Abstract
The proof-of-principle demonstration of rapid whole blood bioassays based on microwave-accelerated metal-enhanced fluorescence (MAMEF) method using silver nanoparticle-deposited surfaces is presented. In this regard, spherical silver nanoparticles were deposited onto glass slides (silver nanoparticle films, SNFs) in a highly reproducible manner, which was assessed by optical absorption spectroscopy. Atomic force microscopy was employed to determine the size of the deposited silver nanoparticles. A model bioassay, based on the well-known interactions of biotinylated bovine serum albumin (b-BSA) and streptavidin was constructed on SNFs. The model bioassay was run at room temperature (metal-enhanced fluorescence (MEF)-based bioassay without microwave heating) for 60 minutes and with microwave heating (MAMEF-based bioassay) for 1 minute. In contrast to MEF-based bioassays that only allowed the use of samples in buffer solution, MAMEF-based bioassays afforded the use of whole blood samples. A lower detection limit of 1 nM and 0.01 nM for b-BSA was determined in MEF-based and MAMEF-based bioassays, respectively.
Keywords: Bioassays, Plasmonics, Surface Plasmons, Silver Nanoparticles, Gold Nanoparticles, Surface Plasmon Resonance, Plasmon Controlled Fluorescence, Metal-Enhanced Fluorescence, Microwave Heating
1. Introduction
Bioassays are commonly used for the detection of biomolecules and analytes present in biologically relevant samples [1]. The quantitative detection of biomolecules and analytes are typically carried out by labeling the antibody/protein with an enzyme, [1] magnetic particles [2, 3] or fluorophores [4, 5] followed by the conversion the measured signal to the concentration of the unknown biomolecules or analytes. Fluorescence-based readout for signal transduction is one of the most widely used technique in bioassays. Although well established, the sensitivity of the fluorescence-based bioassays is mainly affected by the quantum yield of the fluorophore used to label the detector antibody/protein and the type of optical detectors used in collecting the fluorescence readout [6]. In addition, the total assay time is controlled by the binding kinetics of the proteins, which takes up to 20 minutes with automated miniature instruments including sample preparation or several hours in a typical laboratory setting [7]. For whole blood samples, additional time is required to separate whole blood component for the detection of desired biomolecules and analytes.
In this regard, to address the two major shortcomings of fluorescence-based bioassays currently in use today; i.e., bioassay sensitivity and rapidity, a new platform technology called microwave-accelerated metal-enhanced fluorescence (MAMEF), was recently introduced [8, 9]. The MAMEF method couples the benefits of low power microwave heating with metal-enhanced fluorescence (MEF)[10]. In MAMEF, the MEF phenomenon increases the sensitivity of the assays, while the use of low power microwave heating kinetically accelerates assays to completion within a few seconds.[8]
There are three components of the MAMEF method: (I) plasmon-supporting nanoparticles (i.e., silver and gold), (II) electromagnetic energy (microwave region) and (III) an aqueous bioassay medium. The plasmon-supporting nanoparticles serve as (1) a surface for the attachment of one of the biorecognition partners (2) as an enhancer of the fluorescence emission (MEF effect) [11] and (3) a microwave transparent material for the selective heating of the aqueous media with microwave energy. In MAMEF, the most important process is the selective heating of water, which occurs as the aqueous medium is heated by microwaves to a higher temperature than the plasmon-supporting nanoparticles. Subsequently a temperature gradient between the warmer (than room temperature) aqueous medium and the colder nanoparticles (silver nanoparticles are transparent to microwaves at 2.45 GHz and remain at room temperature) is created. This temperature gradient results in mass-transfer of biomolecules from the warmer medium to the surface of the nanoparticles, where the biorecognition events are rapidly driven to completion. Although the MAMEF method provides the means to increasing the sensitivity of the bioassays while shortening the total assay time, to date, it was only demonstrated for bioassays that employ samples in buffer solutions. Given the increasing demand for bioassays for complex biological samples (such as serum, whole blood and saliva), there is still a need for bioassay techniques those routinely evaluates these complex biological samples.
In this study, the proof-of-principle demonstration of whole blood bioassays using MAMEF method is presented. In this regard, first freshly prepared spherical silver nanoparticles were deposited onto glass slides in a highly reproducible manner. Optical absorption spectroscopy was employed to evaluate the reproducibility of the deposition method. The surface plasmon resonance peak at 430 nm for silver nanoparticles deposited onto 10 different glass slides showed a minimal ~2% variation, which provided a direct evidence for the effectiveness of this straightforward deposition method. Atomic force microscopy was employed to determine the size of the silver nanoparticles, where the height of the silver nanoparticles was found to be~100 nm. MEF-based detection of a model protein, b-BSA, in buffer in the concentration range of 0.1–1000 nM was achieved within 60 minutes at room temperature. The incorporation of low power microwave heating into MEF-based bioassays (that is, MAMEF-based bioassay) afforded the detection of < 0.01 nM b-BSA from whole blood samples in 1 minute. In addition to the reduced assay times, the use of microwave heating in MEF-based bioassays also afforded for the expansion of the detectable concentration range of biomolecules/analytes in complex biological samples using MEF-based bioassays without the need for expensive detectors and optics.
2. Materials and Methods
Bovine-biotinamidocaproyl-labeled albumin (b-BSA), Fluorescein isothiocyanate (FITC)-avidin, whole blood, silver nitrate (99.9%), trisodium citrate, press-to-seal silicone isolators (8 well, D × diam. 1.0 mm × 9 mm) and silane-prep™ glass (amino) slides were purchased from Sigma-Aldrich. All chemicals were used as received.
The synthesis of spherical silver nanoparticles was performed using the following procedure: 2 ml of 11.6 mM trisodium citrate solution was added drop wise to a heated (90°C) 98 ml (90 aqueous solution of 6.5 mM of silver nitrate while stirring. The mixture was kept heated for 10 minutes and then it was cooled to room temperature. All glassware used was treated with “piranha solution” (3:7 v/v; 30% hydrogen peroxide/concentrated sulfuric acid: CAUTION! piranha solution reacts violently with most organic materials and should be handled with extreme care) and rinsed with deionized water at least three times before use.
The coating of the amino slides with spherical silver nanoparticles was accomplished by incubating the amino slides in a freshly prepared silver nanoparticle solution for 30 minutes. The amino slides were coated with silver nanoparticles due to the binding of silver to the terminal amine groups of the silane. An 8-well silicon isolator was attached to the SNFs before the construction of the bioassays.
Atomic Force Microscopy (AFM) images were collected with a Veeco Atomic Force Microscope (TMX 2100 Explorer SPM), which is equipped with a dry scanner. Surfaces were imaged in air, in a tapping mode of operation, using non-contact mode cantilever. The AFM scanner was calibrated using a standard calibration grid as well as by using gold nanoparticles, 100 nm in diameter from Ted Pella. Images were analyzed using SPMLab software.
Figure 1A show the construction of the model bioassay used in this paper, which is based on the well-known interactions of biotin and streptavidin. Biotin groups are introduced to the surface of SNFs through incubation of b-BSA at room temperature or using microwave heating. The fluorophore (FITC)-labeled avidin was allowed to bind b-BSA by incubation at room temperature or using microwave heating. In the MAMEF-based whole blood bioassay, a solution of b-BSA in phosphate buffer (pH=7) is mixed with whole blood (50% v/v mixture, final volume: 50 μl, b-BSA final concentration range: 0.01–5000 nM). These mixtures or a buffer solution (control sample, no b-BSA) was placed inside the wells of silicon isolators and the SNFs were heated for 30 seconds in a commercially available microwave oven (Emerson, maximum power 700 W microwave oven, Model: MW8784B, power setting 3 was used). The unbound material was removed by rinsing with phosphate buffer three times. Then, 50 μl of 10 μM FITC-avidin was subsequently added to the b-BSA coated wells and heated for 30 seconds in the microwave cavity, followed by rinsing with buffer to remove the unbound material.
Figure 1.
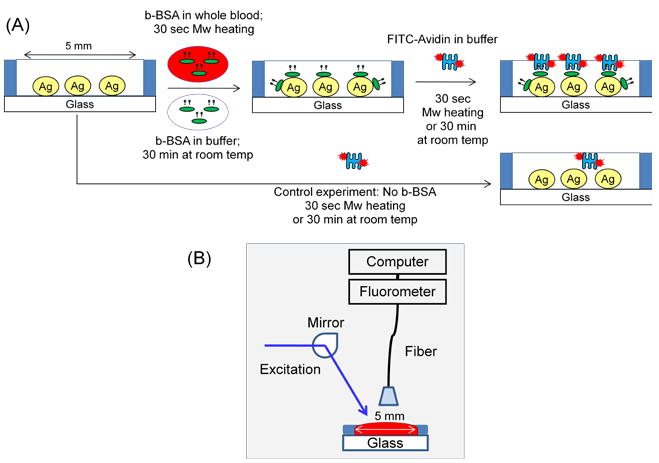
(A) Model bioassay constructed on SNFs. MAMEF-based bioassay was run with 30 seconds of low-power microwave heating in each step of the bioassay (total assay time: 1 minute). MEF-based bioassay was run at room temperature for 30 minutes each step (total assay time: 60 minutes). A control experiment, where b-BSA is omitted from the surface is also run to determine the background emission in the bioassays. (B) Sample geometry for fluorescence emission measurements.
In the bioassay run at room temperature (MEF-based bioassay, no microwave heating), b-BSA in buffer (concentration range: 0.01–5000 nM) was incubated inside the wells of the silicon isolators for 30 minutes. The unbound material was removed by rinsing with phosphate buffer three times. Subsequently, 50 μl of 10 μM FITC-avidin was incubated on biotinylated surfaces at room temperature for 30 minutes. The unbound material was again removed by rinsing with phosphate buffer three times. In all the experiments performed with low power microwaves and SNFs with silicon isolators, there was no evidence of drying of the aqueous media.
The absorption spectrum of SNFs was measured using a Varian Cary 100Bio spectrophotometer. Fluorescence measurements were undertaken using a Jaz™ spectrofluorometer (Ocean Optics, Inc., FL, USA), which allows the collection of fluorescence spectrum of samples using a fiber optic (Figure 1B). A broad spectrum halogen lamp (from Ocean Optics) was used as excitation source. A variable bandwidth filter (Ocean Optics Inc., FL, USA), which has center wavelength of 450 nm and transmission bandwidth at ~25 nm FWHM was placed in front of the excitation source. In addition a 500 nm long pass filter (Ocean Optics Inc., FL, USA) was placed in front of the detector to block the excess excitation light.
3. Results and Discussion
Since the MAMEF-based bioassays employ silver nanoparticles, it is important to validate the reproducibility of the deposition of spherical silver nanoparticles from solution onto glass surfaces. In this regard, 10 different SNFs were prepared and the absorption spectrum of each slide was measured (Figure 2A). As shown in Figure 2A, surface plasmon resonance (SPR) peak for SNFs occur at 430 nm and has the absorption value of 0.28 ± 0.006. The standard deviation for the SPR peak of 10 different samples was calculated to be ~%2.1, proving the highly reproducible deposition of silver nanoparticles onto amino glass slides. Figure 2B shows a typical AFM image (5×5 micrometer2) of SNFs. The horizontal cross-section in the AFM image reveals that the height of the silver nanoparticles is ~100 nm. Previous studies on MEF have shown that the optimum size for silver nanoparticles is ~100 nm [8, 10]. One of the most commonly used methods for the deposition of silver nanoparticles onto planar substrates in literature is called silver island films (SIFs). SIFs are deposited onto glass[10] (and plastic[12]) surfaces in a heterogeneous manner (up to %20 deviation in the wavelength of the SPR peak and broad absorption spectrum at wavelengths >500 nm). SIFs are typically used in the proof-of-principle demonstration of new methodologies, where quantitative measurements are not done [8, 13]. In this regard, the deposition of spherical silver nanoparticles onto glass slides as presented in this work yield highly reliable surfaces for quantitative detection of biomolecules and analytes based on MEF.
Figure 2.
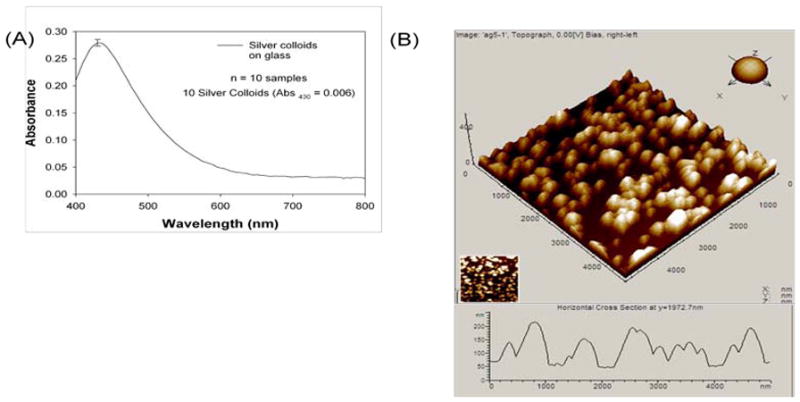
(A) Absorption spectrum for SNFs (the average for 10 different SNFs is plotted). (B) Atomic force microscope image of typical SNFs.
Figure 3 shows the summary of the results of the model bioassay run at room temperature (MEF-based bioassay) and with microwave heating (MAMEF-based bioassay). The total time for the bioassay was 60 minutes and 1 minute for the MEF-based and MAMEF-based bioassays, respectively. Figure 3A-Top shows the fluorescence emission spectrum of FITC measured from SNFs containing various amounts of b-BSA (in buffer concentration range: 0.01–5000 nM) in MEF-based bioassay. A control experiment, where b-BSA was omitted from the surface, was also run to determine the background emission due to the non-specific binding of FITC-Avidin to the surfaces. Figure 3A-Top shows an increase in emission intensity of FITC-avidin as the concentration of b-BSA is increased in buffer. Figure 3A-Bottom shows the emission intensity of FITC at 520 nm levels off when <0.1 nM and >1000 nM of b-BSA was used, indicating the concentration range for b-BSA that can be measured by this method. The background emission intensity was significantly lower than the emission intensities measured for all samples. That is, the extent of non-specific binding of FITC-avidin was minimal.
Figure 3.
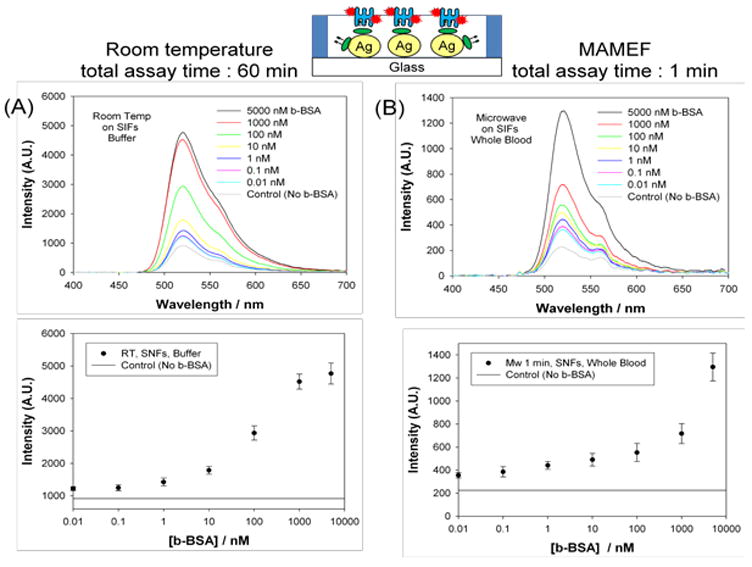
Fluorescence emission spectrum of FITC measured from SNFs containing various amounts of b-BSA (A) in MEF-based bioassay (room temperature; no microwave heating; b-BSA in buffer, total assay time = 60 minutes), (B) in MAMEF-based bioassay (microwave heating; b-BSA in whole blood; total assay time = 1 minute)
Figure 3B-Top shows the fluorescence emission spectrum of FITC measured from SNFs containing various amounts of b-BSA (in whole blood, concentration range: 0.01–5000 nM) in MAMEF-based bioassay. A control experiment, where b-BSA was omitted from the surface (whole blood sample was mixed with buffer without b-BSA), was also run to determine the background emission due to the non-specific binding of FITC-Avidin to the surfaces. Figure 3B-Bottom shows the emission intensity of FITC at 520 nm increases as the concentration of b-BSA is increased in whole blood. The background emission intensity is ~2-fold less than the intensity measured for the lowest b-BSA concentration, which implies that the lower detection limit is expected to be < 0.01 nM. In addition, since the emission intensity value does not level off at 5000 nM, the upper detection limit in the MAMEF-based bioassay is predicted to be >5000 nM. It is important to note that MAMEF-based method is designed for bioassays that employ surfaces plasmon-supporting metal nanoparticles. It was previously shown that the sensitivity of the bioassays carried out using glass surfaces without the metal nanoparticles were significantly less than those surfaces with metal nanoparticles.[8] This is attributed to the fact that the use of plasmon-supporting metal nanoparticles results in the increase of the fluorescence emission and the temperature gradient between the aqueous medium and metal surface, as described in the Introduction section. In this regard, the use of low power microwave heating in bioassays on glass surfaces without metal nanoparticles was not attempted in this study.
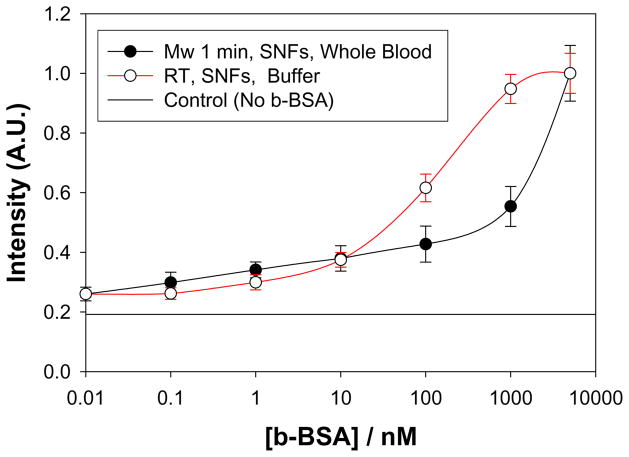
In order to visually compare the results for the MEF-based and MAMEF-based bioassays, the normalized fluorescence emission intensity at 520 nm measured in MEF-based and MAMEF-based bioassays were plotted and shown in Figure 4. Figure 4 reveals that the emission intensity values for the MAMEF-based bioassay (Mw 1 min, SNFs, whole blood) do not level off within the range of concentration of b-BSA studied here. On the other hand, the emission intensity values for the MEF-based bioassay (RT, SNFs, buffer) levels off at <0.1 nM and >1000 nM of b-BSA. That is, the incorporation of microwave heating in MEF-based bioassays afforded for the expansion of the detectable concentration range of biomolecules using MEF-based bioassays without the need for expensive more sensitive detectors and optics. One reason for the observed expansion of the detectable concentration range using microwave heating in MEF-based bioassays is thought to be the significant decrease in the extent of non-specific binding of biomolecules to the surface of the SNFs (and glass) [8].
Figure 4.
Comparison of the normalized fluorescence emission intensity of FITC-streptavidin versus concentration of b-BSA measured in MAMEF-based (Mw, 1min) and MEF-based (RT: room temperature) bioassays. The control experiment, where b-BSA is omitted from the surface, is also shown to indicate the extent of background emission in the bioassays.
It is important to note that whole blood assays were also carried out at room temperature (data not shown). In these experiments, no detectable fluorescence emission was measured from the samples (similar to background emission). This is attributed to the fact that whole blood completely coagulates within ~3 minutes on the surface of SNFs at room temperature. The coagulation of whole blood results in the confinement of the b-BSA molecules in whole blood. That is, the binding of b-BSA to the surface of SNFs was prevented. On the other hand, the MAMEF-based bioassays using whole blood samples were successfully completed due to the reduced assay time (30 seconds). It was previously shown that 30 seconds of microwave heating of the samples in buffer provided sufficient time to allow the binding of b-BSA to the surface of SNFs [8, 14]. It is interesting to note that planar metal thin films were also used in conjunction with microwave heating in bioassays [15]. Subsequently, the data presented in the present work proves that the binding of proteins to the surface of silver nanoparticles using microwave heating is possible even when mixed with whole blood, which was never shown before.
4. Conclusions
The combined use of low power microwave heating and spherical silver nanoparticles deposited to glass slides in MEF-based bioassays for whole blood samples is presented. In this regard, the reproducible deposition of silver nanoparticles onto glass slides were achieved by immersing the amino-coated slides in freshly prepared solution of silver nanoparticles. Optical absorption spectroscopy studies revealed that the surface plasmon resonance peak at 430 nm for silver nanoparticles deposited onto 10 different glass slides showed a minimal ~2% variation, proving the effectiveness of this straightforward deposition method. Atomic force microscopy studies showed the height of the silver nanoparticles deposited onto glass slides were ~100 nm. MEF-based detection of a model protein (b-BSA) in buffer in the concentration range of 0.1–1000 nM was achieved within 60 minutes at room temperature. On the other hand, MEF-based detection of b-BSA in whole blood samples was not successful due to the coagulation of whole blood at room temperature. The incorporation of low power microwave heating into MEF-based detection scheme (MAMEF-based bioassay) afforded the detection of b-BSA from whole blood samples as low as 0.01 nM in 1 minute. In the MAMEF-based bioassay, the use of microwave heating also affords for the expansion of the detectable concentration range of biomolecules/analytes in complex biological samples using MEF-based bioassays without the need for expensive detectors and optics.
Acknowledgments
The project described was supported by Award Number 7-K25EB007565-03 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Van Weemen B, Schuurs A. Immunoassay using antigen-enzyme conjugates. FEBS Letters. 1971;15:232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- 2.Selvaraju T, Das J, Han SW, Yang H. Ultrasensitive electrochemical immunosensing using magnetic beads and gold nanocatalysts. Biosens Bioelectron. 2008;23:932–938. doi: 10.1016/j.bios.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Tang D, Yuan R, Chai Y. Magneto-controlled bioelectronics for the antigen-antibody interaction based on magnetic-core/gold-shell nanoparticles functionalized biomimetic interface. Bioprocess Biosyst Eng. 2008;31:55–61. doi: 10.1007/s00449-007-0145-9. [DOI] [PubMed] [Google Scholar]
- 4.Sheng SL, Bao SH, Huang G, Wang LM. Development of time-resolved immunofluorometric assays for vascular endothelial growth factor and application on plasma of patients with gastric tumours. Clin Exp Immunol. 2008;151:459–466. doi: 10.1111/j.1365-2249.2007.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DS, Eremin SA. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal Bioanal Chem. 2008 doi: 10.1007/s00216-008-1897-z. [DOI] [PubMed] [Google Scholar]
- 6.Lakowicz JR. Principles of Fluorescence Spectroscopy. Kluwer Academic; 1999. p. 2. [Google Scholar]
- 7.Matveeva E, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR. Multi-wavelength immunoassays using surface plasmon-coupled emission. Biochem Biophys Res Commun. 2004;313:721–726. doi: 10.1016/j.bbrc.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslan K, Geddes CD. Microwave-accelerated metal-enhanced fluorescence: Platform technology for ultrafast and ultrabright assays. Analytical Chemistry. 2005;77:8057–8067. doi: 10.1021/ac0516077. [DOI] [PubMed] [Google Scholar]
- 9.Aslan K, Geddes CD. Microwave-accelerated Metal-enhanced Fluorescence (MAMEF): Application to ultra fast and sensitive clinical assays. Journal of Fluorescence. 2006;16:3–8. doi: 10.1007/s10895-005-0026-z. [DOI] [PubMed] [Google Scholar]
- 10.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD. Metal-enhanced fluorescence: an emerging tool in biotechnology. Current Opinion in Biotechnology. 2005;16:55–62. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD. Annealed silver-island films for applications in metal-enhanced fluorescence: Interpretation in terms of radiating plasmons. Journal of Fluorescence. 2005;15:643–654. doi: 10.1007/s10895-005-2970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslan K, Badugu R, Lakowicz JR, Geddes CD. Metal-enhanced fluorescence from plastic substrates. Journal of Fluorescence. 2005;15:99–104. doi: 10.1007/s10895-005-2515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malicka J, Gryczynski I, Lakowicz JR. DNA hybridization assays using metal-enhanced fluorescence. Biochemical and Biophysical Research Communications. 2003;306:213–218. doi: 10.1016/S0006-291X(03)00935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslan K, Holley P, Geddes CD. Microwave-Accelerated Metal-Enhanced Fluorescence (MAMEF) with silver colloids in 96-well plates: Application to ultra fast and sensitive immunoassays, High Throughput Screening and drug discovery. Journal of Immunological Methods. 2006;312:137–147. doi: 10.1016/j.jim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Aslan K, Malyn SN, Geddes CD. Microwave-Accelerated Surface Plasmon-Coupled Directional Luminescence: Application to fast and sensitive assays in buffer, human serum and whole blood. Journal of Immunological Methods. 2007;323:55–64. doi: 10.1016/j.jim.2007.02.010. [DOI] [PubMed] [Google Scholar]