Table 1.
The summary of patient profiles and K14 / p16INK4a expression in each layer
3. Immunohistochemistry
All of the tissues were fixed in formalin, then paraffin-embedded according to conventional procedures. Serial 5-µm sections were cut from each case. The sections were deparaffinized and rehydrated in xylene and a graded series of alcohol solutions. The endogenous peroxidase activity was blocked with 3% hydrogen peroxidase for 10 minutes. For heat-induced epitope retrieval (HIER), the sections were subjected to 0.01 M citrate buffer (pH 6.0) in a microwave at 90℃ for 10 min. A commercially available K14 primary antibody (clone LL002) was purchased from Neomarkers, Inc. (Fremont, CA). A p16INK4a and p16INK4a research Kit (clone E6H4; CINtec, Germany) was purchased and all the tissues were stained. The primary and secondary antibodies were then attached to the sections following the manufacturer's protocol. Labeled avidin-biotin complex (LAB) methods were used. The peroxidase reaction was visualized using diaminobenzidine (DAB). Sections of a normal skin block were stained as a negative control.
4. Scoring of immunohistochemical staining
The cytoplasmic immunoreactivity of K14 and p16INK4a was scored as positive by two experienced dermatopathologists who worked independently. Staining intensity was graded on a 400× magnified view by light microscopy in a blinded fashion according to the following grading system (9): negative, 0 (<1% cells positive); weak, 1+ (1~10% cells positive); moderate, 2+ (10~50% cells positive); or strong, 3+ (>50% cells positive).
5. Statistical analysis
All analyses were performed using PASW statistics 17.0 for Windows. The statistical significance of differences in K14 expression between SCC developed from AK, and AK were tested using a chi-square test. A p-value <0.05 was considered statistically significant.
Results
1. K14 expression in normal tissue
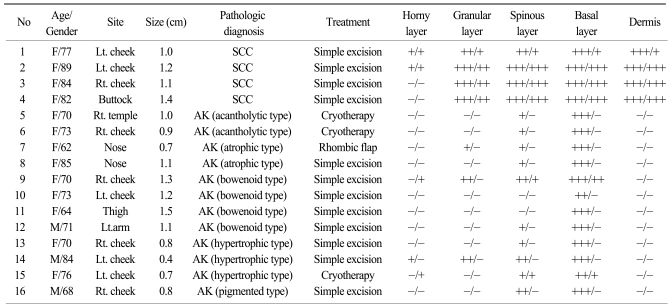
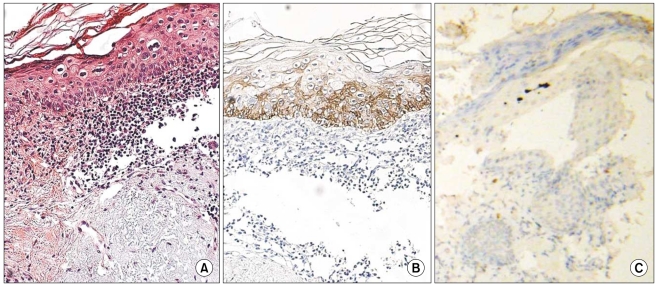
K14 positivity was generally diffuse and cytoplasmic. The basal layer of the epidermis was strongly reactive for K14 (four positive), and the spinous layer of the epidermis showed slight reactivity (one positive). However, the granular and horny layers of the epidermis and dermis exhibited negative reactivity (Fig. 1).
Fig. 1.
The basal layer of the epidermis had strong reactivity for CK14 in normal tissues, but the other layers showed negative or mild reactivity for CK14 (original magnification, ×400).
2. K14 and p16INK4a expression in SCC may have evolved from AK (Table 1)
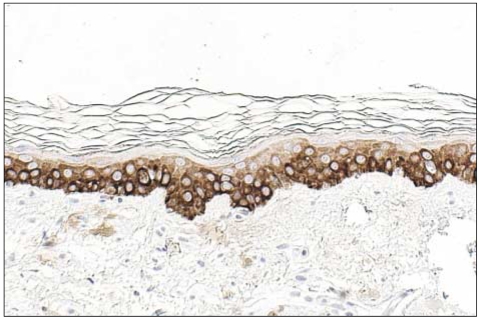
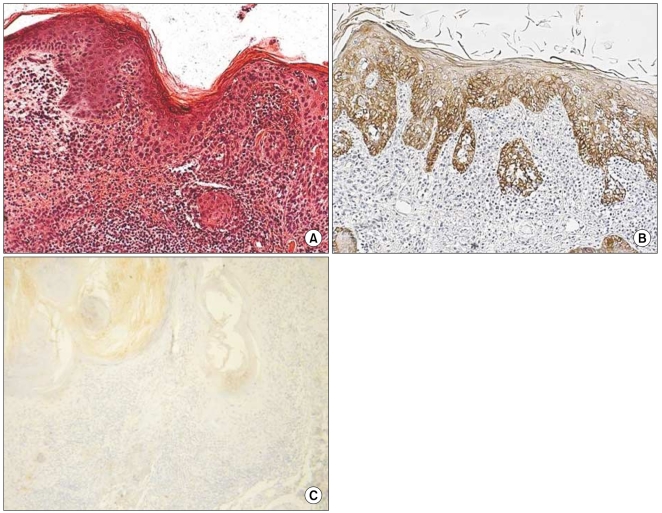
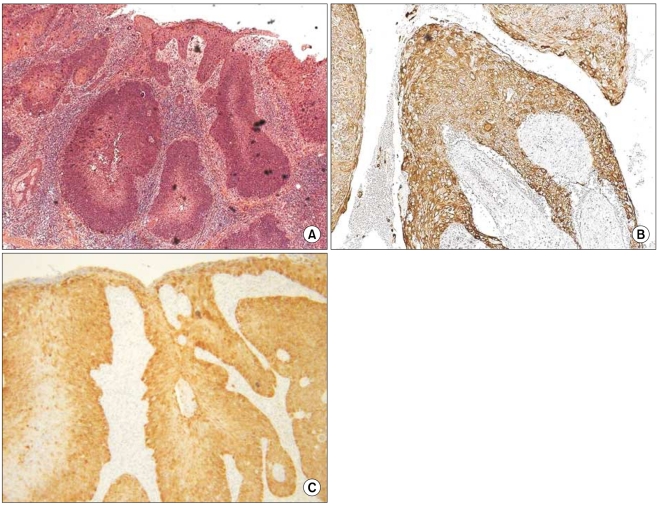
The grade of K14 positivity in SCC tumors is summarized in Table 1. The four cases showed that the basal layer was strongly reactive for K14, as expected, and the expression in the spinous layer in case 1 was moderately reactive (two positive; Fig. 2). The expression in the spinous and granular layers of cases 2, 3, and 4 were strongly reactive for K14, which was unlike the normal tissues (Fig. 3~5). The portions of the tumors that invaded the dermis also showed strong reactivity for K14 in all of the cases. The grades of p16INK4a positivity are summarized in Table 1. With the exception of case 1, all of the SCC tumors had moderate-to-strong reactivity in the basal, spinous, and granular layers. However, weak reactivity was shown in all of the layers of case 1.
Fig. 2.
(A) Histologic findings of case 1 (SCC; H&E, ×200), (B) CK14 expression showed moderate reactivity in the spinous and granular layers and in the tumor cells that invaded the dermis (original magnification, ×200), (C) p16INK4a expression showed weak reactivity in the whole layers (original magnification, ×200).
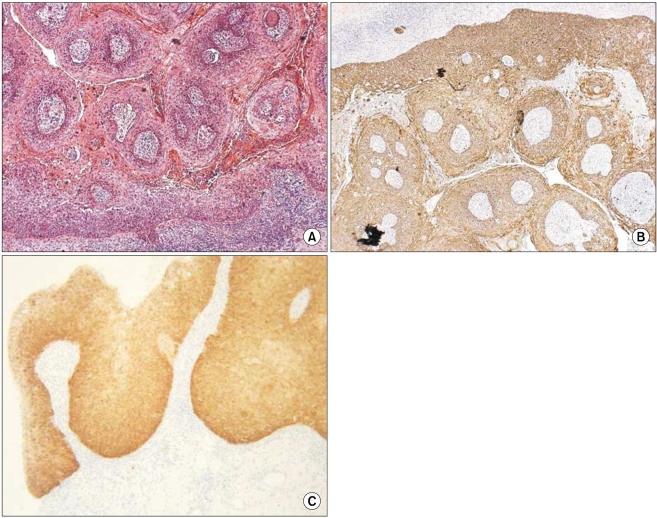
Fig. 3.
(A) Histologic findings of case 2 (SCC; H&E, ×200), (B) CK14 expression showed strong reactivity in the spinous and granular layers and dermal invasion (original magnification, ×100), (C) p16INK4a expression showed a moderate-to-strong reaction in the basal and spinous layers (original magnification, ×200).
Fig. 5.
(A) Histologic findings of case 4 (SCC; H&E, ×200) (B) CK14 expression showed strong reactivity in the spinous and granular layers and dermal invasion (original magnification, ×200), (C) p16INK4a expression showed a moderate-to-strong reaction in the basal and spinous layers (original magnification, ×200).
3. K14 and p16INK4a expression in AK
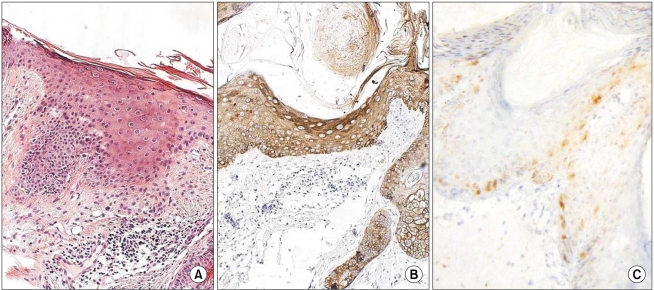
The grades of K14 positivity in the AK lesions are summarized in Table 1. The basal layers of the epidermis in almost all cases had strong reactivity for K14. Also, the basal layers of the adnexa had moderate reactivity in 5 of 12 cases. Mild K14 expression was also detected in the spinous layers in seven cases. The patterns of immunoreactivity were similar with the normal tissues (Fig. 6). However, three cases had mild-to-moderate reactivity in the granular layers, like the SCC lesions (Fig. 7). Also, the grades of p16INK4a positivity are summarized in Table 1. Except for cases 9 and 15, all of the layers of the epidermal lesion were negative. In cases 9 and 15, weak-to-moderate reactivity was shown in the basal, spinous, and horny layers.
Fig. 6.
(A) Histologic findings of case 7 (AK; H&E, ×200), (B) CK14 expression showed negative reactivity in the spinous and granular layers (original magnification, ×200), (C) p16INK4a expression showed negative reactivity in the entire layer (original magnification, ×200).
Fig. 7.
(A) Histologic findings of case 9 (AK; H&E, ×200), (B) CK14 expression showed moderate reactivity in the spinous and granular layers (original magnification, ×200), (C) The p16INK4a expression showed weak-to-moderate reactivity in the basal and spinous layers (original magnification, ×200).
Discussion
SCC and AK are at the opposite ends of the same disease spectrum. There is a continuum from the moment UV light converts keratinocytes into atypical neoplastic cells to the point at which these cells penetrate the dermis (10). It has also been confirmed that the common nature of the atypical keratinocytes is found in both AK and SCC (11). If AK is left untreated for enough time, it is thought that invasive SCC will develop (12). Hurwitz et al. (13) found that 97% of the 459 cutaneous SCCs occurred next to an AK, and Marks et al. (14) estimated that 75~96 AKs per 100,000 AKs convert to SCC per year. However, it has not yet been sufficiently elucidated at which stage in the pathway the critical or irreversible changes from the emergence of pre-cancerous cells to the development of invasive cells occur.
Intermediate filaments have been divided into six distinct classes (I-VI) based on gene sequencing and molecular techniques (6). Keratins belong to class I (acidic keratin, numbers 1~10) and class II (basic keratin, numbers 11~20; 6). In general, most acidic keratins will pair with most basic keratins (5). For example, keratin-1 (K1) and keratin-5 (K5) is paired with keratin-10 (K10) and K14 in the suprabasal or basal layer. According to previous reports, different subsets of keratins are expressed during the course of terminal differentiation and during different stages of development, as well as in different epithelia (5,6). So, the expression of keratin protein could help classify all epithelia, much like fingerprinting (5,6). Specifically, K5 and K14 are more often associated with stratified squamous epithelium, but other keratins could be expressed in other types of epithelial cells and also in carcinomas, such as squamous cell carcinoma, adenocarcinoma, and transitional cell carcinoma (5,15). Several studies have focused on keratin-7 (K7) and keratin-20 (K20) (16). The immunohistochemical reactions for these keratins using the proper antibodies could aid in establishing the differential diagnosis of metastatic carcinoma of unknown origin.
K14 is a basal cell keratin that forms heteropolymers together with keratin-5 (17). The mRNA expression of K5 and K14 are limited to basal cells in normal human skin. Tumor cells that originate from the basal layer contain K14 because the basal cells always contain K14. On the other hand, tumor cells in the suprabasal layer do not contain K14 because the suprabasal layer does not produce K14 mRNA. Jiang et al. (18) reported that K14 promoter expression is induced by transforming growth factor, which is mainly produced from dermal fibroblasts.
Komine et al. (19) demonstrated that K14 expression could be a marker of tumor progression in Bowen's disease. In that report, most of the K14-positive tumor cells, especially in SCC, developed from Bowen's disease, were those that interfaced with the dermis. Marley et al. (20) reported that K14 was significantly over-expressed in pre-cancerous white patches of oral epithelium, and this would eventually evolve into oral SCC. Further, the oral dysplastic epithelium and SCC showed irregular extensions of the K14 proteins into the suprabasal layer, while the normal epithelia showed that K14 proteins are present almost exclusively in the basal layer (21).
p16 protein is a tumor suppressor protein. Alterations in p16 protein have been reported in melanomas, gliomas, and osteosarcomas (22). Also, increased expression of p16INK4a has been described in cervical squamous neoplasms as lesions progress from a premalignant to malignant phase of proliferation (23). The sensitivity and specificity of p16INK4a for a diagnosis of BD versus AK was about 90% in a previous report (8).
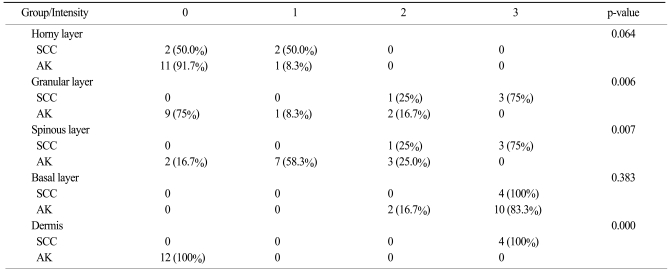
In our study, K14 expression in two groups showed statistically significant differences in the granular (p=.006) and spinous layers (p=.007; Table 2). The four cases of SCCs that might have evolved from AK showed strong reactivity for K14 in the entire epidermal layer, with the exception of the horny layer. Also, all basal layers in the dermal portions of the tumor cells were positive for K14. This means that the tumor cells originated from the basal layer. As AK tumor cells progresses to invasive SCC, tumor cells have direct contact with the dermis. The expression of K14 in these tumor cells is induced by the dermal cell cytokines, such as transforming growth factor-β, which is primarily produced from dermal fibroblasts (18). Taking these results into consideration, it was hypothesized that the high-risk groups of patients with AK that would evolve to SCC are positive for K14 in the spinous and granular layers. Moreover, if the AK tumor cells are positive for K14, then they would have a higher risk of dermal invasion than the K14-negative tumor cells. The results of staining for p16INK4a were similar with that for K14 in the current study. This means that K14 could be another prognostic factor for SCC proliferation.
Table 2.
Comparison of number (%) of cases with K14 staining intensity between SCC which developed from AK, and AK
We studied the expression of K14 in 12 cases of AK and found that three cases of AK were weakly or moderately positive for K14 in the spinous and granular layers. Therefore, we suggest that these cases have a higher risk of evolving to SCC than the other cases that had negative reactivity for K14.
Conclusion
K14 protein expression in spinous and granular layers may be a prognostic factor for tumor progression and invasion in AK.
Fig. 4.
(A) Histologic findings of case 3 (SCC; H&E, ×200), (B) CK14 expression showed strong reactivity in the spinous and granular layers and dermal invasion (original magnification, ×200), (C) p16INK4a expression showed moderate-to-strong reaction in the basal and spinous layers (original magnification, ×200).
Footnotes
This work was supported by the 2007 Catholic University of Korea, Dermatology Research Fund (D0217-50006).
References
- 1.Duncan OK, Geisse JK. Epithelial precancerous lesions. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2007. pp. 1007–1028. [Google Scholar]
- 2.Giles GG, Marks R, Foley P. Incidence of non-melanocytic skin cancer treated in Australia. Br Med J (Clin Res Ed) 1988;296:13–17. doi: 10.1136/bmj.296.6614.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukamizu H, Inoue K, Matsumoto K, Okayama H, Moriguchi T. Metastatic squamous-cell carcinomas derived from solar keratosis. J Dermatol Surg Oncol. 1985;11:518–522. doi: 10.1111/j.1524-4725.1985.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 4.Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42:23–24. doi: 10.1067/mjd.2000.103339. [DOI] [PubMed] [Google Scholar]
- 5.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 6.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: pattern of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 7.Chu PG, Lyda MH, Weiss LM. Cytokeratin 14 expression in epithelial neoplasms: a survey of 435 cases with emphasis on its value in differentiating squamous cell carcinomas from other epithelial tumours. Histopathology. 2001;39:9–16. doi: 10.1046/j.1365-2559.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- 8.Salama ME, Mahmood MN, Qureshi HS, Ma C, Zabro RJ, Ormsby AH. p16INK4a expression in actinic keratosis and Bowen's disease. Br J Dermatol. 2003;149:1006–1012. doi: 10.1111/j.1365-2133.2003.05654.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi YD, Park JN, Kang MS, Cho SH, Park SW. Expression of cytokeratin and Ki-67 in development of the pilomatricoma. Korean J Dermatol. 2003;41:1619–1626. [Google Scholar]
- 10.Anwar J, Wrone DA, Kimyai-Asadi A, Alam M. The development of actinic keratosis into invasive squamous cell carcinoma: Evidence and evolving classification schemes. Clin Dermatol. 2004;22:189–196. doi: 10.1016/j.clindermatol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Fu W, Cockerell CJ. The actinic (solar) keratosis: a 21st century perspective. Arch Dermatol. 2003;139:66–70. doi: 10.1001/archderm.139.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Lober BA, Lober CW. Actinic keratosis is squamous cell carcinoma. South Med J. 2000;93:650–655. [PubMed] [Google Scholar]
- 13.Hurwitz RM, Monger LE. Solar keratosis: an evolving squamous cell carcinoma. Benign or maligmamt? Dermatol Surg. 1995;21:184. doi: 10.1111/j.1524-4725.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squmaous cell carcinoma. Lancet. 1988;1:795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 15.Harnden P, Southgate J. Cytokerain 14 as a marker of squmaous differention in transitional cell carcinoma. J Clin Pathol. 1997;50:1032–1033. doi: 10.1136/jcp.50.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson AR, Smoller BR. Update on dermatopathology. Dermatol Clin. 1999;17:667–689. doi: 10.1016/s0733-8635(05)70115-7. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs E, Weber K. Intermediated filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 18.Jiang CK, Tomic-Canic M, Lucas DJ, Simon M, Blumenberg M. TGF beta promotes the basal phenotype of epidermal keratinocytes: transcriptional induction of K5 and K14 keratin genes. Growth Factors. 1995;12:87–97. doi: 10.3109/08977199509028955. [DOI] [PubMed] [Google Scholar]
- 19.Komine M, Okinaga M, Takeda F, Nashiro K, Kikuchi K, Murakami T, et al. Patterns of basal cell keratin 14 expression in Bowen's disease: a possible marker for tumor progression. Br J Dermatol. 2001;145:223–228. doi: 10.1046/j.1365-2133.2001.04338.x. [DOI] [PubMed] [Google Scholar]
- 20.Marley JJ, Robinson PA, Hume WJ. Expression of human cytokeratin 14 in normal, premalignant and malignant oral tissue following isolation by plaque differential hybridization. Eur J Cancer B Oral Oncol. 1994;30B:305–311. doi: 10.1016/0964-1955(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 21.Su L, Morgan PR, Lane EB. Keratin 14 and 19 expression in normal, dysplastic and malignant oral epithelia. A study using in situ hybridization and immunohistochemistry. J Oral Pathol Med. 1996;25:293–301. doi: 10.1111/j.1600-0714.1996.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 22.Piccinin S, Doglioni C, Maestro R, Vukosavljevic T, Gasparotto D, D'Orazi C, et al. p16/CDKN2 and CDK4 gene mutations in sporadic melanoma development and progression. Int J Cancer. 1997;74:26–30. doi: 10.1002/(sici)1097-0215(19970220)74:1<26::aid-ijc5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]