Abstract
A convenient GC–MS/MS-based method was developed for the simultaneous measurement of 17β-estradiol, 17α-estradiol and estrone using liquid-liquid extraction, a single-step derivatization with N-(trimethylsilyl)imidazole and the corresponding deuterated estrogens as internal standards. Separation of these estrogens was achieved on a 50% phenyl polysilphenylene-siloxane bonded phase column. MS/MS response factors for the derivatized analytes and their corresponding internal standards were found to be practically identical. Therefore, analyte concentrations could be determined by multiplying the measured analyte to internal standard ion-current ratio with known molar concentration of the corresponding deuterated internal standards. Assay accuracies, determined from the analyses of quality control samples obtained by spiking known concentrations of analytes into charcoal-stripped human serum, were in the −11% to +14% range. Limits of quantitations were between 13 pg/mL and 21 pg/mL from this biological medium.
Keywords: Endogenous estrogens, Gas chromatography –tandem mass spectrometry, Trimethylsilylation, Isotope dilution
Introduction
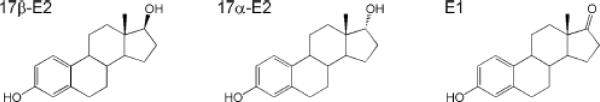
A lesser-known natural estrogen, viz. 17α-estradiol (17α-E2) has been recognized to induce genomic effects such as weak estrogenic activity [1–3], besides possessing important non-genomic actions such as neuroprotection [4–8] and relaxation of various smooth muscles [9]. 17α-E2 is the C17 diastereomer or epimer of 17β-estradiol (17β-E2), the most potent endogenous estrogen that can be interconverted to estrone (E1) by 17β-hydroxysteroid dehydrogenase [10]. The biosynthesis of 17α-E2 has not been fully elucidated, but E1 and epitestosterone [11] have been proposed as possible precursors for its endogenous formation. 17α-E2 occurs naturally in some ungulates and rodents [12]; large quantities of this estrogen is present in the urine of pregnant mares and, therefore, it is a constituent of conjugated estrogen-containing hormone replacement therapies [13]. Exposure of humans to 17α-E2 is also possible via environmental sources, such as consuming certain foods [14]. In postnatal and adult rodent brain, the existence of a novel, developmentally regulated estrogen receptor has also been proposed with 17α-E2 which is apparently synthesized at high levels locally in the brain, as its own ligand [15–17]. 17α-E2 is believed to be important in neurogenesis and elevated brain levels of this weak estrogen may act as an endogenous antidepressant. Therefore, identification and quantitation of this steroid from various biological matrices are essential to further elucidate the role of this underappreciated estrogen. Due to the potential interconversion among 17α-E2, 17β-E2 and E1 (Fig. 1), methods for the simultaneous determination of these estrogens are also necessary.
Fig. 1.
Chemical structure of 17β-estradiol (17β-E2), 17α-estradiol (17α-E2), and estrone (E1)
Quantitation of estrogens and related compounds from various matrices usually employs either liquid chromatography–tandem mass spectrometry (LC–MS/MS) or gas chromatography–mass spectrometry (GC–MS) [18,19]. Each method offers distinct advantages and disadvantages. By using the well-established dansylation as the derivatization of choice [20], an LC-MS/MS method has been developed for the simultaneous determination of 17α-E2, 17β-E2 and E1 [16]. However, conflicting data regarding limits of detection (LODs) and limits of quantification (LOQs) of these estrogens have been reported in this publication. To our knowledge, there is only one reported method [21] that has utilized stable-isotope dilution for the simultaneous measurement of these steroids based on GC–MS and selected ion monitoring (SIM) [22]. This GC–MS-SIM procedure relies on tedious sample processing involving mixed derivatization with 3,5-bis(trifluoromethyl)benzoyl chloride and N-methylbis(trifluoroacetamide), as well as liquid-liquid extraction between the two derivatization steps. To avoid such cumbersome sample preparation, we intended to develop a simplified assay to simultaneously quantify these steroids from various tissues such as serum or brain for our ongoing research involving estrogens as neuroprotectants [23,24]. Our procedure reported here utilizes only a single-step trimethylsilyl (TMS) derivatization followed by GC–MS/MS that relies on electron ionization (EI), which offers a convenient and robust assay for the simultaneous determination of 17α-E2, 17β-E2 and E1. By employing MS/MS, we wished to take advantage of the capability of this method to eliminate the need for extensive sample work-up necessary to minimize interference that may occur with the SIM method by co-eluting compounds from a complex biological matrix [25, 26].
Experimental
Chemicals
Estrogens (17α-E2, 17β-E2 and E1) were obtained from Steraloids, Inc. (Newport, RI, USA). Deuterated estrogens; 17β-estradiol-2,4,16,16,17-d5 (d5-17β-E2), 17α-estradiol-2,4-d2 (d2-17α-E2) and estrone-2,4,16,16-d4 (d4-E1) were purchased from C/D/N Isotopes (Pointe-Claire, Quebec, Canada). Charcoal-stripped human serum was obtained from Sigma-Aldrich. (St. Louis, MO, USA). All other chemicals were of analytical grade and obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Samples and Sample Preparation
Calibration solutions containing 17β-E2, 17α-E2 and E1 were prepared by serial dilutions in acetonitrile starting with 1 mg/mL stock solutions to get 300 pg/ μL, 100pg/ μL, 30 pg/ μL and 10 pg/μL working solutions. The internal standard (IS) stock solution was prepared by mixing equal volumes of d5-17β-E2, d2-17α-E2 and d4-E1 stock solutions of 1mg/mL concentrations, followed by dilution with acetonitrile to a 100 pg/μL-working solution. Calibration samples were generated at 0:1; 0.1:1; 0.3:1; 1:1; 3:1 analyte/IS molar ratios, respectively, for all three estrogens. The quantity of ISs was kept constant at 30pg/μl injection. The appropriate calibration solution (12 μL) and the IS working solution (12 μL) were evaporated under a nitrogen stream into a glass insert with bottom spring (50 μL, Grace Davidson Discovery Sci., Deerfield, IL) placed into an autosampler vial (Alltech Associates, Deerfield, IL, USA), and the residue was derivatized with 40 μL of N-(trimethylsilyl)imidazole for 30 min at 60 °C without the use of auxiliary organic solvent. Quality control (QC) samples were obtained by spiking the analytes into charcoal-stripped human serum at five different concentrations (25, 50, 100, 150, and 200 pg/mL). After adding a fixed quantity of ISs (100 pg/mL ~ 365 pM of d4-E1 or d2-17α-E2 and 361 pM of d5-17β-E2, respectively), the samples (1.0 mL) were diluted with 9.0 mL of water and extracted with 3 × 5 mL of ethyl acetate. The organic phase was separated by centrifugation, and then the combined organics were dried over anhydrous Na2SO4 and evaporated to dryness under a nitrogen stream. Derivatization was carried out analogously to that of calibration series and samples were analyzed without further dilution. Each experiment was done in five replicates to ensure the reproducibility and reliability of the results obtained.
GC–EI-MS/MS
Analyses were performed on a TRACE GC interfaced to a PolarisQ ion-trap mass spectrometer (Thermo Fisher Scientific) equipped with an AI 3000 autosampler (Thermo Electron Corp., Milan, Italy). Samples were separated on a 30 m × 0.25 mm i.d., 0.25-μm TR-50MS (Thermo Fisher Scientific) 50% phenyl polysilphenylene-siloxane bonded stationary phase. Helium (1.5 mL min−1) was used as a carrier gas. Column temperature was held at 200 °C for 1 min, and then raised to 250 °C at 15 °C min−1, followed by an increase to 260 °C at 2 °C min−1 and, finally, at 30°C min−1 to 300 °C where column temperature was held for 4 min. One μL of the derivatized sample was injected in splitless mode with injector temperature set to 250 °C. Transfer line and ion source temperatures were 300 °C and 250 °C, respectively. Electron ionization (EI, 70 eV) was employed to obtain mass spectra. Collision-induced dissociation (CID) product-ion tandem mass spectra (MS-MS) were collected using 1.0 Th parent ion isolation width and 12 ms isolation time, followed by 2.5 V applied to the endcap electrodes for 15 ms (excitation time) with 0.3 selected as resonance parameter (q). Data acquisition and processing were performed by the manufacturer's XCalibur (version 1.4) software. Selected-reaction monitoring (SRM) transitions chosen for quantitative assay development were m/z 416 → 285 for the bis-silyl derivatives of estradiols, while m/z 342 → 257 was used as SRM signal for TMS-E1. SRMs for the TMS derivatives of d5-17β-E2, d2-17α-E2, and d4-E1 were 421 → 287, 418 → 286+287 and 346 → 261, respectively. Assay calibration was done by fitting the measured analyte to IS integrated SRM ion-current ratios (Ranal/IS) with their molar ratios (nanal/nIS) using the following linear equation [22]: Ranal/IS = Rb + k·(nanal/nIS), where k is the MS response factor, and Rb is the ion-current ratio measured by the MS when the IS is measured by itself (i.e., nanal = 0).
Assay Validation
Recovery experiments were done at 3 different concentrations of estrogens (25 pg/mL, 100 pg/mL and 200 pg/mL) in charcoal-stripped human serum spiked with 100pg/mL of ISs and repeated 5 times. Recovery of ISs was also done separately using 100 pg/mL of each IS. LOD and LOQ were estimated as 3.3σ/S and 10σ/S, respectively, where σ is the standard deviation of the response (approximated by the standard deviation of y-intercept of the regression) and S is the slope of the calibration line [27]. Accuracy, indicating the extent of agreement between measured (CM) and nominal concentrations (CQ) of each estrogens in the sera, was estimated at five different analyte concentrations with 100 pg/mL of each IS and experiments repeated five times. Percentage accuracy was expressed as [(CM-CQ)/CQ] × 100 [28]. Precision, expressed as percentage coefficient of variation (% CV) was obtained from five runs, as (standard deviation/mean) × 100.
Results and Discussion
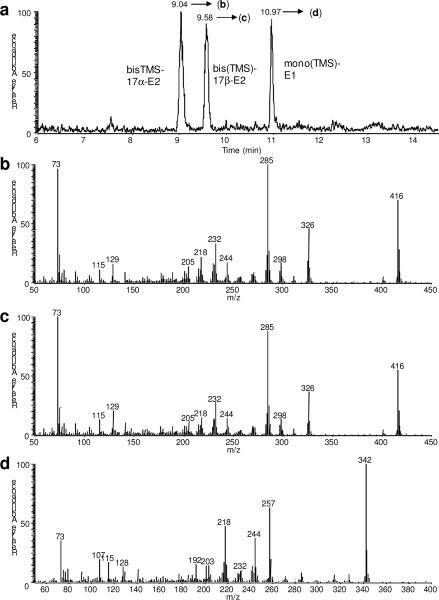
For the separation of 17α-E2, 17β-E2 and E1, we used 50% phenyl polysilphenylenesiloxane bonded stationary phase (TR-50MS) and a single-step derivatization using N-(trimethylsilyl)imidazole. As shown in Fig. 2a, the TR-50MS column yielded an excellent separation of the bis-TMS derivatives of 17α-E2 and 17β-E2, as well as of a mono-silylated E1. Mono-silylated estradiols and bis-silylated E1 were not detected. The relative abundances of the molecular ions (M+•) of the bis-TMS derivatives of 17α-E2 (Fig. 2b) and 17β-E2 (Fig. 2c) were 60% and 75%, respectively, of that of the most intense fragment ions (base peak = 100), while M+• was the most abundant in the EI mass spectrum of the mono-silylated E1 (Fig. 2d).
Fig. 2.
GC–EI-MS analysis of the TMS-derivatives of 17β-E2, 17α-E2 and E1: ion chromatogram (a) and full-scan mass spectra of bis(TMS)-17α-E2 (b), bis(TMS)-17β-E2 (c), and TMS-E1 (d).
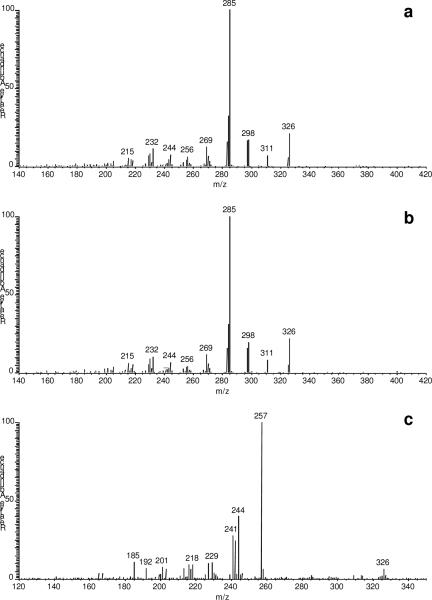
The full scan MS/MS spectra of the M+• for bis(TMS)-17α-E2 and bis(TMS)-17β-E2 (m/z 416) displayed m/z 285 as their major fragment ion, as shown in Fig. 3a and Fig. 3b, respectively. M+• of the single, mono-TMS derivative for E1 (m/z 342) gave major product ions at m/z 257, 244, and 241 upon CID (Fig. 3c), which confirmed trimethylsilylation on the A-ring of the steroid according to fragmentation patterns reported earlier [29]. The total run time per sample was 12 min.
Fig. 3.
MS/MS product-ion spectra of the molecular ions (M+•) of (a) bis(TMS)-17α-E2 (precursor: m/z 416), (b) bis(TMS)-17β-E2 (precursor: m/z 416), and (c) TMS-E1 (precursor: m/z 342).
Since the labeled ISs did not produce significant signal at the SRMs for the analytes, we considered Rb=0. For bis-TMS derivative of d2-17α-E2, the MS/MS spectrum indicated the involvement of the hydrogen in the labeled position into the fragmentation induced by CID to yield the fragment ion at m/z 285 in the unlabeled analyte (Fig. 3a). Therefore, the sum of the intensities for two fragment ions (m/z 286 and 287) was chosen to create the SRM chromatogram of this IS and, thus, maximize its SRM signal, which is an option that can be exploited by using ion traps for the purpose of quantitative analyses [30]. To obtain the MS response factor (k) that relates the measured Ranal/IS from the SRM chromatograms to the nanal/nIS molar ratios of the analytes and their labeled IS by linear fitting in a calibration series (with nanal/nIS 0, 0.3, 1.0, 2.0 and 3.0, respectively), the equations were as follows: Ranal/IS =1.092 (± 0.020) · nanal/nIS for E1 (r2=0.9990); Ranal/IS =1.063 (± 0.020) · nanal/nIS for 17β-E2 (r2=0.9989); and Ranal/IS =0.990 (± 0.025) · nanal/nIS for 17α-E2 (r2=0.9998). Accordingly, the k was considered 1 and, therefore, the molar concentrations (nanal) of estrogens could simply be calculated by multiplying the Ranal/IS with the known molar concentration of the IS (nIS) added.
About 80–90% of estrogens were recovered by liquid-liquid extraction from the spiked charcoal-stripped human serum. There was no significant difference in recovery between the analytes and their corresponding IS (data not shown). Recoveries for all three estrogens were linear. We estimated [27] 4 pg/mL for 17β-E2, 5 pg/mL for 17α-E2 and 7 pg/mL for E1 as LODs, respectively (Table 1). Consequently, the LOQ was around 13pg/mL for 17β-E2, 15 pg/mL for 17α-E2, and 21 pg/mL for E1. These values of merit were significantly lower than those reported for GC–MS-SIM [21], and comparable to those of reported for an LC/MS/MS assay involving these estrogens [16]. Precision (expressed in percentage from coefficients of variation, CVs) and accuracy (Table 1) were also within the acceptable range [22].
Table 1.
Precision and accuracy of the developed GC-EI-MS-MS assay for the simultaneous measurement of 17α-E2, 17β-E2 and E1 in human serum
| Nominal quantity of QC (pg mL-1) | E1 | 17α-E2 | 17β-E2 | Accuracy (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| CM (pg mL-1) | C.V. (%) | CM (pg mL-1) | C.V. (%) | CM (pg mL-1) | C.V. (%) | E1 | 17α-E2 | 17β-E2 | |
| 25 | 27.3 | 9.0 | 22.2 | 9.8 | 28.4 | 9.4 | 9.2 | −11.2 | 13.6 |
| 50 | 52.6 | 7.1 | 51.5 | 5.2 | 53.4 | 5.6 | 5.2 | 3.0 | 6.8 |
| 100 | 101.8 | 2.4 | 103.7 | 3.3 | 103.4 | 4.1 | 1.8 | 3.7 | 3.4 |
| 150 | 147.6 | 4.4 | 160.0 | 7.1 | 158.0 | 3.6 | −1.6 | 6.6 | 5.1 |
| 200 | 223.7 | 9.9. | 216.6 | 9.1 | 229.0 | 7.9 | 11.8 | 8.3 | 14.5 |
In conclusion, we developed and validated a GC-MS/MS-based method for the simultaneous determination of 17β-E2, 17α-E2 and E1 from human serum. The procedure relies on liquid-liquid extraction, involves only a single-step derivatization and employs the principle of isotope dilution to obtain quantitative data, which makes it straightforward and convenient to employ as a routine assay.
Acknowledgment
Financial support for this work was provided in part by the grant AG027956 from the National Institute of Health (Bethesda, MD, USA). Laszlo Prokai is the Robert A. Welch Professor of the University of North Texas Health Science Center (endowment number BK-0031).
References
- 1.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 2.Hajek RA, Robertson AD, Johnston DA, Van NT, Tcholakian RK, Wagner LA, Conti CJ, Meistrich ML, Contreras N, Edwards CL, Jones LA. Environ Health Perspect. 1997;105:577–581. doi: 10.1289/ehp.97105s3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. New Eng J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 4.Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, Holsboer F. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 5.McClean J, Nunez JL. Exp Neurol. 2008;210:41–50. doi: 10.1016/j.expneurol.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin C, Zinia R, Simon N, Tillementa JP. Neuroscience. 2002;115:415–424. doi: 10.1016/s0306-4522(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 7.Gelinas S, Bureau G, Valsatro B, Massicotte G, Cicchetti F, Chiasson K, Gagne B, Blanchet J, Matinoli MG. Neurotox Res. 2004;6:141–148. doi: 10.1007/BF03033216. [DOI] [PubMed] [Google Scholar]
- 8.Barha CK, Lieblich SE, Galea LAM. J Neuroendocrinol. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 9.Perusquia M, Navarrete E. Reprod Biol Endocrinol. 2005;3:30–40. doi: 10.1186/1477-7827-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labile FL, Luu-The V, Lin S, Labile C, Simard J, Breton R, Bélanger A. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Williams KIH, Layne DS. J Clin Endocrinol. 1967;27:159–164. doi: 10.1210/jcem-27-2-159. [DOI] [PubMed] [Google Scholar]
- 12.Sievernich A, Wildt L, Lichtenberg-Fraté H. J Steroid Biochem Mol Biol. 2004;94:444–463. doi: 10.1016/j.jsbmb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Bhavnani BR. Proc Soc Exp Biol Med. 1998;217:6–16. doi: 10.3181/00379727-217-44199. [DOI] [PubMed] [Google Scholar]
- 14.Rizzati V, Rathahao E, Gamet-Payrastre L, Delous G, Jouanin I, Guéraud F, Paris A. Steroids. 2005;70:161–172. doi: 10.1016/j.steroids.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 17.Toran-Allerand CD. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 18.Giese RW. J Chromatogr A. 2003;1000:401–412. doi: 10.1016/s0021-9673(03)00306-6. [DOI] [PubMed] [Google Scholar]
- 19.Díaz-Cruz MS, López de Alda MJ, López R, Barceló D. J Mass Spectrom. 2003;38:917–923. doi: 10.1002/jms.529. [DOI] [PubMed] [Google Scholar]
- 20.Roos RW, Medwick TJ. Chromatogr Sci. 1980;18:626–630. doi: 10.1093/chromsci/18.11.626. [DOI] [PubMed] [Google Scholar]
- 21.Hobe G, Schöna R, Goncharov N, Katsiya G, Koryakin M, Gesson-Cholat I, Oettele M, Zimmermann M. Steroids. 2002;67:883–893. doi: 10.1016/s0039-128x(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 22.MacCoss MJ, Matthews DE. Anal Chem. 2005;77:294A–302A. doi: 10.1021/ac053431e. [DOI] [PubMed] [Google Scholar]
- 23.Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez E, Liu R, Simpkins JF. Proc Nat Acad Sci USA. 2003;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prokai-Tatrai K, Perjesi P, Rivera-Portalatinc NM, Simpkins JW, Prokai L. Steroids. 2008;73:280–288. doi: 10.1016/j.steroids.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraf A, Park JY, Milton DK, Larsson L. J Environ Monit. 1999;1:163–168. doi: 10.1039/a809019j. [DOI] [PubMed] [Google Scholar]
- 26.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Thanikachalam S, Rajappan M, Kannappan V. Chromatographia. 2008;67:41–47. [Google Scholar]
- 28.Canson RJ. J Chromatogr B. 1997;689:175–180. doi: 10.1016/s0378-4347(96)00297-6. [DOI] [PubMed] [Google Scholar]
- 29.Zuo Y, Zhang K, Lin Y. J Chromatogr A. 2007;1148:211–218. doi: 10.1016/j.chroma.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Tiller PR, Cunniff JB, Land AP. Rapid Commun Mass Spectrom. 1997;11:1151–1153. [Google Scholar]