Abstract
Mallory-Denk body (MDB) formation is a component of alcoholic and non alcoholic hepatitis. In the present study, the role of the toll-like receptor (TLR) signaling pathway was investigated in the mechanism of MDB formation in the DDC-fed mouse model. Microarray analysis data mining, performed on the livers of drug primed mice refed DDC, showed that TLR2/4 gene expression was significantly up regulated by DDC refeeding. SAMe supplementation prevented this up regulation and prevented the formation of MDBs. qRT-PCR analysis confirmed these results. TLR2/4 activates the adapter protein MyD88. The levels of MyD88 were increased by DDC refeeding. The increase of MyD88 was also prevented by SAMe supplementation. Results showed that MyD88-independent TLR3/4-TRIF-IRF3 pathway was not up regulated in the liver of DDC refed mice. Tumor necrosis factor receptor-associated factor 6 (TRAF6) is the down stream protein recruited by the MyD88/IRAK protein complex, and is involved in the regulation of innate immune responses. Results showed a significant increase in the levels of TRAF-6. TRAF-6 activation leads to activation of NFkB and the mitogen-activated protein kinase (MAPK) cascade. The TRAF-6 increase was ameliorated by SAMe supplementation. These results suggest that DDC induces MDB formation through the TLR2/4 and MyD88-dependent signaling pathway. In conclusion, SAMe blocked the over-expression of TLR2/4, and their downstream signaling components MyD88 and TRAF-6. SAMe prevented the DDC-induced up regulation of the TLR signaling pathways, probably by preventing the up regulation of INF-γ receptors by DDC feeding. INFγ stimulates the up regulation of TLR2. The ability of SAMe feeding to prevent TLR signaling up regulation has not been previously described.
Keywords: TLRs, 26s proteasome, immunoproteasome, interferon γ, proinflammatory cytokines
INTRODUCTION
Toll like receptor (TLR) signaling pathway has been shown to be up regulated in chronic alcoholic liver disease (Nath and Szabo 2009). TLR signaling is part of the innate immune system where NFkB activation results in an up regulation of proinflammatory cytokines like TNFα down stream in response to endotoxemia (Seki and Brenner, 2008). TLR signaling also activates the mitogen-activated protein kinase (MAPK) cascade (i.e., ERK1/2, p38, JNK) to activate AP1 up stream, up regulating genes controlling cell proliferation (Testro and Visvanathan, 2009). Both p38, JNK, ERK, AP1 pathway for cell growth and TNFα, NFkB proinflammatory pathway are up regulated in the DDC refeeding mouse model of MDB pathogenesis (Nagao et al., 1998a; Nagao et al., 1998b; Yuan et al., 2000; Nan et al., 2005; Wu et al., 2005; Nan et al., 2006;). In this DDC model of MDB pathogenesis, both the proinflammatory and proliferation pathways are prevented when the methyl donors S-adenosylmethionine or betaine are fed with DDC (Oliva et al., 2009a, 2009b; Li et al., 2008). This indicates that the MDB formation response to DDC is the result of epigenetic changes in gene expression where histone methylation is decreased and as a result genes are up regulated (Bardag-Gorce et al., 2007, 2008; Oliva et al., 2008). The question now is, will SAMe prevent the TLR signaling-induced proinflammatory and liver cell proliferation outcome that is stimulated by DDC refeeding. SAMe feeding blocks the up regulation of TLR signaling in DDC refed mice has already been reported in part in an abstract (Bardag-Gorce et al., 2009).
MATERIAL & METHODS
Refed DDC drug-primed mouse model, which induces MDB formation, was used (Yuan et al, 1996). Three control C3H male mice (Harlan Sprague-Dawley, San Diego, CA) were fed the control high protein complete diet (Teklad, Madison, WI) (Group 1). Group 2 (6 mice) was fed the control diet with diethyl-1,4-dihydro-2,4,6-trimethyl-3,5-pyridine-decarboxylate (DDC) 0.1% (Aldrich, St Louis, MO) added. The 2 groups were fed the diets for 10 weeks. At this time, group 2 was switched to the control diet for 1 month. After 1 month of control diet feeding, most of the MDBs, which had formed by 10 weeks of DDC feeding, disappeared (drug primed mice). Three of the drug-primed mice were refed DDC for 7 days (group 2). Three of the drug-primed mice were refed DDC plus SAMe (SAMe tosylate disulfate, Nature Made Mission Hills, CA), 4 gm/day by gavage, for 7 days (group 3). At this time, the 3 groups were anesthetized with ketamine, and liver tissue was fast frozen with liquid nitrogen. A portion of the liver was fixed in 10% buffered zinc formalin for histologic studies. All mice were treated in accordance with the guidelines of the National Academy of Science and with approval by the Animal Care Committee at Harbor-UCLA LABioMed Research Institute.
Immunohistochemistry
Liver sections were immunostained with primary antibodies. The liver sections were double stained with a mouse monoclonal antibody to ubiquitin to stain MDBs (CHEMICON, Millipore, Billerica, MA) and an antibody to UbD (FAT10) (BIOMOL International, L.P., Plymouth Meeting, PA). Texas-red and FITC-conjugated secondary antibodies were used. DAPI was used as the nuclear stain. Fluorescent antibody stains were viewed using a Nikon 400 fluorescent microscope with a FITC filter cube and a triple color band filter cube to detect FITC and Texas-red labeled antibody staining and DAPI.
Liver Homogenates
Mouse liver homogenates were prepared by homogenizing 100 mg of liquid nitrogen frozen liver in 2 ml of 20 mM Tris-HCl pH 7.5; glycerol 10%; EGTA 1 mM; DTT 1 mM; sodium-fluoride 50 mM; protease and phosphatases inhibitor cocktail (Sigma, St Louis. MO). The livers were homogenized using the Ultra-Turrax T25 homogenizer. Protein concentrations were quantitated using the Bradford method (Bradford, 1976).
Western Blot Analysis
Proteins (50 µg) from liquid nitrogen frozen stored livers were separated by SDS-PAGE gels and transferred to a PVDF membrane (Bio-Rad, Hercules, CA) for 1 hr. in 25 mM Tris-HC1 (pH 8.3), 102 mM glycine and 20% methanol. The membranes were stained using primary antibodies against CD14, MyD88, TRAF6 and IL-1β (Santa Cruz, Biotechnology, Inc., Santa Cruz, CA). Appropriate species polyclonal and monoclonal HRP-conjugated antibodies were used as second antibodies. The membranes were examined for chemiluminescence using luminal, according to the manufacturer’s instructions (Amersham Pharmacia Biotech, Piscataway, NJ). The results were normalized by stripping the membranes and staining for GAPDH (Santa Cruz, Biotechnology, Inc., Santa Cruz, CA).
Quantitative Real-time RT-PCR
Total liver RNAs were extracted with Trizol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA). Synthesis of cDNAs was performed with 5 µg total RNA, and 50 ng random hexamer primers, using SuperSriptIII RNase H Reverse Transcriptase (Invitrogen, Carlsbad, CA). RT-PCR primers were designated using Primer Express software (Applied Biosystems, Foster City, CA).
Sense and anti-sense: Quantitative PCR was done using the SYBR Green JumpStart™ Tag ReadyMix (Sigma, St. Louis, MO) on an ABI PRISM 7700 Sequence Detector System (Applied Biosystems, Foster City, CA). The thermal cycling consists of an initial step at 50°C for 2 min., followed by a denaturation step at 95°C for 10 min., then 40 cycles at 95°C for 15 s and 60°C for 1 min. Single PCR product was confirmed with the heat dissociation protocol at the end of the PCR cycles. Quantitative values were obtained from the threshold PCR cycle number (Ct) at which point the increase in signal associated with an exponential growth for PCR product starts at ΔCt = Cttarget gene –Ct 18S. For each target gene, the highest ΔCt was assigned as ΔCtmax. The relative mRNA levels were calculated as a 2ΔΔCt, ΔΔCt = ΔCtmax - ΔCt.
List of Primer Sequences Used for RT-PCR
TLR2 NM_011905
Forward:AAGATGCGCTTCCTGAATTTG
Reverse: CCAGCGTCTGAGGAATGCA
TLR4 NM_021297.2
Forward: CATGGAACACATGGCTGCTAA
Reverse: GTAATTCATACCCCTGGAAAGG
CD14 NM_009841.3
Forward: CAGCCCTCTGTCCCCTCAA
Reverse: TCCATCCCCGCGTTACG
Statistical Analysis
Data were obtained from 3 animals for each group. Bars represent mean values ± SEM. P values were determined by one-way ANOVA and Student-Newman Keuls for multiple group comparisons (Sigma-Stat software, San Francisco, CA). p= < 0.05 was used for establishing a significant difference.
RESULTS
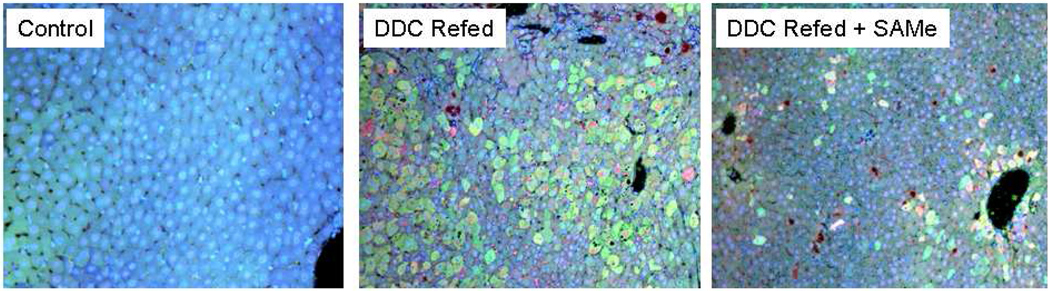
The livers from control group 1 mice showed normal histology without UbD or ubiquitin positive individual hepatocytes. No MDBs were formed (Fig 1). The livers of the mice refed DDC for 7 days (group 2) showed numerous UbD positive hepatocytes scattered among non staining normal appearing hepatocytes throughout the lobule (Fig 1). The livers from the mice refed DDC plus SAMe for 7 days (group 3) showed a few scattered residual UbD positive liver cells, which did not disappear after the DDC 1 month withdrawal. This is to be expected if DDC is not refed, and indicates that SAMe completely prevented the proliferation of UbD positive cells as previously reported (Oliva et al, 2008).
Figure 1.
Hepatocytes from a mouse refed DDC and a mouse refed DDC + SAMe stained with Antibodies to UbD (green) and Ubiquitin (red). Note that the cytoplasm of MDB forming cells stained positive for UbD (green). The UBD positive MBD forming cells increased in the livers of DDC refed mice. This increase was prevented in the liver of DDC refed mice with SAMe. Tricolor filter ×10.
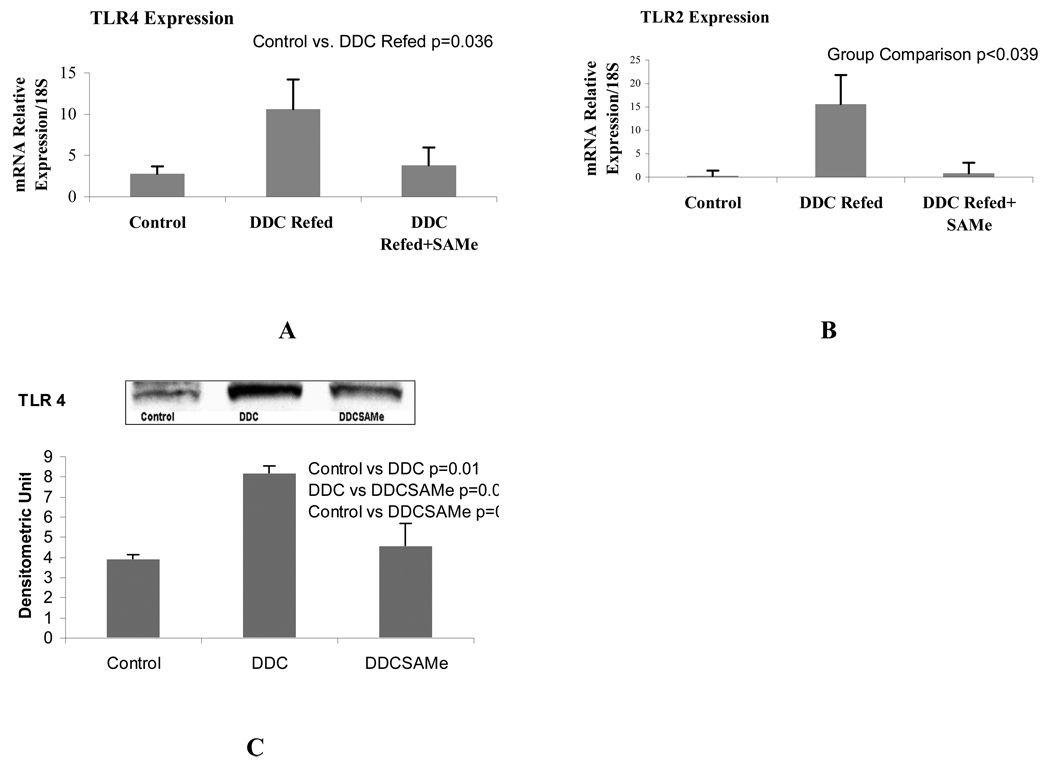
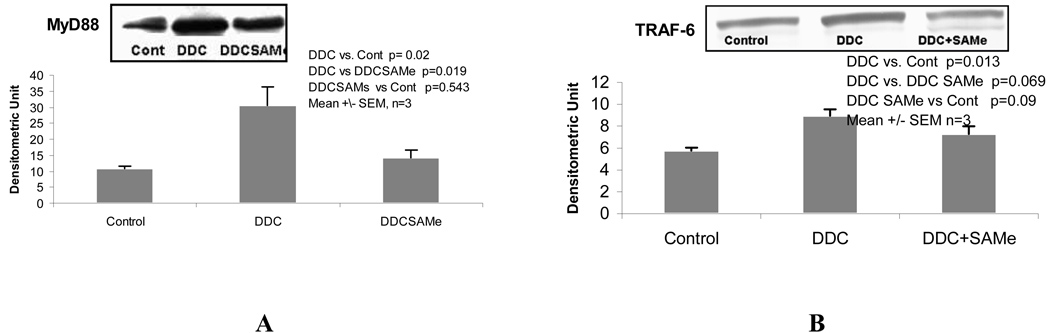
When the expression of TLR2 and 4 were measured by qPCR, both TLR2 and 4 were significantly up regulated by DDC refeeding 7 days (group 2). SAMe feeding (group 3) prevented this up regulation (Fig 2). When MyD88 and TRAF-6 were measured by Western blot, they were significantly increased by DDC refeeding (group 2) (Fig 3). SAMe feeding (group 3) prevented the DDC-induced increase in MyD88. SAMe feeding also tended to prevent the increase in TRAF-6 (p<0.069) (group 3) (Fig 3). These changes are consistent with the up regulation of the TLR4-MyD88 dependent signaling pathway caused by DDC refeeding.
Figure 2.
qRT-PCR analysis showed that TLR4 (A) and TLR4 (B) receptors were up regulated in the livers of mice refed DDC and SAMe prevented it (Mean+SEM, n=3). Western blot analysis (C) confirmed the result of PCR.
Figure 3.
The protein level of MyD88 (A) and TRAF-6 (B) were increased by DDC refeeding and SAMe prevented this change for MyD88. SAMe tended to prevent the increase in TRAF6 induced by DDC refeeding (Mean±SEM, n=3).
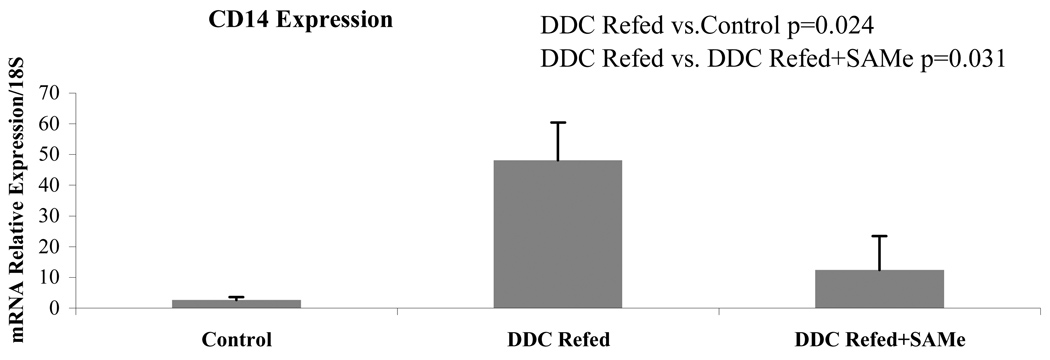
When CD14 expression was measured by qPCR as an indication of increased sensitivity to LPS it was shown to be up regulated by DDC refeeding (group 2). This was prevented by SAMe feeding (Fig 4).
Figure 4.
Mice refed DDC showed up regulation of CD14 expression in their livers, as shown by qRT-PCR. SAMe feeding prevented this up regulation (Mean±SEM, n=3).
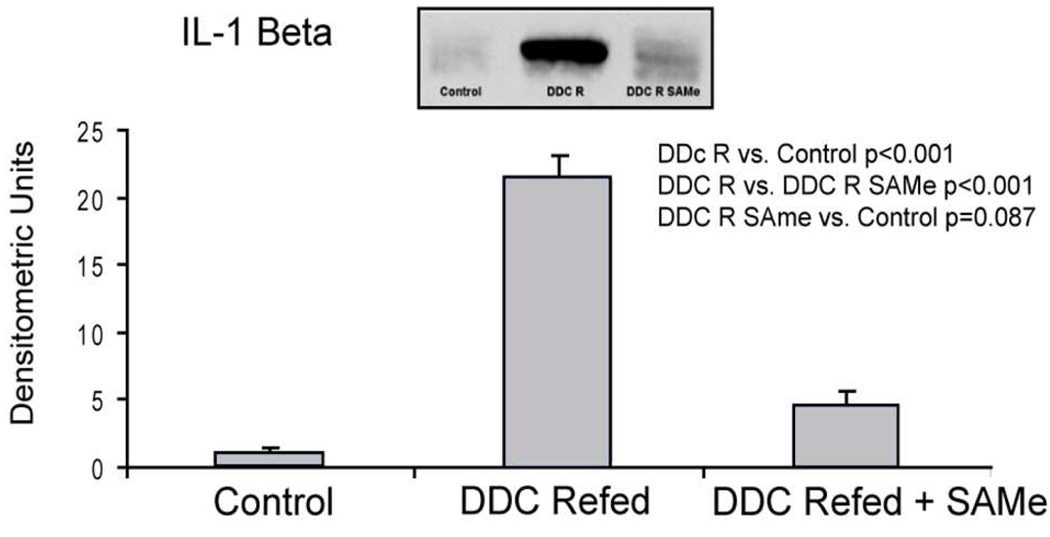
As a down stream outcome of proinflammatory up regulation induced by the TLR4/2 signaling pathway, the levels of IL-1β were measured by western blot. DDC refeeding (group 2) markedly increased the levels of 1L-1β in the liver and SAMe feeding prevented this up regulation (Fig 5).
Figure 5.
DDC refeeding caused a significant increase in IL-1B in liver and SAMe feeding prevented this increase as shown by Western blot (Mean±SEM, n=3).
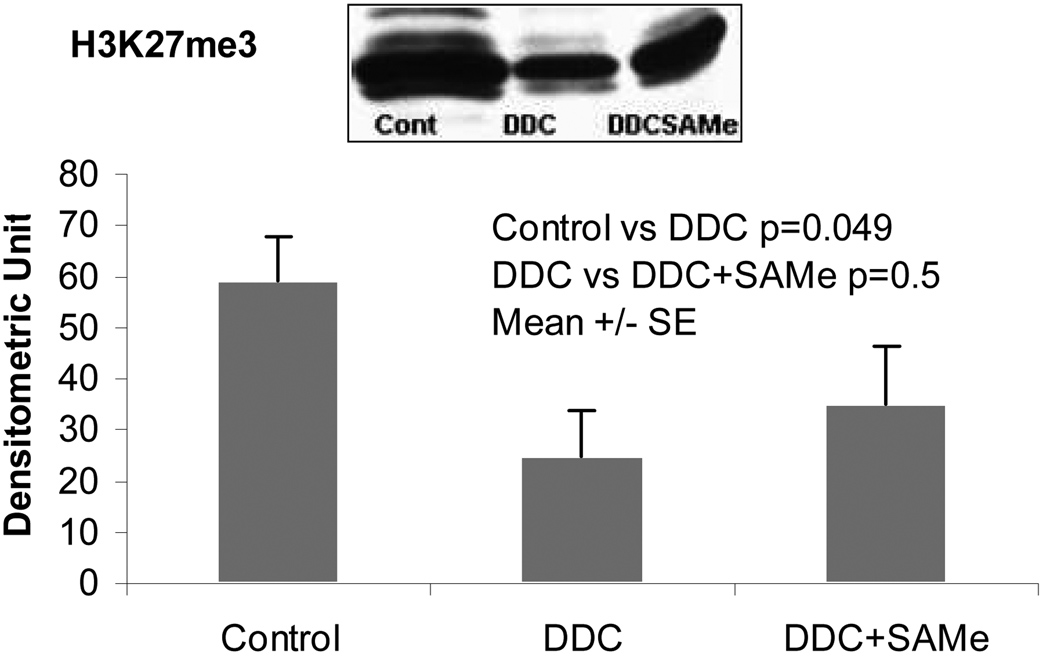
Figure 6 showed that DDC decreases significantly the methylation of H3K27me3. SAMe supplementation tended to increase the methylation of H3K27me3 which support the hypothesis that the methyl donor by SAMe could be the mechanism of the protective effects of SAMe
Figure 6.
Western blot analysis of trimethylated histone 3 lysine 24 (H3K27me3) in the liver samples from DDC Refed mice and DDA+SAMe mice.
DISCUSSION
DDC feeding up regulated TLR 2 and TRL4 signaling pathways. The two pathways join at the MyD88 juncture (Beutler, 2004) leading to cytokine up regulation through NFkB activation including TNFα, IL-Iβ, IL-6, IL-12, IL-18 and IL-10 (Testro and Visvanathan, 2009). IL-1β was up regulated in the present study. Previously TNFα levels were shown to be up regulated in the same DDC model (Oliva et al., 2009a, 2009c). Likewise, DDC refeeding induced an increase in UbD expressing hepatocytes that form MDBs (Oliva et al., 2008) associated with AP-1 up regulation (Nagao et al., 1998a) which promotes liver cell growth (Testro and Visvanathan, 2009). The UbD positive cells that form MDBs have a growth advantage over the intervening normal hepatocytes (Nagao et al., 1998; Roomi et al., 2006; Oliva et al., 2008).
DDC induces a shift in the 26s proteasome to form the immunoproteasome causing a reduction in the 26s proteasome activity and the formation of aggresomes (MDBs) (Oliva et al., 2009a). This is associated with the induction of TNFα and INFγ reactors (Oliva et al., 2009a). INFγ and TNFα induce the TLR2 receptor (Winder et al., 2009). INFγ and TNFα induce the overexpression of FAT10 and the expression of the immunoproteasome catalytic subunits (Aki et al., 1994; Lukasiak S, et al., 2008; Oliva et al., 2009a). SAMe feeding prevents all of these responses to DDC. Therefore, we postulate that MDB formation in FAT10 positive hepatocytes is the result of DDC induction of IFNγ and TNFα up regulation of the TLR signaling and conversion of the 26S proteasome to the immunoproteasome.
SAMe feeding prevented the proliferation of UbD positive hepatocytes caused by the DDC refed mice as reported before (Oliva et al., 2008). Likewise, SAMe prevented the up regulation of TLR2/4 signaling pathways and the activation of proinflammatory cytokine induction by DDC. The mechanism of SAMe action is probably by its methyl donor function. Methylation of histones causes gene silencing. For example, methylation of H3K27 by Ezh2 leads to gene silencing by associating with polycomb complex binding (Lennartsson and Ekwall, 2009).
SAMe is unstable and converts to MTA spontaneously. Both MTA and SAH are stable and cell permeable. The mechanism of SAMe’s pharmacologic effect on proinflammatory mediators is mainly mediated by MTA and SAH at the level of histone methylation (Ara et al., 2008).
Epigenetic codes often come in patterns of modification (Lennartsson and Ekwall, 2009). In the case of DDC refeeding several histone methylation modifications were observed. Of the changes observed several were prevented by SAMe feeding including H3K4me3, H3K9me3 demethylation. Also prevented were changes in methylating and acetylating enzymes, ubiquitination of H2A and numerous intermediates and enzymes involved in methionine metabolism (Bardag-Gorce et al., 2007, 2008; Li et al., 2008). Betaine, another methyl donor, affects the changes in methionine metabolism caused by DDC refed mice in a way that is similar to SAMe. SAH levels were changed (decreased) by DDC and this was prevented by betaine (Oliva et al., 2009c).
Acknowledgements
We would like to thank Adriana Flores for typing the manuscript. This study was supported by a grant from NIH/NIAAA 8116 and an Alcohol Center Grant, Liver and Pancreas PA50-011999 morphology core
Financial Support: NIH/NIAAA Grant 8116 and Alcohol Center Grant, Liver and Pancreas PA50-011999 morphology core
Abbreviations
- TLR
Toll-like receptors
- UbD
ubiquitin D (FAT10)
- SAMe
S-adenosylmethionine
- DDC
diethyl-1-4-dihydro-2, 4, 6-trimethyl-3,5-pyridine-decarboxylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ara AI, et al. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aki M, Shimbara N, et al. Interferon-γ induces different subunits organizations and functional diversity of proteasomes. J Biochem. 1994;115:257–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, et al. Mallory body formation is associated with epigenetic phenotypic change in hepatocytes in vivo. Exp Mol Pathol. 2007;83:160–168. doi: 10.1016/j.yexmp.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, et al. Epigenetic mechanisms regulate Mallory Denk body formation in the livers of drug-primed mice. Exp Mol Pathol. 2008;94:113–121. doi: 10.1016/j.yexmp.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, et al. SAMe blocks the up regulation of toll-like receptors signaling in Mallory-Denk body forming hepatocytes. Hepatocyte. 2009;50(50) Suppl:867A. doi: 10.1016/j.yexmp.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions, and possibilities in Toll-like receptor signaling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Lennartsson A, Ekwall K. Histone modification patterns and epigenetic codes. Biochem Biophys Acta. 2009;1790:863–868. doi: 10.1016/j.bbagen.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Li J, et al. S-adenosylmethionine prevents Mallory body formation in drug-primed mice by inhibiting epigenetic memory. Hepatology. 2008;47:613–624. doi: 10.1002/hep.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiak S, et al. Proinflammatory cytokines cause FAT10 up regulation in cancers of the liver and colon. Oncogene. 2008;27:6068–6074. doi: 10.1038/onc.2008.201. [DOI] [PubMed] [Google Scholar]
- Nagao Y, et al. Pathogenesis of Mallory bodies formation studies using the drug-primed mouse model. Hepatol Res. 1998a;13:42–54. [Google Scholar]
- Nagao Y, et al. Inhibition of PPAR alpha/RXR alpha-mediated direct hyperplasia pathways during griseofulvin-induced hepatocarcinogenesis. J Cell Biochem. 1998b;69:18–20. doi: 10.1002/(sici)1097-4644(19980501)69:2<189::aid-jcb9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Nan L, et al. the p105/50 NF-kB pathway is essential for Mallory body formation. Exp Mol Pathol. 2005;78:198–206. doi: 10.1016/j.yexmp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Nan L, et al. Mallory body (cytokeratin aggresomes) formation is prevented in vitro by p38 inhibitor. Exp Mol Pathol. 2006;80(3):228–240. doi: 10.1016/j.yexmp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Nath B, Szabo G. Alcohol-induced modulation of signaling pathway in liver parenchymal and nonparenchymal cells: Implications for immunity. Seminars. 2009;29:166–177. doi: 10.1055/s-0029-1214372. [DOI] [PubMed] [Google Scholar]
- Oliva J, et al. FAT10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Exp Mol Pathol. 2008;84:102–112. doi: 10.1016/j.yexmp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, et al. Betaine prevents Mallory-Denk body formation in drug-primed mice by epigenetic mechanisms. Exp Mol Pathol. 2009c;86:77–86. doi: 10.1016/j.yexmp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, et al. Mallory-Denk body formation is associated with an increase of the immunoproteasome and a decrease of the 26s proteasome. FASEB J. 2009a Suppl:219A. [Google Scholar]
- Oliva J, et al. Betaine prevents Mallory-Denk body formation in drug-primed mice by epigenetic mechanisms. Ex Mol Pathol. 2009b;86:77–86. doi: 10.1016/j.yexmp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi MW, et al. Preneoplastic liver foci expansion by thioacetamide toxicity in drug-primed mice. Exp Mol Pathol. 2006;81:8–14. doi: 10.1016/j.yexmp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Seki SS, et al. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- Testro AG, Visvanathan K. Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009;24:943–954. doi: 10.1111/j.1440-1746.2009.05854.x. [DOI] [PubMed] [Google Scholar]
- Winder AA, et al. Differential effects of cytokines and corticosteroids on Toll-like receptor Z expression and activity in human airway epithelia. Respiratory Res. 2009;10:96. doi: 10.1186/1465-9921-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. The role of laminin-integrin signaling in triggering (MB) formation: An in vivo and in vitro study. Exp Mol Pathol. 2005;79:1–8. doi: 10.1016/j.yexmp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Yuan QX, et al. Mallory body induction in drug-primed mouse liver. Hepatology. 1996;24:603–612. doi: 10.1002/hep.510240324. [DOI] [PubMed] [Google Scholar]
- Yuan QX. Dexamethasone enhances Mallory body formation in drug-primed mouse liver. Exp Mol Pathol. 2000;69:202–210. doi: 10.1006/exmp.2000.2320. [DOI] [PubMed] [Google Scholar]