Abstract
Biosynthesis of the anticancer drug Taxol in Taxus (yew) species involves 19 steps from the universal diterpenoid progenitor geranylgeranyl diphosphate derived by the plastidial methyl erythritol phosphate pathway for isoprenoid precursor supply. Following the committed cyclization to the taxane skeleton, eight cytochrome P450-mediated oxygenations, three CoA-dependent acyl/aroyl transfers, an oxidation at C9, and oxetane (D-ring) formation yield the intermediate baccatin III, to which the functionally important C13-side chain is appended in five additional steps. To gain further insight about Taxol biosynthesis relevant to the improved production of this drug, and to draw inferences about the organization, regulation, and origins of this complex natural product pathway, Taxus suspension cells (induced for taxoid biosynthesis by methyl jasmonate) were used for feeding studies, as the foundation for cell-free enzymology and as the source of transcripts for cDNA library construction and a variety of cloning strategies. This approach has led to the elucidation of early and late pathway segments, the isolation and characterization of over half of the pathway enzymes and their corresponding genes, and the identification of candidate cDNAs for the remaining pathway steps, and it has provided many promising targets for genetically engineering more efficient biosynthetic production of Taxol and its precursors.
Keywords: baccatin, cytochrome P450 taxoid hydroxylases, paclitaxel, Taxaceae, taxadiene synthase, taxane diterpenoids, taxoid acyl transferases, taxoids, Taxol, Taxus, yew
Taxol, arguably the most successful anti-cancer drug of all time, was structurally defined by Wall and Wani and their colleagues in 1971 (Wani et al., 1971) and gained first marketing approval from the U.S. Food and Drug Administration for the treatment of refractory ovarian cancer in 1992 and metastatic breast cancer in 1994 (Suffness and Wall, 1995). The very long development time for this drug was a consequence of limited supply from the original source, the bark of the Pacific yew Taxus brevifolia (the harvest was destructive, the purification was complicated, and the yields were low but, conversely, the politics of supply were exceedingly abundant (Goodman and Walsh, 2001)), coupled to formulation problems due to the very hydrophobic nature of this diterpenoid natural product and concerns about side effects (Arbuck and Blaylock, 1995). Were it not for the encouragement of Matt Suffness and others at the National Cancer Institute (Wall and Wani, 1995; Rowinsky, 1997), the discovery of the novel mode of action of Taxol in binding β-tubulin to promote microtubule assembly and disrupt mitosis (Schiff et al., 1979) and, ultimately, the efforts of many clinical scientists in developing suitable formulations and treatment regimes (Adams et al., 1993; Straubinger, 1995), this plant-derived drug and its relatives would not likely have achieved its present commercial success as the world’s leading anticancer agent (estimated annual sales exceeding three billion USD worldwide).
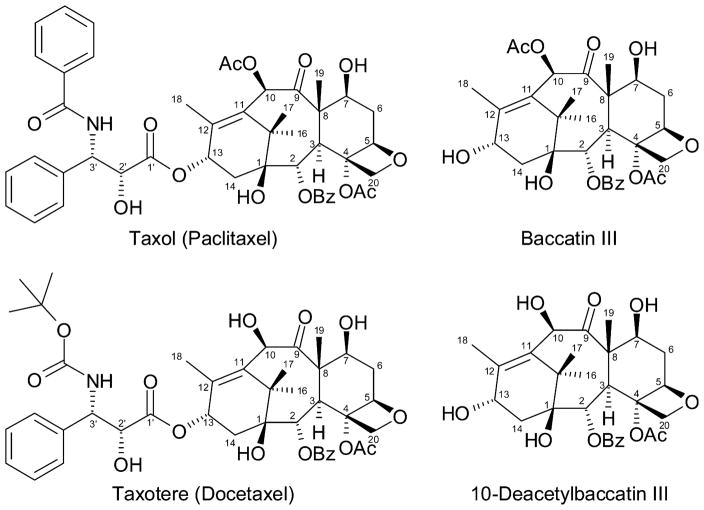
Taxol (generic name paclitaxel; Figure 1)1 entered the generic drug market with much fanfare and some controversy in 2000 (Walsh and Goodman, 2002), and is now largely produced by Taxus cell culture methods (reviewed in Tabata, 2004) or by semi-synthetic means (reviewed in Wuts, 1998) from advanced precursors (e.g., baccatin III) that are more readily available from the needles of various yew species as a renewable resource. The closely related drug Taxotere (generic name docetaxel; Figure 1) is prepared semi-synthetically from 10-deacetyl baccatin III. Several elegant total syntheses of Taxol have been devised (reviewed in Kingston et al., 2002; Xiao et al., 2003), but this approach is not commercially viable due to low yield and high cost considerations. With the increasing utilization of Taxol for the treatment of additional cancer types and other human diseases, for application much earlier in the course of intervention, for combination therapies with other antineoplastic agents (e.g., with anthracyclines and platinum compounds) (Goldspiel, 1997; Brown, 2003), and as the platform for the development of the next generation of more efficacious drugs and prodrugs (Wang et al., 2003), the market for Taxol and its congeners is expected to expand by three-fold within the next 4 years (McCoy, 2004). Drug sourcing and patient treatment costs will clearly remain important issues.
Figure 1.
Structures of Taxol, the related semi-synthetic drug Taxotere, and their respective precursors. Ac and Bz denote acetyl and benzoyl groups, respectively. The taxane A ring in this figure is illustrated as found in much of the earlier literature. Subsequent renderings illustrate the A ring as suggested by Kingston et al. (1993) to more accurately depict the 16β- and 17α-methyl groups.
The supply of Taxol and its precursors for semi-synthesis will continue for the foreseeable future to rely upon biological methods of production, involving intact Taxus plants (Croom, 1995; Kikuchi and Yatagai, 2003) for which there remain some concerns about sustainable harvest (Rikhari et al., 1998) or cell cultures derived therefrom (Gibson et al., 1995; Takeya, 2003) which have now been shown to be commercially viable (Tabata, 2004). Improving the biological production yields of Taxol depends critically upon a detailed understanding of the relevant biosynthetic pathway(s), the enzymes that catalyze the sequence of reactions, especially the slow steps, and the genes encoding these enzymes, because only this approach can usefully guide efforts to increase yield by classical genetic manipulation or by molecular engineering of the producing organism.
Biogenetic considerations
Taxol is but one of the structurally more complex representatives of the approximately 400 defined taxoids (i.e., taxane diterpenoids) of Taxus species (Baloglu and Kingston, 1999; Itokawa, 2003), all of which are based upon the unique taxane (pentamethyl [9.3.1.0]3,8 tricyclopentadecane) skeleton (see Figure 2) or rearrangement products of this tricyclic scaffold (i.e., the abeo-taxoids derived by rearrangement at the A/B or B/C ring junctures). The taxane nucleus bears three stereocenters and Taxol itself bears 11 such centers, the large number of possible stereoisomers affording some appreciation of the difficulty of Taxol total syntheses.
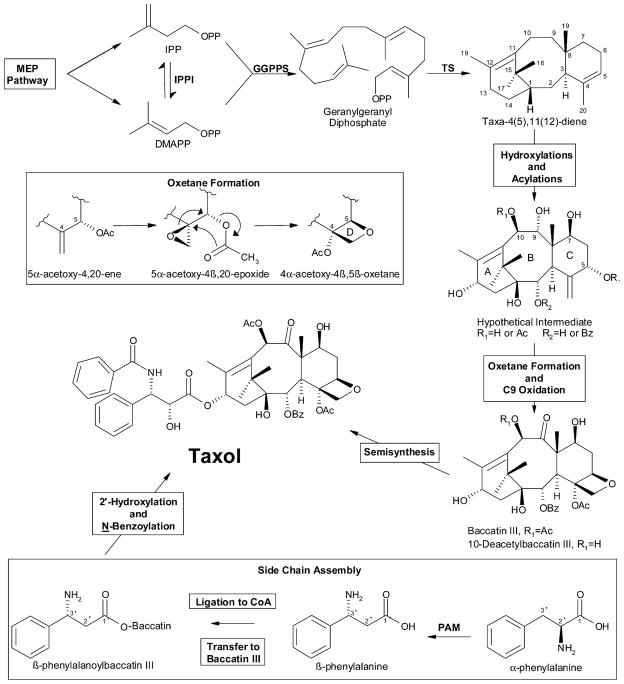
Figure 2.
Overview of the Taxol biosynthetic pathway. The boxed MEP Pathway is the plastidial route from pyruvate and glyceraldehyde 3-phosphate (via methylerythritol phosphate) for C5 (IPP and DMAPP) precursor supply. The abbreviations are: IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; IPPI, isopentenyl diphosphate isomerase; GGPPS, geranylgeranyl diphosphate synthase; TS, taxadiene synthase; and PAM, phenylalanine aminomutase. OPP denotes the diphosphate moiety; Ac and Bz denote acetyl and benzoyl groups, respectively. Because the relative order of C1 hydroxylation and oxetane formation is uncertain, the hypothetical intermediate illustrated could be at the level of an acylated hexaol rather than a heptaol bearing the C1 hydroxyl.
The rationale for the biosynthesis of such a vast assortment of structurally diverse taxoids by Taxus species is unknown. A small number of these 400 or so taxoid metabolites are almost certainly relevant Taxol intermediates (the pathway to Taxol is considered to involve 19 steps from primary plant metabolism (Jennewein et al., 2004b)). Many others may simply represent the consequences of promiscuous oxygenase and acyltransferase activities, several likely play a role in plant defense in possessing antifeedant (Daniewski et al., 1998) or antibiotic activity (Young et al., 1992; Elmer et al., 1994), and others are toxic to mammals (Odgen, 1988). What is clear is that Taxus species, both intact plants and derived cell cultures (Ketchum et al., 2003), direct considerable pathway flux to the production of taxoids other than Taxol, and that any approach to improving the production yields of Taxol and its immediate precursors must take into account these numerous and apparently diversionary taxoid biosynthetic side-routes and dead-ends.
The biogenesis of Taxol (Figure 2) can be conceptually divided into several discrete processes, the first being the construction of the taxane skeleton that is followed by the addition of eight oxygen functional groups to the core. Not surprisingly, these added functions have been shown to arise via atmospheric O2 (Eisenreich et al., 1998) indicating that the reactions involved are indeed oxygenations and not double bond hydrations. Two acetylations and a benzoylation (at C2) decorate the oxygenated intermediate. Based on a survey of the positional frequency of oxygen functional groups in the extant taxoids, Floss and Mocek (1995) have proposed the order of oxygenation of the taxane core to be C5 and C10, followed by C2 and C9, then C13 followed by C7, and finally C1 hydroxylation which occurs very late in the pathway. Acylation reactions almost certainly intervene en route to the hypothetical heptaol intermediate (Figure 2), and the order of oxygenation deduced may be somewhat biased by inclusion in the survey of taxoid metabolites most unlikely to reside on the pathway( s) to Taxol (e.g., 14β-hydroxy taxoids and 13-acetyl derivatives).
Several more auxiliary reactions are required to reach baccatin III, including an oxidation at C9 to the ketone function and the formation of the oxetane (D ring), both processes of which are thought to occur late in the pathway (Figure 2). Several proposals based on sound chemical reasoning have been put forward for construction of the oxetane (reviewed in Floss and Mocek, 1995; see also Giner and Faraldos, 2003), all involving the progression from the 4,20-ene-5α-oxy functional grouping to the 4β,20-epoxide-5α-oxy group to the oxetane (i.e., ring expansion of the C4,C20- epoxide to the C4,C20-O-C5 oxetane).
The final steps of the pathway likely involve the assembly of the C13-side chain appended to baccatin III. Little by way of chemical inference can be said about this process, except that the last step may be the N-benzoylation to Taxol; related reactions would afford cephalomannine (N-tigloyl) or taxol C (N-hexanoyl). The N-benzoyl phenylisoserine C13-side chain, the acetate at C4, the benzoate group at C2, the oxetane ring, and the cup-shaped taxane core itself are all important structural elements contributing to the Taxol pharmacophore for tubulin binding (Georg et al., 1995; Kingston, 1995; Jiménez-Barbero et al., 2002; Wang et al., 2003); the bioactive conformation of Taxol bound to β-tubulin has recently been described (Ganesh et al., 2004).
In this paper, we employ this descriptive outline of Taxol biosynthesis (Figure 2) to review, in approximate sequence and with some personal accounting, the defined pathway steps, enzymes and structural genes of Taxol formation, with attention, where possible, to related organizational, regulatory, and evolutionary features of the pathway, and with equal emphasis on those aspects of Taxol metabolism that are still unknown. Progress in this research area was last briefly reviewed in 2001 (Jennewein and Croteau, 2001; Walker and Croteau, 2001).
Experimental systems
Significant early work, including feeding experiments and time-course studies, cell-free enzymology, cDNA library construction and pathway gene cloning (Hezari et al., 1995; Koepp et al., 1995; Hefner et al., 1996; Wildung and Croteau, 1996), was conducted using mature Taxus plants as a tissue source. The forests of northern Idaho offer nearly limitless biomass of T. brevifolia and a very scenic environment; however, the logistics of such research in the forest setting coupled to seasonal variation in co-worker enthusiasm (particularly during the winter) rapidly brought this approach to a halt. Taxus saplings raised in the greenhouse offer an alternative source material but immature plants, like their mature counterparts, are slow-growing, phenolic-laden, woody (stems) or oleaginous (needles), and thus hardly ideal for biochemical or molecular biological study. With the development of Taxol-producing Taxus cell cultures (Gibson et al., 1995), an immediate switch was made to this experimental system. Taxus cell suspension cultures, especially those that are inducible with methyl jasmonate for increased taxoid production (Ketchum et al., 1999), are highly amenable to biochemical and molecular study. The approach also offers a viable commercial production platform for the pharmaceutical industry that is more controllable (Tabata, 2004) and that is free of the environmental and political issues which may attend tissue collection (Cragg et al., 1993; Rikhari et al., 1998; Goodman and Walsh, 2001), and that also has the potential for molecular genetic manipulation of taxoid composition and yield with relatively short development times.
All Taxus species produce Taxol (Itokawa, 2003) and, although the mixture of taxoids accumulated can vary widely between species (and between tissues of the same species), the basic taxoid biosynthetic pathways are thought to be universal in the genus. It was once considered that all Taxus species may be subspecies of T. baccata (the European yew) (see, for example, the discussion in Hartzell (1991) based on the early work of Pilger (1903)); however, more recent molecular taxanomic evidence (Collins et al., 2003) has demonstrated the existence of at least several distinct, but very closely related, species. Limited biochemical evidence indicates that the pathways and enzymes of Taxol biosynthesis are indistinguishable between Taxus species but, more tellingly, perusal of GenBank for acquisitions, for example, of taxadiene synthase (TS), taxoid 10β-hydroxylase, or taxadien-5α-ol-O-acetyl transferase from different Taxus species (for the significance of these enzymes, see below) indicates that these genes are nearly identical (>95%I). This essential identity at the molecular level has permitted switching between the various available Taxus species (both intact tissue and cell cultures) in pursuit of the enzymology and molecular genetics underlying the pathway and its induction, as well as compositional and developmental variations, with the expectation that broad, generally applicable conclusions about taxoid metabolism in the genus can be drawn.
Precursor supply and early pathway steps
The diterpenoid taxane core is derived via the plastidial 2-C-methyl-D-erythritol phosphate (MEP) pathwaywhich supplies the C5 isoprenoid precursors isopentenyl diphosphate (IPP, three units are required) and dimethylallyl diphosphate (DMAPP, one starter unit is required) (Eisenreich et al., 1996). Partial sequencing of nearly 8500 anonymous cDNA clones from a methyl jasmonate-induced T. cuspidata cell library (Schoendorf et al., 2001) revealed (Jennewein et al., 2004b) at least one EST encoding each of the seven enzymes of the plastidial MEP pathway for production of IPP and DMAPP from pyruvate and glyceraldehyde 3-phosphate (for reviews of this pathway, see Rohmer, 1999; Eisenreich et al., 2001; Kuzuyama and Seto, 2003); see also Rodríguez-Concepción elsewhere in this issue. Given that taxoid biosynthesis is substantially induced in the source Taxus cells (Ketchum et al., 1999), the up-regulation of this pathway for precursor supply might be anticipated. ESTs encoding 1-deoxy-D-xylulose 5-phosphate (DXP) reductoisomerase were substantially more abundant than acquisitions encoding other MEP pathway enzymes, although transcripts encoding all but MEP cytidyltransferase and hydroxymethyl-butenyl diphosphate reductase were well represented (Jennewein et al., 2004b). DXP reductoisomerase and DXP synthase have both been implicated as catalyzing slow steps in the biosynthesis of plastid-derived terpenoids (Estevez et al., 2001; Mahmoud and Croteau, 2001).
The parental taxane clearly arises in plastids (see below), as almost certainly do all plant diterpenes, from the universal, acyclic (C20) precursor geranylgeranyl diphosphate (GGPP) (Figure 2). GGPP synthase from cell cultures of both T. canadensis (Hefner et al., 1998) and T. baccata (Laskaris et al., 2000) has been isolated and partially characterized, and it appears typical of this class of prenyltransferases in properties. Consideration of the GGPP synthase activity profile measured in vitro and taxoid accumulation in T. baccata cells led Laskaris et al. (1999) to suggest that GGPP synthase may play a regulatory role in taxoid production; however, in T. canadensis cells (the two experimental systems differ substantially in detail), consideration of in vitro determined rates of GGPP synthase versus the immediate downstream step (the committed cyclization) during the time course of taxoid production indicated that GGPP synthase is very unlikely to have much regulatory influence on the pathway (Hefner et al., 1998).
A cDNA encoding GGPP synthase from T. canadensis cells has been isolated and confirmed by heterologous functional expression (Hefner et al., 1998). The sequence encodes an apparent plastidial transit peptide, as expected, and translates a mature protein of ~32 kDa that functions as a homodimer of ~60 kDa. The sequence also resembles those of angiosperm GGPP synthases but, not surprisingly given the phylogenetic distance between this ancient gymnosperm and the angiosperms, it is clearly distinguishable from them; the recombinant enzyme has become the prototype of the class from gymnosperms that has proved useful for a range of studies (Burke and Croteau, 2002). The time-course of transcript abundance in developing T. canadensis cells compared to that for the immediate cyclization step also supports the notion that GGPP synthase is probably not rate limiting in either constitutive or induced biosynthesis of taxoids in this system (Hefner et al., 1998); this earlier work on Taxus GGPP synthase appears to have been overlooked by Laskaris et al. (1999, 2000). ESTs encoding GGPP synthase were quite abundant in the induced Taxus cell cDNA library (1.7‰), but only one acquisition for IPP isomerase was noted (Jennewein et al., 2004b). Because the MEPpathway seemingly yields IPP andDMAPP in a 5:1 ratio (Rohdich et al., 2002), conversion of IPP to DMAPP by plastidial IPP isomerase (Figure 2) could establish a more appropriate 3:1 ratio for GGPP synthesis since isomerization to DMAPP is favored (Ramos-Valdivia et al., 1997). IPP isomerase could be a useful gene for overexpression in Taxus, as would be the homologous GGPP synthase, for the sake of compatibility, when attempting to reconstruct Taxol biosynthesis in a heterologous host (DeJong et al., 2005).
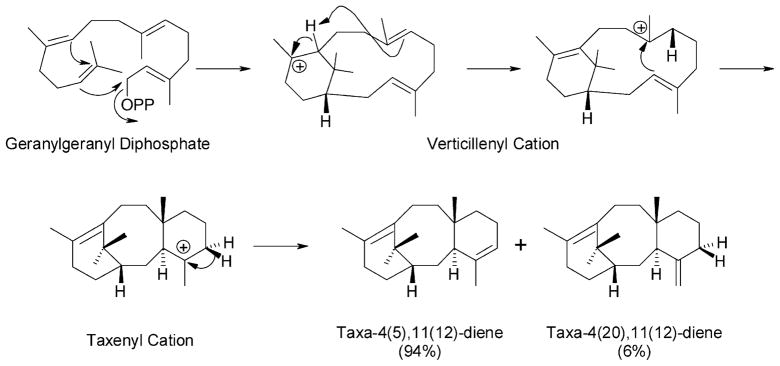
The formation of the taxane skeleton was initially, and presciently, suggested by Lythgoe and his colleagues (Harrison et al., 1966) to involve cyclization of the geranylgeranyl skeleton to taxa- 4(20),11(12)-diene by an electrophilic mechanism now known to be characteristic of this enzymatic reaction type. The 4(20),11(12)-isomer of taxadiene was the proposed product based on the observation that many taxoids bear double bonds in these positions. Work on the responsible cyclization enzyme was first carried out with cell-free extracts prepared from T. brevifolia stem tissue, with which it was shown that the product of the reaction was in fact the isomeric taxa-4(5),11(12)- diene that was confirmed as the precursor of Taxol by feeding studies (Koepp et al., 1995); migration of the double bond from the 4(5)- to the 4(20)-position occurs during the subsequent hydroxylation step (see below). Taxa-4(5),11(12)-diene was isolated as a natural product (in very small amounts) from T. brevifolia bark (Koepp et al., 1995) and both positional isomers of taxadiene were subsequently prepared by total synthesis (Rubenstein and Williams, 1995). The operationally soluble TS clearly catalyzes the committed step of taxoid biosynthesis by constructing the taxane nucleus from the branch-point intermediate geranylgeranyl diphosphate. The enzyme is similar in properties to other diterpenoid cyclases from angiosperms and gymnosperms (relatively low substrate Km, requirement for divalent metal ion, size of roughly 80 kDa, but with an alkaline pH optimum (Hezari et al., 1995)).
Based on the assumption that TS would also resemble other terpene synthases in structure, a homology-based cloning strategy was employed to acquire the corresponding cDNA from a T. brevifolia stem library (Wildung and Croteau, 1996). The sequence encodes a 98 kDa preprotein that bears a plastidial targeting peptide and contains all of the other structural elements typical of this enzyme class (Davis and Croteau, 2000). The mature enzyme of nearly 85 kDa functions as a monomer (Williams et al., 2000b), and TSs from other Taxus species appear to be essentially identical (Hezari et al., 1997). The mechanistic and stereochemical details of this complex cyclization (Figure 3) have been explored (Lin et al., 1996; Williams et al., 2000a, b; Jin et al., 2005a, b), and the reaction, in which three rings and three chiral centers are generated, shown to involve inversion of configuration at C1 of the geranylgeranyl precursor in the initial bicyclization to the verticillenyl carbocation, followed by a unique intramolecular proton migration from C11 to the re-face of C7 to complete the A-ring and promote closure of the B/C-ring juncture, with a terminating deprotonation from the C5β-face of the tricyclic taxenyl carbocation to yield taxa-4(5),11(12)-diene as the principal product (94%). Minor products of this electrophilic cyclization cascade include taxa-4(20), 11(12)-diene (5%) and verticillene (1%); interesting, the kinetic isotope effect resulting from the forced elimination of deuterium at C5β (from the 4R–2H of GGPP) even allows formation (by isotopically sensitive branching) of the unusual 3(4),11(12)-isomer of taxadiene in small amounts (Williams et al., 2000b).
Figure 3.
Cyclization of geranylgeranyl diphosphate by taxadiene synthase involving ionization of the diphosphate with closure of the first ring, intramolecular transfer of a proton in the resulting verticillenyl cation to promote the second closure, and deprotonation of the resulting taxenyl cation to yield taxa-4(5),11(12)-diene (major product) and taxa-4(20),11(12)-diene (minor product).
The time-course of TS activity versus Taxol production in T. canadensis cell cultures suggests that the committed cyclization is a slow step of the pathway; that neither taxadiene nor other early pathway intermediates accumulate to any appreciable level in Taxus tissues or cell cultures further indicates rapid conversion of these metabolites by downstream reactions (Koepp et al., 1995; Hefner et al., 1996; Hezari et al., 1997). Nevertheless, TS is apparently not rate limiting, and slower steps reside further downstream on the pathway to Taxol (Hezari et al., 1997). ESTs corresponding to two catalytically comparable, minor variants of TS (Jennewein et al., 2004a) were very abundant (~5‰) in the induced T. cuspidata cell library (Jennewein et al., 2004b). Despite the relatively high level of expression of this gene, the cyclization is a slow step of the pathway, perhaps reflecting the low turnover rate of this enzyme (0.01 s−1) (Williams et al., 2000b).
Cytochrome P450 taxoid oxygenases
The survey of functionalized taxoids by Floss and Mocek (1995) has suggested the approximate order of hydroxylation steps on the taxane core. However, the dearth of lightly functionalized taxoids described in the literature (the first to appreciably accumulate in any Taxus tissue is at the level of a 5,9,10,13-tetraol) (Baloglu and Kingston, 1999; Itokawa, 2003) gave little guidance to the exact sequence of the initial reactions which obviously must proceed from taxa-4(5),11(12)-diene (Hezari and Croteau, 1997). The observations that no oxygenated taxoids bearing the 4(5)-double bond had yet been reported, whereas taxoids with the exo-methylene at the 4(20)-position and that also bore on oxygen function at C5 were exceedingly common (Kingston et al., 1993), suggested that hydroxylation at C5 of taxa-4(5),11(12)-diene, with migration of the double bond, must occur as an early, if not the first, oxygenation step of the pathway.
To explore this possibility, cell-free preparations from T. brevifolia stems and T. cuspidata cell cultures were examined for their ability to transform biosynthetically prepared [2-3H]taxa-4(5),11(12)-diene to more polar products under a range of oxygenation conditions. Microsomal preparations were shown to convert taxadiene to a monool under cytochrome P450 reaction conditions (i.e., O2 and NADPH-dependence, CO-inhibition and blue light reversal); the product was identified as taxa-4(20), 11(12)-dien-5α-ol (by synthesis of the authentic standard), shown to be an intermediate of Taxol biosynthesis by feeding studies, and identified as a naturally occurring metabolite by isolation (albeit in very small amounts) from Taxus (Hefner et al., 1996). Subsequent studies (Lovy Wheeler et al., 2001) demonstrated that Taxus microsomes were capable of converting taxadiene, taxadienol and related simple taxoids to the level of a hexaol under identical conditions, suggesting that most, if not all, of the relevant pathway oxygenation steps were cytochrome P450-mediated and localized to the endoplasmic reticulum. This general approach of employing cell-free systems for initial demonstration of activity with defined substrates, or with surrogate substrates when the predicted substrate is not readily available, coupled to feeding studies to demonstrate pathway relevance, synthesis of the authentic reaction product, and isolation of the corresponding metabolite from Taxus has guided all subsequent biochemical and molecular exploration of the pathway from simpler precursors to more complex taxoids.
Several cloning strategies have been employed to acquire cDNAs encoding the cytochrome P450 taxoid oxygenases. Initially, a differential display of mRNA-reverse transcription-polymerase chain reaction (DD-RT-PCR) method was employed (Schoendorf et al., 2001) using methyl jasmonate induced T. cuspidata cells versus uninduced cells as source material (Ketchum et al., 1999). This approach was subsequently supplemented with a classical homology-based search (Jennewein et al., 2004a) and, ultimately, random sequencing of the same induced-cell library (Jennewein et al., 2004b) to yield a family of nearly 20 cytochrome P450 clones that display high similarity (>70%) within the group but appear to be only distantly related to other cytochrome P450s of plant origin (<35% similarity). The selection of new candidate clones as potential taxoid oxygenases could thus be made by sequence relatedness. For functional assessment of activity, clones were initially expressed in the WATII Saccharomyces cerevisiae cell line that coexpresses an Arabidopsis NADPH:cytochrome P450 reductase for improved redox coupling (Pompon et al., 1996), and then screened by a method involving feeding of the intact transformed yeast with the appropriate radiolabeled taxoid precursors (Schoendorf et al., 2001). This approach avoided the uncertainties of microsome preparation prior to clone identification and more detailed cell-free studies to characterize the recombinant cytochrome. For clones that did not express well in yeast, or were unstable in this host (as determined by C-terminal epitope tagging and/or CO-difference spectrometry), the more tedious but highly reliable baculovirus-Spodoptera expression system (Kutchan et al., 1994) was employed.
The first oxygenase of the Taxol pathway (cytochrome P450 taxa-4(5),11(12)-diene 5α-hydroxylase) was acquired by homology-based screening (Jennewein et al., 2004a), this clone having been missed in the initial DD-RT-PCR-based approach because the gene is not highly induced by methyl jasmonate in T. cuspidata cells (Lovy Wheeler et al., 2001). The cDNA encodes a protein of about 56 kDa, an N-terminal membrane (endoplasmic reticulum) insertion sequence, and all of the other conserved structural elements (for heme, oxygen and reductase binding (von Wachenfeldt and Johnson, 1995)) typical of a cytochrome P450 and that are found in all of the other taxoid oxygenases defined thus far. The recombinant microsomal enzyme, functionally expressed in both yeast and Spodoptera, is also typical in properties, like the native enzyme, but the turnover number is seemingly low (Jennewein et al., 2004a) and the reaction is unusual, but not unprecedented (Groves and Subramanian, 1984; Groves, 2005), in involving an allylic transposition (Figure 4). Interestingly, the enzyme also utilizes taxa-4(20),11(12)-diene as substrate to produce the same product with comparable kinetics (to the 4(5),11(12)-diene isomer which is the major product of the TS reaction). This observation is consistent with a reaction mechanism involving promiscuous hydrogen radical abstraction from the C20 position, or the C5 position of the 4(20),11(12)-isomer, to afford from either olefin the same delocalized radical, to which oxygen is ultimately delivered to the C5α-face to yield taxa-4(20), 11(12)-dien-5α-ol. The gene is induced roughly twofold by methyl jasmonate, and the abundance in the EST library is modest (0.8‰). This, coupled to the apparently low turnover number of the enzyme, perhaps as a consequence of the unusual reaction catalyzed, suggests that the 5α-hydroxylation is a slow step of the pathway. Taxus cell cultures accumulate but exceedingly low levels of taxa-4(5),11(12)-diene (4.8 μg/g dry wt.) and taxa- 4(20),11(12)-diene (0.33 μg/g dry wt.), and taxa- 4(20),11(12)-dien-5α-ol is rarely detectable (1.5 μg/g dry wt.), implying that the 5α-ol intermediate produced is rapidly consumed by downstream hydroxylation/acylation reactions (Ketchum and Croteau, 2005).
Figure 4.
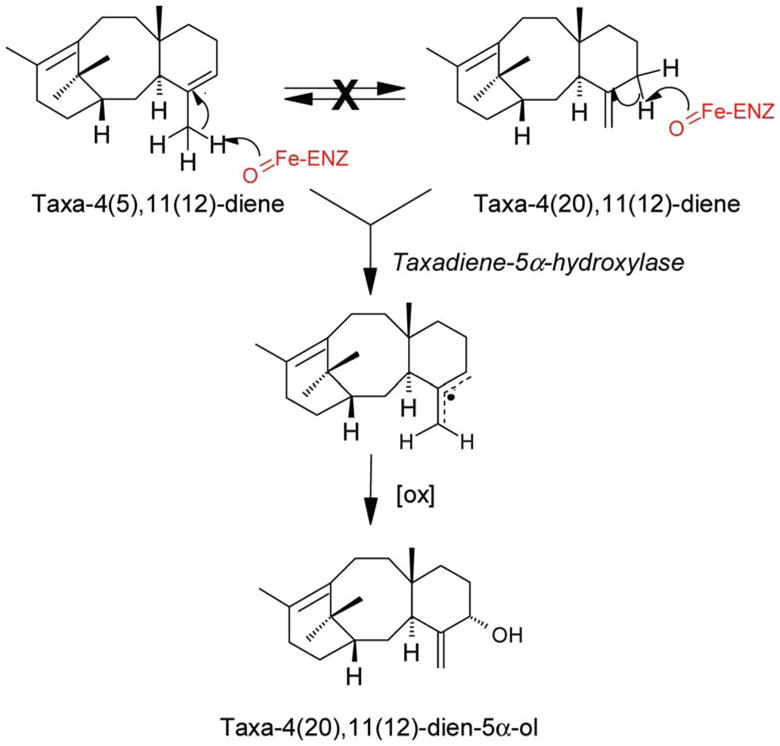
Proposed mechanism for cytochrome P450 taxadiene 5α-hydroxylase involving two modes of hydrogen abstraction to a common allylic radical followed by stereoselective oxygen insertion at the α-face of the common intermediate. Isomerization of the taxadiene isomers is not observed.
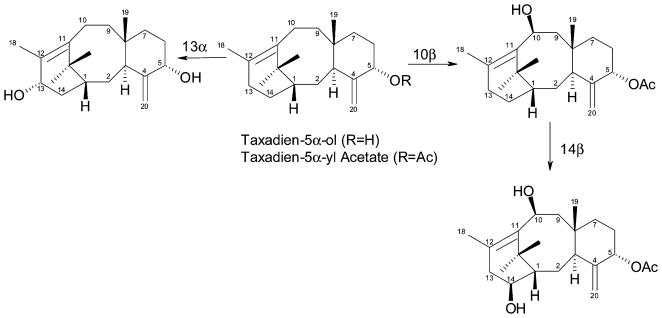
The taxoid 13α-hydroxylase was acquired by the DD-RT-PCR screen via functional expression in Spodoptera fugiperda cells using labeled taxa- 4(20),11(12)-5α-ol as substrate (Jennewein et al., 2001), and the taxoid 10β-hydroxylase was similarly acquired but with functional confirmation in yeast using labeled taxa-4(20),11(12)-dien-5α-yl acetate as substrate (Schoendorf et al., 2001) (Figure 5). Both are typical cytochrome P450s in structure and properties, and both exhibit some plasticity in substrate utilization (i.e., with lower efficiency, the 10β-hydroxylase can utilize the 5α-alcohol and the 13α-hydroxylase can utilize the 5α-acetate). These results, coupled to precursor evaluation with microsomal preparations from Taxus cells (Lovy Wheeler et al., 2001), suggest bifurcation of the taxoid biosynthetic pathway at a very early stage, leading from the 5α-ol via the 5α,13α-diol or via acetylation at C5 (see below) and 10β-hydroxylation. Feeding studies of Taxus cells with these various early precursors indicate that 5α-acetoxytaxadien-10β-ol yields a higher proportion of diversionary products (of the taxoid 14β-hydroxy type, see below) than Taxol and its congeners compared to, for example, taxadien-5α-ol itself (Ketchum and Croteau, in preparation). While the full implications of these findings are not yet known, they do suggest multiple anastomosing routes to Taxol that diverge early in the pathway and compete at some level with several side routes to other, very numerous taxoid derivatives.
Figure 5.
Reactions catalyzed by the taxoid 10β-hydroxylase, taxoid 13α-hydroxylase and taxoid 14β-hydroxylase. All of these hydroxylases can employ the 5α-alcohol or the corresponding acetate ester as alternate substrates but only the kinetically most favorable routes are illustrated.
In addition to these demonstrated hydroxylation (and acylation) steps, oxygenation at C9 is also presumed to occur fairly early in the pathway (Floss and Mocek, 1995). A clone for the cytochrome P450 taxoid 9α-hydroxylase has been tentatively identified in the initial family of sequence acquisitions by functional expression in yeast and testing the 5α,13α-diol and the 5α,10β-diol (and the corresponding 5α-acetates) as substrates; however, sufficient biosynthetic products have not yet been prepared to permit NMR-based confirmation of structures. Should the taxoid-9α-hydroxylase be verified, the above four cytochrome P450 clones would suffice to convert taxa-4(5),11(12)-diene to the level of a taxa-4(20),11(12)-dien-5α,9α,10β, 13α-tetraol.
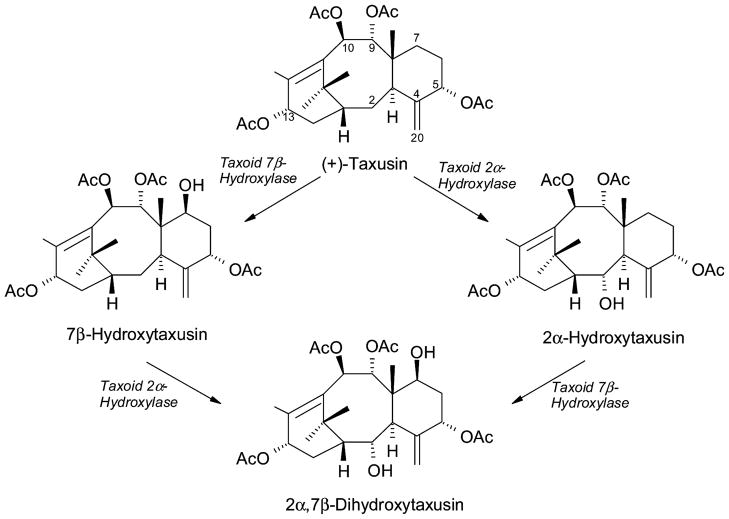
Intermediate oxygenation steps of the pathway (i.e., from the level of a taxadien tetraol onward) have been more difficult to approach. This midsection of the pathway is not well defined in reaction order, and the intermediates are not known or, if predicted, are not so readily available for testing. This difficulty necessitates the use of accessible “surrogate” substrates to explore these “central” hydroxylations. For this purpose, taxusin (the tetraacetate of taxa-4(20),11(12)-dien-5α,9α,10β,13α-tetraol) was employed as a surrogate substrate to test microsomal preparations and to functionally evaluate the extant cytochrome P450 clones for taxoid C1, C2 and C7 hydroxylase activities, and for the presumed C4,C20-epoxidase. (+)-Taxusin (Figure 6) is a prominent metabolite of yew heartwood (Miyazaki et al., 1968; Della Casa de Marcano and Halsall, 1969) in which it is considered a dead-end metabolite, not a possible intermediate in Taxol formation (Floss and Mocek, 1995). Nevertheless, the natural occurrence in Taxus of a broad range of taxusin-like metabolites bearing additional oxygen functional groups at C1, C2 or C7 (Baloglu and Kingston, 1999) encouraged the use of this material as an alternate substrate for testing microsomal oxygenase activities and for screening cytochrome P450 clones in yeast. This approach led to the acquisition and characterization of the seemingly regioselective taxoid- 2α-hydroxylase (Chau and Croteau, 2004) and taxoid-7β-hydroxylase (Chau et al., 2004a).
Figure 6.
Conversions of the surrogate substrate (+)-taxusin mediated by taxoid 2α-hydroxylase and taxoid 7β-hydroxylase.
Both hydroxylases exhibit excellent kinetics for the surrogate substrate in the production of the respective 2α- and 7β-hydroxytaxusins (Figure 6) and are otherwise typical in structure and properties compared to the other taxoid hydroxylases. Selectivity studies indicated preference for polyoxygenated and acylated taxoid substrates, consistent with the operation of these enzymes in the central portion of the Taxol biosynthetic pathway. The transformation of taxusin in Taxus microsomes, and the reciprocal conversion in yeast of their respective hydroxy tetraacetoxy products to the common dihydroxy tetraacetoxy product (i.e., 2α,7β-dihydroxy taxusin) (Figure 6) indicated that these hydroxylases are capable of operating sequentially in the possible order of 7β-hydroxylation followed by 2α-hydroxylation on the way to the level of a taxadien-2α, 5α, 7β, 9α, 10β, 13α-hexaol at some level of acylation (Chau and Croteau, 2004).
In addition to the six taxoid hydroxylases (the 2α-, 5α-, 7β-, 9α-(tentative), 10β- and 13α-hydroxylases) described above, a seventh hydroxylase was discovered by in vivo screening the cytochrome P450 clones in yeast using radiolabeled 5α-acetoxytaxadien-10β-ol and taxadien-5α, 13α-diol as test substrates. This oxygenase clone, which resembles the other family members in structure and properties, efficiently converted 5α-acetoxytaxadien- 10β-ol (but not the taxadien diol) to 5α-acetoxytaxadien- 10β, 14β-diol (Jennewein et al., 2003) (Figure 5). Because Taxol is unsubstituted at C14, this cytochrome P450 taxoid 14β-hydroxylase cannot reside on the pathway to the target drug but rather appears to be responsible for early diversion of the pathway to 14β-hydroxy taxoids (e.g., of the taxuyunnanine C type) that are prominent metabolites of Taxus cell cultures (Menhard et al., 1998; Ketchum et al., 2003). That the 14β-hydroxylation branch pathway appears to diverge early in taxoid metabolism is consistent with precursor feeding studies in cell culture, and suggests that transgenic down-regulation of this hydroxylase gene could permit significant redirection of the pathway to increase flux toward Taxol.
Most of the previously identified hydroxylases of taxoid metabolism were well represented in the induced T. cuspidata cell EST library (Jennewein et al., 2004b), including the taxoid 14β-hydroxylase (1.5‰ abundance) involved in the production of a family of side-route metabolites. Curiously, not a single EST was identified that corresponded to the original taxoid 10β-hydroxylase clone previously isolated by the DD-RT-PCR approach and homology-based screen (Schoendorf et al., 2001; Jennewein et al., 2004a). An alternative taxoid 10β-hydroxylase was therefore sought among the new EST acquisitions, and such a gene was found (by functional expression in yeast) that was rather similar (68% identity; 82% similarity) to the previously isolated taxoid 10β-hydroxylase from the same cDNA library (Jennewein et al., 2004b). This result indicated at least some redundancy in enzymes mediating this presumptive early step of taxoid metabolism. Although the regioselectivity and substrate specificity of the recombinant taxoid hydroxylases have not been fully assessed, the demonstration of redundancy in at least one case, the above-noted multiple routes from taxadienol, the observed plasticity in substrate utilization by some hydroxylases, and the fact that the same surrogate substrate can be utilized by more than one hydroxylase suggest that the Taxol biosynthetic pathway is not so linear as initially imagined but likely involves multiple routes perhaps converging at a late stage intermediate.
Two oxygenation reactions remain to be defined, and the corresponding genes are still missing; these are the taxoid C1β-hydroxylase and the C4β, C20-epoxidase leading to oxetane formation (see Figure 2). Both reactions are presumed to be cytochrome P450-mediated (cytochrome P450 epoxidases have ample precedent (Ortiz de Montellano and De Voss, 2005)), as are the other taxoid oxygenases, and the corresponding genes are presumed to reside within the extant family awaiting extrication using the appropriate substrate to query these remaining P450 clones for function. Little guidance is available to suggest the precise order of C1 hydroxylation, epoxidation and ring expansion steps, and alternate reaction sequences may prevail in the main and side-routes for taxoid metabolism. It is certainly conceivable that oxirane/oxetane formation precedes installation of the C1 hydroxyl on route to Taxol, such that the hypothetical intermediate illustrated in Figure 2 may be an acylated derivative of a taxadien hexaol, rather than a heptaol functionalized at C1.
It seemed reasonable to assume that the family of cytochrome P450 taxoid oxygenases derived from a common progenitor by gene duplication and differentiation (Pichersky and Gang, 2000) to evolve alternative substrate selectivities and new regio- and stereochemistries of oxygenation on the core taxadiene structure. In the context of establishing the reaction sequence, it also seemed possible that the pattern of descent from the parental gene (as determined by sequence relatedness) might reflect the order of oxygenations in the Taxol biosynthetic pathway starting from the presumptive initial hydroxylation at C5α of the committed taxadiene precursor. A cladogram of the taxoid oxygenases was constructed with rooting at the C5α-hydroxylase (Jennewein et al., 2004b), and the pattern was consistent with the preliminary hydroxylation steps leading from C5 hydroxylation to C10 or C13 hydroxylation and with the early emergence of C14 hydroxylation after C5 hydroxylation as a major side route. Phylogenic considerations placed the taxoid C7 hydroxylase closer to the C5 hydroxylase than might have been anticipated based on the proposed sequence of hydroxylations deduced from the relative abundances of taxoid metabolites functionalized at the various positions (Floss and Mocek, 1995) but in agreement with recent experimental implications for the relative placement of the 7β-hydroxylase (Chau and Croteau, 2004; Chau et al., 2004a). With at least two pathway oxygenases still unaccounted for, and many of the cytochrome P450 genes in the group of still uncertain function, this preliminary phylogeny of pathway steps can only be regarded as approximate.
Although not strictly a component of the Taxol pathway, the NADPH:cytochrome P450 reductase (required for electron transfer) is nevertheless important, given that nearly half of the pathway steps are cytochrome P450-mediated oxygenations. A cDNA encoding the reductase was isolated from induced T. cuspidata cells and the recombinant enzyme characterized (Jennewein et al., 2005). The gene is reasonably well represented (0.6‰) in the induced-cell library (Jennewein et al., 2004b), and has been co-expressed in yeast and Spodoptera cells (Jennewein et al., 2004a) as an improved aid for characterizing Taxus oxygenase clones, and as a preliminary to reconstructing taxoid biosynthesis in the microbial host (DeJong et al., 2005; Jennewein et al., 2005).
Acyl and aroyl transferases
Baccatin III (Figure 1) contains three ester functions (C2 benzoate, C4 acetate and C10 acetate), and two additional transfers are required for C13-side chain assembly to reach Taxol (Figure 2). The first of the responsible biosynthetic enzymes to be approached was that for the 5-O-acetyl transfer to taxadienol, the route to taxa-4(20),11(12)-dien-5α-ol having been recently established as described above, and the C5α-acetoxy function being considered important in oxetane ring formation involving the proposed intramolecular transfer of this function to the C4α-position (Figure 2). The requisite activity was first demonstrated in soluble protein extracts from T. canadensis suspension cells, and the 50 kDa, acetyl CoA-dependent enzyme was partially purified and fully characterized with respect to pH optimum (~9.0), pI (~5.0), kinetics (low μM Km values for both co-substrates) and selectivity (10-deacetylbaccatin III was not a substrate but simple terpenols were competently acetylated) (Walker et al., 1999). Because the gene had no homologs (i.e., no other terpenoid O-acetyl transferases) in the databases to permit similarity-based cloning approaches, a protein-based cloning strategy was adopted. The enzyme was purified to near homogeneity (to permit microsequencing) from T. canadensis cells because these cells yielded the highest starting levels of activity. With primers designed for PCR amplification, a cDNA library as target was constructed using T. cuspidata suspension cells because these cells afforded the highest levels of induced Taxol production (Walker et al., 2000). This approach yielded a number of probes with which library screening ultimately provided eight full-length transferase-like sequences; the EST project provided an additional seven acquisitions, bringing the total of related cDNAs encoding this enzyme type to 15 (Walker et al., 2000; Jennewein et al., 2004b). Functional assessment of these clones was conducted by heterologous expression in Escherichia coli and assay of the derived soluble, recombinant enzyme preparations for the relevant acyl and aroyl transferase activities. By this means, cDNAs encoding the taxadien-5α-ol-O-acetyl transferase, the taxoid-2α-O-benzoyl transferase, the taxoid-10β-O-acetyl transferase, and the two transferases involved in C13-side chain assembly were obtained.
The cDNA encoding the taxadien-5α-ol-O-acetyl transferase (ex. T. cuspidata) corresponds to a deduced amino acid sequence of 439 residues that exhibits high sequence identity to the proteolytic fragments of the native enzyme (ex. T. canadensis), which the recombinant acyl transferase resembles closely in properties (Walker et al., 2000). Consistent with the size of the operationally soluble native enzyme, the DNA appears to encode a monomeric protein of molecular weight 49,079 that bears no N-terminal organellar targeting information.
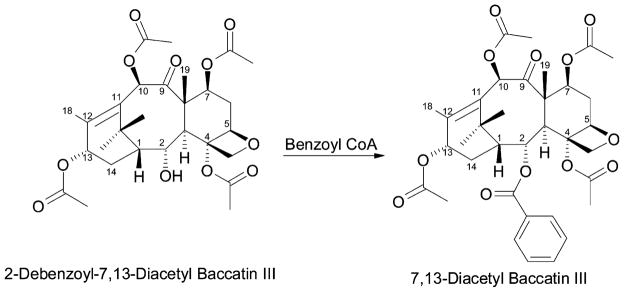
The second transfer to the taxane core, presumed to occur roughly mid-pathway, involves benzoylation at the C2α-position, and this gene was acquired by the identical functional screen of the family of clones using labeled 2-debenzoyl-7, 13-diacetylbaccatin III as a surrogate substrate (Figure 7) because the actual substrate was uncertain, and the surrogate was accessible semi-synthetically (Walker and Croteau, 2000a). The cDNA, like that for the 5α-O-acetyl transferase, codes for a protein of ~50 kDa that lacks apparent targeting information, resembles other members of the transferase family (with about 65%I), and bears conserved sequence elements thought to be involved in acyl (aroyl) group transfer from the CoA-ester to the substrate alcohol. The monomeric recombinant enzyme has a pH optimum of 8.0, sub-mM Km values for the taxoid substrate and benzoyl CoA (acetyl CoA is a very inefficient donor), and is apparently regiospecific for benzoylation of the 2α-hydroxyl group of the functionalized taxane nucleus (Walker and Croteau, 2000a).
Figure 7.
Benzoylation of the surrogate substrate 2-debenzoyl-7,13-diacetylbaccatin III by the taxoid 2α-O-benzoyl transferase.
The taxoid 10β-O-acetyl transferase (i.e., 10-deacetyl baccatin III-10-O-acetyl transferase), thought to catalyze formation of the last diterpenoid intermediate in the Taxol biosynthetic pathway (just prior to C13-side chain addition), was acquired by the same cloning strategy, with functional evaluation by expression in E. coli as before (Walker and Croteau, 2000b). The full-length cDNA encodes a deduced protein of 440 residues with a calculated molecular weight of 49,052, consistent with the size of the operationally soluble, monomeric native acetyl transferase. The recombinant enzyme has a pH optimum of 7.5, Km values of about 10 μM for both cosubstrates, and is apparently regiospecific toward the 10β-hydroxy group of the taxoid core.
A 10β-hydroxytaxane-O-acetyl transferase activity was first reported in extracts of T. baccata and T. cuspidata (Zocher et al., 1996; Pennington et al., 1998), and an enzyme of this type was purified to apparent homogeneity from the soluble protein fraction of T. chinensis cell cultures and characterized in some detail (Menhard and Zenk, 1999). The C10-O-acetyl transferase from T. chinensis differs somewhat in properties from the corresponding native and recombinant acetyl transferase from T. cuspidata (Walker and Croteau, 2000b), most notably in the size of the former which was found to be a monomer of 71 kDa. All 15 acyl (aroyl) transferase cDNA clones from T. cuspidata code for proteins of about 50 kDa, and all of the native and recombinant T. cuspidata and T. canadensis transferases thus far examined are functional monomers of this size. It is worth noting that all of the transferases are apparently translated without N-terminal targeting information, and so all are presumed to be cytosolic enzymes consistent with their operationally soluble nature. Thus, the Taxol pathway originates in plastids and involves hydroxylation at the endoplasmic reticulum and acylation in the cytosol. Whether interorganellar trafficking of these relatively hydrophobic metabolites relies on diffusional control, or is transport protein-mediated, is presently unknown.
The five defined acyl (aroyl) transferases of the 19-step Taxol biosynthetic pathway from GGPP (three for modifying the core and two for C13-side chain assembly) are remarkably well represented in the induced Taxus cell cDNA library (59 total acquisitions of 8424 sequences). The 10 other distinct transferases observed comprise a total of 60 ESTs. A very large number of taxoid side chain variants are known that differ in position on the hydroxylated taxane core and in the type of acyl/aroyl substitution, including tiglate, butanoate, hexanoate, cinnamate, and other aromatic and aminoacyl esters in addition to the more common acetates and benzoates (Baloglu and Kingston, 1999). Taxoid acetate esters are particularly common. Thus, Taxol bears an acetate at C10 and another at C4 thought to originate by intramolecular migration of a C5 acetate function in the process of oxetane ring formation (Figure 2), but many other naturally occurring taxoids bear acetate groups at the C1, C2, C7, C9 (of 9α-dihydro derivatives) and C13 positions that would appear to block pathway progression to Taxol. These “inappropriately” acetylated metabolites can accumulate to significant levels in cell culture (over 30% of total taxoids) and thus represent considerable diversion of pathway flux (Ketchum and Croteau, 2005). It is also possible that some of these acylated metabolites are true intermediates, since it is conceivable that the Taxol pathway involves transient acylation/deacylation for the purposes of trafficking and organellar targeting, or flux regulation. Such processes could greatly increase the number of biosynthetic steps and pathway complexity. It is assumed that the remaining 10 transferase genes that have been isolated are responsible for the production of these numerous taxoid side chain structural and regiochemical variants.
To explore this issue, in particular the origins of the large number of variously acetylated taxoids, the group of recombinant Taxus acyl transferases was investigated with a range of polyhydroxylated taxoids as substrates (Chau et al., 2004b). Fromthis survey, a new acetyl transferase clone was identified that was capable of acetylating taxadien-5α-ol with activity comparable to that of the previously identified 5α-O-acetyl transferase. However, when these two recombinant enzymes were presented with taxadien triol and tetraol substrates, they exhibited different regiospecificities for acetylation of the “northern” hemisphere hydroxyls at C9 and C10 and the “east-west” pole positions at C5 and C13 (Chau et al., 2004b). These results clearly indicate some redundancy in transferases for the C5 acetylation step and that regioselectivity of acylation depends in part on the substitution pattern of the taxoid substrate. Although the sequence of the C2 benzoylation and C10 acetylation steps on route to Taxol seems reasonably secure, the precise timing of C5 acetylation on the Taxol pathway is now less certain for two reasons: assessment of the transacylases indicates that acetylation at C5 can occur at kinetically very competent rates at the level of a taxadien polyol; and feeding studies suggest that acetylation of taxadien-5α-ol as a very early step (as originally proposed (Walker et al., 1999)) more favorably promotes side route diversion, via 10β- and 14β-hydroxylation, than direct progression to Taxol. This question of precise timing (C5 acetylation is still likely the first acylation) may be resolved by further feeding studies with more advanced precursors, and more detailed characterization of the selectivity and kinetic competence of the extant acyl transferases.
As with the cytochrome P450 taxoid oxygenases, it seemed reasonable to assume that this family of taxoid acyl and aroyl transferases (showing >65% similarity within the 15-member group) also derived from a common ancestral gene by duplication and differentiation to evolve alternative acyl/aroyl CoA substrate selectivities and new regio-chemistries for ester synthesis at the various hydroxylated positions on the taxane core, and for amidation of the C13-phenylisoserinoyl side chain. Here also, it might be expected that the pattern of descent as gauged by sequence relatedness could reflect the order of acylation in the Taxol biosynthesis pathway, which the current data suggest is acetylation at C5 of the taxane core (i.e., taxadienol or a derived polyol), benzoylation at C2, acetylation at C10 (ultimately leading to baccatin III), addition of the side chain at C13, and N-benzoylation of the C13-side chain. To evaluate the possibility of such a relationship, a cladogram of the defined acyl transferases was constructed with rooting at the 5α-O-acetyl transferase as the first probable acylation (Jennewein et al., 2004b). The relative phylogenic placement of these transferases is generally consistent with the predicted order of acylation in Taxol biosynthesis, but the placement of the recently acquired 5-O-acetyl transferase is apparently anomalous. As with the cytochrome P450 oxygenases, there are still too many genes of uncertain function in the transferase group to regard the suggested phylogeny as anything but approximate.
Additional steps to baccatin III
As indicated above, two presumptive cytochrome P450 oxygenases on the route to baccatin III remain to be acquired; these are the C1β-hydroxylase and the C4β,C20-epoxidase which modify the taxane core prior to C13-side chain transfer. Uncertainties about the exact timing of these steps within the pathway provide little guidance as to the true substrates of these reactions. Such uncertainties have limited synthetic efforts directed to the preparation of potential “surrogate” substrates for test of function of candidate genes by expression and in vivo feeding studies; the general approach of employing accessible surrogates has proved successful recently in the acquisition of the C2-Obenzoyl transferase and the C2α- and C7β-hydroxylases. Similar efforts based on surrogates of the taxadien pentaol and hexaol type (e.g., the2α, 5α, 7β, 9α, 10β, 13α-hexaol) hold promise for acquiring the taxoid epoxidase, whereas surrogates of the baccatin I type (devoid of the 1β-hydroxyl but with the 4,20-epoxide) and 1β-dehydroxy-baccatin VI type (devoid of the 1β-hydroxyl but with the oxetane function) would seem most suitable for approaching the 1β-hydroxylation for which the timing (before or after oxetane formation) is uncertain based on the metabolite survey of Floss and Mocek (1995).
The remaining two steps to yield baccatin III, the oxidation at C9 and oxetane (D-ring) construction, also occur relatively late in the pathway where uncertainties about the preceding steps (epoxidation and oxetane formation likely precede oxidation at C9; see Floss and Mocek (1995)) similarly constrain substrate design for test of function. The oxidation of the taxane C9α-hydroxyl function to the corresponding ketone (Figure 1) could, like the initial hydroxylation at this position, be mediated by a cytochrome P450 hydroxylase (via the ketone hydrate), and there is precedent for this reaction type in the biosynthesis of other diterpenoids (resin acids and gibberellins) (MacMillan and Beale, 1999). This oxidation step may also be catalyzed by a more typical pyridine nucleotide-dependent dehydrogenase. The EST acquisitions from T. cuspidata cells revealed many undefined candidate dehydrogenases for this step, and it is clear that biochemical studies, with 9α-dihydrobaccatin III type derivatives as surrogates, to define the type of enzyme involved in C9-oxidation must precede a cloning effort.
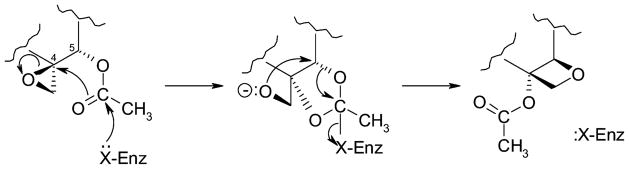
Several groups have proposed chemically feasible mechanisms for construction of the oxetane (D-ring) of Taxol and related compounds, all of which implicate the progression from the 4,20-ene-5α-oxytaxoid functional grouping through the 4β, 20-epoxide-5α-oxy derivative to the 4α-oxy-4β,20-O-5-oxetane (see Floss and Mocek, 1995; Walker and Croteau, 1999 for review of these proposals) but none of which have been experimentally tested. A simple and quite plausible proposal has been put forward by Potier and colleagues (Guéritte-Voegelein et al., 1987) in which the 4β,20-epoxide-5α-acetoxy grouping undergoes rearrangement, by protonation and opening of the epoxide, with the acetoxy group migrating from C5 to C4 in the ring expansion process (i.e., along the lines shown in Figure 2 via the intermediate dioxonium ion (Giner and Faraldos, 2003)). At the other extreme, this reaction could also be easily rendered as an intramolecular migration catalyzed by a transferase-type mechanism involving initial nucleophilic displacement of the 4β-epoxide (Figure 8), which similarly accounts for the correct stereochemistry and the observation that the only known esters at C4 are acetates. From the Taxus cell EST project, the number of candidate “oxomutases” for this process is quite large, given that many of the potential suspects could be disguised as proteases, esterases or other hydrolases; potentially, this enzyme could be of a new type. The biochemistry of the reaction, a necessary foundation to narrow the number of candidate enzyme/gene types, can most likely be approached with baccatin I and 1β-hydroxybaccatin I as surrogate substrates (i.e., highly functionalized taxoid 4β,20-epoxides).
Figure 8.
A proposal for the oxomutase reaction involving intramolecular exchange of the C5α-acetoxy group and the C4β-oxide function of an advanced taxoid catalyzed by a transferase-type mechanism.
C13-side chain assembly
The origin of the β-phenylalanoyl-type side chains of the taxoids has been of interest for some time; feeding studies by Leete and Bodem (1966) established α-phenylalanine as the precursor, and subsequent work by Haslam and coworkers (Platt et al., 1984) showed that β-phenylalanine arose from α-phenylalanine by an aminomutase-type reaction. Floss and associates have conducted a more recent series of illuminating feeding studies with Taxus (Fleming et al., 1993, 1994) directed to the sequence of reactions for constructing the 13-O-(N-benzoyl-3-phenylisoserinoyl) side chain; this work is reviewed in Floss and Mocek (1995). These results clearly confirmed that the phenylisoserine side chain is formed from α-phenylalanine via β-phenylalanine. A phenylalanine aminomutase activity, as the committed step of side chain assembly, was subsequently demonstrated in Taxus stem extracts (Walker and Floss, 1998). Interestingly, the N-benzoate moiety of the side chain was also shown to be derived from β-phenylalanine rather than via cinnamic acid as might have been expected. Feeding studies with baccatin III and various side chain precursors indicated that β-phenylalanine was roughly three-times more efficiently incorporated into Taxol than was phenylisoserine, and that N-benzoyl phenylisoserine was only poorly incorporated and mostly after hydrolysis of the benzoate group. The combination of these results suggested that side chain transfer to the diterpenoid moiety occurs at the level of baccatin III (i.e., very late in the pathway) by way of β-phenylalanine and/or phenylisoserine, and that N-benzoylation of the C13-side chain is the terminal step of Taxol biosynthesis (Figure 2). These feeding studies could not address the issues of the timing or the specific precursor of the side chain 2′-hydroxylation step.
All indications are that, as a prelude to side chain assembly, α-phenylalanine is separately converted to β-phenylalanine by an aminomutase. A gene cloning approach to this target was based on the assumption that phenylalanine aminomutase would resemble the well known plant enzyme phenylalanine ammonia lyase, and a phenylalanine ammonia lyase-like sequence was acquired from the T. cuspidata cDNA library, functionally expressed in E. coli, and confirmed as the target aminomutase (Walker et al., 2004). The recombinant enzyme and clone are virtually identical to those acquired from T. chinensis cells by a reverse genetics approach (Steele et al., 2003). The cDNA encodes a 698 residue enzyme (76.5 kDa) with Km of 70 μM, kcat of 30 s−1 and pH optimum at 8.5. The aminomutase requires no cofactors to conduct the reaction but relies for this purpose solely on the electronegativity of an autocatalytically formed methylideneimidazol-5-one prosthetic group within the active site that is derived from a signature Ala-Ser-Gly motif characteristic of this enzyme type (Walker et al., 2004). The mechanism of this aminomutase involves removal and interchange of the pro-3S-hydrogen and the amino group of 2S-α-phenylalanine to yield 3R-β-phenylalanine.
As indicated previously, the two aroyl CoA transferase clones required for C13-side chain assembly were acquired by functional screening of the original set of T. cuspidata transferases expressed in E. coli. The final step of Taxol biosynthesis is catalyzed by the side chain N-benzoyl transferase (Figure 2), and a cDNA encoding this stereoselective and regiospecific enzyme was located using the surrogate substrate N-debenzoyl- (3′-RS)-2′-deoxytaxol and benzoyl-CoA as cosubstrate to yield the product 2′-deoxytaxol (Walker et al., 2002a). The actual substrate for this reaction in planta is considered to be N-debenzoyltaxol, the penultimate product of the pathway that also gives rise to cephalomannine (by N-tigloyl transfer) and Taxol C (by N-hexanoyl transfer). Whether the same or different transferases catalyze these alternate amidation reactions is not presently known. The full-length cDNA coding for the N-benzoyl transferase translates a 441-residue protein (~49 kDa) without apparent targeting information. The recombinant enzyme has a pH optimum at 8.0, Km values for the N-deacylated taxoid and benzoyl-CoA of about 0.4 mM, and operates with kcat of about 1.5 s−1 with the surrogate substrate. Preliminary studies indicate that this enzyme can be exploited to attach modified aroyl groups to taxoid precursors for the purpose of improving drug efficacy.
The C13-phenylpropanoyl-CoA transferase that initiates side chain assembly on baccatin III (Figure 2) is a catalyst of considerable interest. The cDNA clone was obtained from the original transferase set by functional screening in E. coli, as before, using β-phenylalanoyl CoA as the acyl donor for transfer to baccatin III to form N-debenzoyl-2′-deoxytaxol (Walker et al., 2002b). The full-length cDNA encodes a 445-amino acid protein (50.5 kDa) without targeting information, and the soluble recombinant enzyme has a pH optimum at 6.8 with Km values of 2.4 and 4.9 μM for baccatin III and β-phenylalanoyl-CoA, respectively. The side chain transferase resembles the four other acyl/aroyl transferases involved directly in Taxol biosynthesis (i.e., between 71 and 74% similarity at the amino acid level compared to the others), but is the only one of the five that contains a G163XXXDA168 motif instead of the typical acyl transferase HXXXDG(A) element of which the His and Asp side chains along with a conserved upstream cysteine residue (Cys-95 in the present case) form a catalytic triad thought to be involved in acyl group transfer (St-Pierre and De Luca, 2000). The Gly-163 for His-163 substitution in the side chain transferase would almost certainly disrupt the proposed triad function (Brown et al., 1994); however, the free β-amino group of the CoA ester cosubstrate in this instance could, through hydrogen bonding, function as a surrogate intramolecular general acid/base in place of the normal histidine at this position of the enzyme.
This transferase appears to be highly selective for esterification at the 13-O-position but lacks absolute specificity for the aroyl CoA donor, in that, while preferring β-phenylalanoyl-CoA (Vrel= 100), the enzyme can also transfer from 3-phenylisoserinoyl- CoA at lower efficiency (Vrel=40). Neither α-phenylalanoyl-CoA nor N-benzoyl phenylisoserinoyl-CoA are productive acyl donors (Vrel <1), consistent, in the first case, with the requirement for a 3-amino group (not 2-amino group) of the 3-phenylpropanoyl donor to serve as the surrogate internal acid/base, and, in the second case, with the separate N-benzoyl transfer as the last operation of the pathway (i.e., the complete N-benzoyl phenylisoserine side chain is not transferred as a unit).
The productive, but less efficient, transfer of phenylisoserine to baccatin III, compared to the transfer of β-phenylalanine, is of particular note in suggesting that 2′-hydroxylation in the side chain could occur before transfer (i.e., hydroxylation at the level of β-phenylalanine to yield on transfer phenylisoserinoyl-baccatin III=N-debenzoyltaxol) rather than after transfer of β-phenylalanine (to β-phenylalanoyl-baccatin III=N-debenzoyl-2′-deoxytaxol) with subsequent 2′-hydroxylation and terminal N-benzoylation. This issue of timing of the side chain 2′-hydroxylation step remains untested by feeding studies with doubly labeled β-phenylalanoyl-baccatin III (labeling in both side chain and baccatin core is required to monitor for disassembly and resynthesis of Taxol from the modified side chain precursor and baccatin III) but preliminary evidence has been obtained for 2′-hydroxylation of β-phenylalanoyl-baccatin III to phenylisoserinoyl-baccatin III by Taxus microsomes under cytochrome P450 reaction conditions (Long and Croteau, 2005). Such an enzyme might resemble a typical “taxoid hydroxylase” and could be presumed to reside within the family of cytochrome P450 cDNA clones already on hand. No evidence has thus far been obtained for the direct hydroxylation of β-phenylalanine in cell-free enzyme systems under reaction conditions described for other amino acid hydroxylases (Silverman, 2000). β-Phenylalanoyl-CoA has not yet been evaluated as a substrate for 2′-hydroxylation; yet, consideration of the possible use of the CoA-ester in the 2′-hydroxylation reaction raises the larger issue of the role of the enabling aroyl CoA ligase in the side chain assembly process.
Database searching of the induced Taxus cell library revealed 22 total ESTs encoding seven distinct CoA ligases of unknown function (ranging from 0.1 to 0.7‰ abundance), in addition to the well known coumaroyl CoA ligase (14 ESTs, 1.7‰ abundance). Given the variety of acyl and aroyl substitutions found in the naturally occurring taxoids (Baloglu and Kingston, 1999), it is not surprising that a number of CoA ligases are expressed in this system for the purpose of activating the various acyl and aroyl groups before transfer to the taxane core or C13-side chain. Evaluation of the full-length expressed forms with all conceivable side chain precursors (β-phenylalanine, phenylisoserine, and N-benzoyl phenylisoserine) should allow determination of relevant types and their specificity; note here that Floss and associates have demonstrated incorporation of both β-phenylalanine and phenylisoserine (less efficiently), but not the N-benzoate, into Taxol by Taxus feeding studies (see Floss and Mocek, 1995), suggesting that both of these 3-amino-3-phenyl-propanoic acids can be activated to the CoA-ester before transfer. It is anticipated that a combined assessment of selectivity of both the aroyl CoA ligase and C13-side chain aroyl CoA transferase, along with additional feeding studies with the relevant precursors and direct evaluation of β-phenylalanoyl CoA as a possible 2′-hydroxylase substrate, will reveal the timing of the 2′-hydroxylation step and give further guidance to the 2′-hydroxylase cloning effort.
Conclusions
Intense activity in developing new uses of Taxol in cancer chemotherapy, and in the treatment of other human maladies ranging from polycystic kidney disease (Woo et al., 1994), to coronary restenosis (Park et al., 2003), to Alzheimer’s disease (Zhang et al., 2005), will ensure continued interest in the supply and cost of this valuable natural product, and in its biosynthesis in yew species as the only viable source. A range of studies described here have revealed several of the early and late steps of the Taxol biosynthetic pathway, and over half of the genes (14 out of 20) required for the biosynthesis of Taxol from primary metabolites have now been cloned. Although the sequence of reactions, especially those comprising the mid-section of the pathway, is still uncertain, and several steps for modification of the core and for side chain assembly are still unknown at the enzyme and gene level, the broad outlines of taxoid metabolism have emerged, as have some details of the organization, regulation and evolutionary origins of the pathway that may serve as a model for the formation of other complex natural products.
With the biochemical and molecular tools currently in hand, it is anticipated that the remaining enzymes and genes of Taxol biosynthesis will be defined in the not too distant future. However, as indicated throughout this chapter, the complexity of taxoid metabolism, the limitations of feeding studies (in availability of relevant labeled precursors, and in precursor uptake and in the sometimes non-physiological disposition of exogenous precursors), and the shortcomings of inference from the in vitro properties of native and recombinant enzymes, may defy our best efforts to extricate the kinetically most efficient pathway to Taxol from multiple competing routes to the target, and from numerous side reactions and metabolic dead-ends; additional approaches will be necessary.
Expression profiling of pathway genes in induced Taxus cells relative to their constitutive counterparts will certainly provide important biochemical context and can assist in revealing flux-restricting metabolic steps. However, transgenic manipulation of the pathway in cultured cells would appear to provide the most productive means of delineating precursor–product relationships (and the true substrates for reactions thus far probed only with surrogates), sequencing reaction steps, and defining flux controls. The transgenic upregulation and knock-out of each gene of the pathway should result, respectively, in a decreased level of upstream metabolites and increased level of immediate precursor(s) relative to the metabolite profile of controls to reveal placement and relative contribution to pathway (and off-pathway) flux. A systematic approach of this type, in addition to revealing the role of each gene product, is central to defining the most direct route to Taxol and has many practical consequences for improving production yield. Thus, increasing precursor supply by manipulation of the plastidial MEP pathway, increasing flux through the early slow steps of the reaction sequence (e.g., TS, taxadiene 5α-hydroxylase) and eliminating limitations to side chain assembly on baccatin III can all be expected to increase Taxol production. An alternative approach directed to knock-out of major diversionary routes (e.g., to 14β-hydroxy taxoids, and C9- and C13-acetate derivatives) should similarly increase Taxol yield. Redirection of the pathway by transgenic means may also offer a complementary approach to the difficult semi-synthesis of new, second generation drugs, such as C9-dihydrotaxoids and C13,C14-dihydroxytaxoids, that hold promise for improved efficacy (Alder et al., 1996; Distefano et al., 1997; Polizzi et al., 1999).
The coming years are certain to witness a continuation of feeding studies and other classical biochemical approaches to understanding the origins of Taxol. Interesting new enzymology will likely be revealed, and understanding substrate selectivity and the regio and stereochemistry of the target enzymatic reactions (and their placement on the pathway) will assist in finding an efficient road to Taxol that must exist among the many diversions and dead-ends along the way. Cloning the missing biosynthetic genes will also remain a high priority, but the major thrust in the future will be transgenic manipulation of Taxus cells to fully define the pathway and its controls, and to exploit the tools and information gained to increase Taxol yields.
Acknowledgments
Studies on the origin of Taxol in our laboratory were supported by a grant from the National Institutes of Health (National Cancer Institute; CA-55254), a research agreement with Cytoclonal Pharmaceutics (eXegenics Inc.)/Bristol-Myers Squibb, and McIntire-Stennis Project 0967 from the Agricultural Research Center, Washington State University. We are indebted to a group of dedicated coworkers for their many efforts, as indicated in the appropriate references, and to our expert collaborators Robert M. Williams (Colorado State University), Robert M. Coates (University of Illinois) and Heinz G. Floss (University of Washington).
Abbreviations
- DD-RT-PCR
differential display of mRNA-reverse transcription-polymerase chain reaction
- DMAPP
dimethylallyl diphosphate
- DXP
1-deoxy-D-xylulose-5-phosphate
- EST
expressed sequence tags
- GGPP
geranylgeranyl diphosphate
- GGPPS
geranylgeranyl diphosphate synthase
- IPP
isopentenyl diphosphate
- IPPI
isopentenyl diphosphate isomerase
- MEP
2-C-methyl-D-erythritol phosphate
- NMR
nuclear magnetic resonance spectroscopy
- PAM
phenylalanine aminomutase
- TS
taxadiene synthase
Footnotes
Paclitaxel is the generic name for Taxol, which is now a registered trademark of Bristol-Myers Squibb. Because of the greater familiarity with the word Taxol, we use it here instead of paclitaxel. The full systematic name of this natural product, from the 11th edition of the Merck Index, is [2aR-[2aα, 4β, 4aβ, .6β, 9α(αR*, βS*), 11α, 12α, 12aα, 12bα]]-β–(benzoylamino)–α-hydroxybenzenepropanoic acid 6,12b-bis-(acetyloxy)-12-(benzoyloxy)- 2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4, 11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1 H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester.
References
- Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG, Finley R. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. Monograph Natl Cancer Inst. 1993;15:141–147. [PubMed] [Google Scholar]
- Alder JD, Jarvis KP, Marsh KC, Klein LL, Clement JJ. Preclinical in vivo efficacy of two 9-dihydrotaxane analogues against human and murine tumours. Brit J Cancer. 1996;73:560–564. doi: 10.1038/bjc.1996.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuck SG, Blaylock BA. Taxol: clinical results and current issues in development. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 379–415. [Google Scholar]
- Baloglu E, Kingston DGI. The taxane diterpenoids. J Nat Prod. 1999;62:1448–1472. doi: 10.1021/np990176i. [DOI] [PubMed] [Google Scholar]
- Brown DT. Preclinical and clinical studies of the taxanes. In: Itokawa H, Lee K-H, editors. Taxus – The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 387–435. [Google Scholar]
- Brown NF, Anderson RC, Caplan SL, Foster DW, McGarry JD. Catalytically important domains of rat carnitine palmitoyltransferase II as determined by site-directed mutagenesis and chemical modification. Evidence for a critical histidine residue. J Biol Chem. 1994;269:19157–19162. [PubMed] [Google Scholar]
- Burke C, Croteau R. Interaction with the small subunit of geranyl diphosphate synthase modifies the chain length specificity of geranylgeranyl diphosphate synthase to produce geranyl diphosphate. J Biol Chem. 2002;277:3141–3149. doi: 10.1074/jbc.M105900200. [DOI] [PubMed] [Google Scholar]
- Chau M, Croteau R. Molecular cloning and characterization of a cytochrome P450 taxoid 2α-hydroxylase involved in taxol biosynthesis. Arch Biochem Biophys. 2004;427:48–57. doi: 10.1016/j.abb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Chau M, Jennewein S, Walker K, Croteau R. Taxol biosynthesis: molecular cloning and characterization of a cytochrome P450 taxoid 7β-hydroxylase. Chem Biol. 2004a;11:663–672. doi: 10.1016/j.chembiol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Chau M, Walker K, Long R, Croteau R. Regioselectivity of taxoid-O-acetyltransferases: heterologous expression and characterization of a new taxadien-5α-O-acetyltransferase. Arch Biochem Biophys. 2004b;430:237–246. doi: 10.1016/j.abb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Collins D, Mill RR, Möller M. Species separation of Taxus baccata, T. canadensis and T. cuspidata (Taxaceae) and origins of their reputed hybrids inferred from RAPD and cpDNA data. Am J Bot. 2003;90:175–182. doi: 10.3732/ajb.90.2.175. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Schepartz SA, Suffness M, Grever MR. The Taxol supply crisis. New NCI policies for handling the large-scale production of novel natural product anticancer and anti-HIV agents. J Nat Prod. 1993;56:1657–1668. doi: 10.1021/np50100a001. [DOI] [PubMed] [Google Scholar]
- Croom EM., Jr . Taxus for taxol and taxoids. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 37–70. [Google Scholar]
- Daniewski WM, Gumulka M, Anczewski W, Masnyk M, Bioszyk E, Gupta KK. Why the yew tree (Taxus baccata) is not attacked by insects. Phytochemistry. 1998;49:1279–1282. [Google Scholar]
- Davis EM, Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Top Curr Chem. 2000;209:53–95. [Google Scholar]
- DeJong JM, Liu Y, Bollon AP, Long RM, Jennewein S, Williams D, Croteau RB. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol Bioeng. 2005 doi: 10.1002/bit.20694. (in press) [DOI] [PubMed] [Google Scholar]
- Della Casa de Marcano DP, Halsall TG. The isolation of seven new taxane derivatives from the heartwood of yew (Taxus baccata L.) Chem Commun. 1969:1282–1283. [Google Scholar]
- Distefano M, Scambia G, Ferlini C, Gaaini C, De Vincenzo R, Riva A, Bombardelli E, Ojima I, Fattorossi A, Panici PB, Mancuso S. Anti-proliferative activity of a new class of taxanes (14β-hydroxy-10-deacetylbaccatin III derivatives) on multidrug-resistance-positive human cancer cells. Int J Cancer. 1997;72:844–850. doi: 10.1002/(sici)1097-0215(19970904)72:5<844::aid-ijc22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Menhard B, Hylands PJ, Zenk MH, Bacher A. Studies on the biosynthesis of taxol: the taxane carbon skeleton is not of mevalonoid origin. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Menhard B, Lee MS, Zenk MH, Bacher A. Multiple oxygenase reactions in the biosynthesis of taxoids. J Am Chem Soc. 1998;120:9694–9695. [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001;6:78–84. doi: 10.1016/s1360-1385(00)01812-4. [DOI] [PubMed] [Google Scholar]
- Elmer WH, Mattina MJI, MacEachern GI. Sensitivity of plant pathogenic fungi to taxane extracts from ornamental yews. Phytopathology. 1994;84:1179–1185. [Google Scholar]
- Estevez JM, Cantero A, Reindl A, Reichler S, Leon P. 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem. 2001;276:22901–22909. doi: 10.1074/jbc.M100854200. [DOI] [PubMed] [Google Scholar]
- Fleming PE, Mocek U, Floss HG. Biosynthesis of taxoids. Mode of formation of the Taxol side chain. J Am Chem Soc. 1993;115:805–807. [Google Scholar]
- Fleming PE, Knaggs AR, He X-G, Mocek U, Floss HG. Biosynthesis of taxoids. Mode of attachment of the taxol side chain. J Am Chem Soc. 1994;116:4137–4138. [Google Scholar]
- Floss HG, Mocek U. Biosynthesis of taxol. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 191–208. [Google Scholar]
- Ganesh T, Guza RC, Bane S, Ravindra R, Shanker N, Lakdawala AS, Snyder JP, Kingston DGI. The bioactive taxol conformation on beta-tubulin: experimental evidence from highly active constrained analogs. Proc Natl Acad Sci USA. 2004;101:10006–10011. doi: 10.1073/pnas.0403459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georg GI, Boge TC, Cheruvallath ZS, Clowers JS, Harriman GCB, Hepperle M, Park H. The medicinal chemistry of Taxol. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 317–375. [Google Scholar]
- Gibson DM, Ketchum REB, Hirasuna TJ, Shuler ML. Potential of plant cell culture for taxane production. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 71–95. [Google Scholar]
- Giner J-L, Faraldos JA. Facile orthoester formation in a model compound of the taxol oxetane: are biologically active epoxy esters, orthoesters and oxetanyl esters latent electrophiles? Helv Chem Acta. 2003;86:3613–3622. [Google Scholar]
- Goldspiel BR. Clinical overview of the taxanes. Pharmacotherapy. 1997;17:110S–125S. [PubMed] [Google Scholar]
- Goodman J, Walsh V. The Story of Taxol. Cambridge University Press; Cambridge, UK: 2001. p. 282. [Google Scholar]
- Groves JT, Subramanian DV. Hydroxylation by cytochrome P-450 and metalloporphyrin models. Evidence for allylic rearrangement. J Am Chem Soc. 1984;106:2177–2181. [Google Scholar]
- Groves JT. Models and mechanisms of cytochrome P450 action. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3. Kluwer Academic/Plenum Press; New York, NY, USA: 2005. pp. 1–43. [Google Scholar]
- Guéritte-Voegelein F, Guénard D, Potier P. Taxol and derivatives: a biogenetic hypothesis. J Nat Prod. 1987;50:9–18. doi: 10.1021/np50049a002. [DOI] [PubMed] [Google Scholar]
- Harrison JW, Scrowston RM, Lythgoe B. The constitution of taxine-I. J Chem Soc (C) 1966:1933–1945. [Google Scholar]
- Hartzell H., Jr . The Yew Tree. Hulogosi Press; Eugene, OR, USA: 1991. p. 319. [Google Scholar]
- Hefner J, Rubenstein SM, Ketchum REB, Gibson DM, Williams RM, Croteau R. Cytochrome P450-catalyzed hydroxylation of taxa-4(5),11(12)-diene to taxa- 4(20),11(12)-dien-5α-ol: the first oxygenation step in taxol biosynthesis. Chem Biol. 1996;3:479–489. doi: 10.1016/s1074-5521(96)90096-4. [DOI] [PubMed] [Google Scholar]
- Hefner J, Ketchum REB, Croteau R. Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Arch Biochem Biophys. 1998;360:62–74. doi: 10.1006/abbi.1998.0926. [DOI] [PubMed] [Google Scholar]
- Hezari M, Lewis NG, Croteau R. Purification and characterization of taxa-4(5),11(12)-diene synthase from Pacific yew (Taxus brevifolia) that catalyzes the first committed step of taxol biosynthesis. Arch Biochem Biophys. 1995;322:437–444. doi: 10.1006/abbi.1995.1486. [DOI] [PubMed] [Google Scholar]
- Hezari M, Croteau R. Taxol biosynthesis: an update. Planta Med. 1997;63:291–295. doi: 10.1055/s-2006-957684. [DOI] [PubMed] [Google Scholar]
- Hezari M, Ketchum REB, Gibson DM, Croteau R. Taxol production and taxadiene synthase activity in Taxus canadensis cell suspension cultures. Arch Biochem Biophys. 1997;337:185–190. doi: 10.1006/abbi.1996.9772. [DOI] [PubMed] [Google Scholar]
- Itokawa H. Taxoids occuring in the genus Taxus. In: Itokawa H, Lee K-H, editors. Taxus – The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 35–78. [Google Scholar]
- Jennewein S, Croteau R. Taxol: biosynthesis, molecular genetics and biotechnological applications. Appl Microbiol Biotechnol. 2001;57:13–19. doi: 10.1007/s002530100757. [DOI] [PubMed] [Google Scholar]
- Jennewein S, Rithner CD, Williams RM, Croteau R. Taxol biosynthesis: taxane 13α-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci USA. 2001;98:13595–13600. doi: 10.1073/pnas.251539398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein S, Rithner CD, Williams RM, Croteau R. Taxoid metabolism: taxoid 14β-hydroxylase is a cytochrome P450-dependent monooxygenase. Arch Biochem Biophys. 2003;413:262–270. doi: 10.1016/s0003-9861(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Jennewein S, Long RM, Williams RM, Croteau R. Cytochrome P450 taxadiene 5α-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chem Biol. 2004a;11:379–387. doi: 10.1016/j.chembiol.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Jennewein S, Wildung MR, Chau M, Walker K, Croteau R. Random sequencing of an induced Taxus cell cDNA library for identification of clones involved in taxol biosynthesis. Proc Natl Acad Sci USA. 2004b;101:9149–9154. doi: 10.1073/pnas.0403009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein S, Park H, DeJong JM, Long RM, Bollon AP, Croteau RB. Coexpression in yeast of Taxus cytochrome P450 reductase with cytochrome P450 oxygenases involved in taxol biosynthesis. Biotechnol Bioeng. 2005;89:588–598. doi: 10.1002/bit.20390. [DOI] [PubMed] [Google Scholar]
- Jiménez-Barbero J, Amat-Guerri F, Snyder JP. The solid state, solution and tubulin-bound conformations of agents that promote microtubule stabilization. Curr Med Chem Anti-Cancer Agents. 2002;2:91–122. doi: 10.2174/1568011023354416. [DOI] [PubMed] [Google Scholar]
- Jin Q, Williams DC, Hezari M, Croteau R, Coates RM. Stereochemistry of the macrocyclizaton and elimination steps in taxadiene biosynthesis through deuterium labeling. J Org Chem. 2005a;70:4667–4675. doi: 10.1021/jo0502091. [DOI] [PubMed] [Google Scholar]
- Jin Y, Williams DC, Croteau R, Coates RM. Taxadiene synthase-catalyzed cyclization of 6-fluorogeranylgeranyl diphosphate to 7-fluoroverticillenes. J Am Chem Soc. 2005b;127:7834–7842. doi: 10.1021/ja050592r. [DOI] [PubMed] [Google Scholar]
- Ketchum REB, Gibson DM, Croteau R, Shuler ML. The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol Bioeng. 1999;62:97–105. doi: 10.1002/(sici)1097-0290(19990105)62:1<97::aid-bit11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ketchum REB, Rithner CD, Qiu D, Kim YS, Williams RM, Croteau RB. Taxus metabolomics: methyl jasmonate preferentially induces production of taxoids oxygenated at C-13 in Taxus×media cell cultures. Phytochemistry. 2003;62:901–909. doi: 10.1016/s0031-9422(02)00711-2. [DOI] [PubMed] [Google Scholar]
- Ketchum REB, Croteau R. The taxoid metabolome and the elucidation of the paclitaxel biosynthetic pathway in cell suspension cultures of Taxus. In: Saito K, Dixon R, Willmetzer L, editors. Biotechnology in Agriculture and Forestry. Springer Heidelbergin press; 2005. [Google Scholar]
- Kikuchi Y, Yatagai M. The commercial cultivation of Taxus species and production of taxoids. In: Itokawa H, Lee K-H, editors. Taxus – The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 151–178. [Google Scholar]
- Kingston DGI, Molinero AA, Rimoldi JM. The taxane diterpenoids. Prog Chem Org Nat Prod. 1993;61:1–206. doi: 10.1007/978-3-7091-9242-9_1. [DOI] [PubMed] [Google Scholar]
- Kingston DGI. Recent advances in the chemistry and structure–activity relationships of paclitaxel Am. Chem Soc Symp Ser. 1995;583:203–216. [Google Scholar]
- Kingston DGI, Jagtop PG, Yuan H, Samala L. The chemistry of Taxol and related taxoids. Prog Chem Org Nat Prod. 2002;84:53–225. doi: 10.1007/978-3-7091-6160-9_2. [DOI] [PubMed] [Google Scholar]
- Koepp AE, Hezari M, Zajicek J, Stofer Vogel B, LaFever RE, Lewis NG, Croteau R. Cyclization of geranylgeranyl diphosphate to taxa-4(5),11(12)-diene is the committed step of Taxol biosynthesis in Pacific yew. J Biol Chem. 1995;270:8686–8690. doi: 10.1074/jbc.270.15.8686. [DOI] [PubMed] [Google Scholar]
- Kutchan TM, Bock A, Dittrich H. Heterologous expression of the plant proteins strictosidine synthase and berberine bridge enzyme in insect cell culture. Phytochemistry. 1994;35:353–360. doi: 10.1016/s0031-9422(00)94763-0. [DOI] [PubMed] [Google Scholar]
- Kuzuyama T, Seto H. Diversity of the biosynthesis of the isoprene units. Nat Prod Rep. 2003;20:171–183. doi: 10.1039/b109860h. [DOI] [PubMed] [Google Scholar]
- Laskaris G, DeJong CF, Jaziri M, van der Heijden R, Theodoridis G, Verpoorte R. Geranylgeranyl diphosphate synthase activity and taxane production in Taxus baccata cells. Phytochemistry. 1999;50:939–946. [Google Scholar]
- Laskaris G, van der Heiden R, Verpoorte R. Purification and partial characterization of geranylgeranyl diphosphate synthase from Taxus baccata cell cultures. An enzyme that regulates taxane biosynthesis. Plant Sci. 2000;51:97–105. doi: 10.1016/s0168-9452(99)00263-0. [DOI] [PubMed] [Google Scholar]
- Leete E, Bodem GB. The biosynthesis of 3-dimethylamino- 3-phenylpropanoic acid in yew. Tetrahedron Lett. 1966:3925–3927. [Google Scholar]
- Lin X, Hezari M, Koepp AE, Floss HG, Croteau R. Mechanism of taxadiene synthase, a diterpene cyclase that catalyzes the first step of taxol biosynthesis in Pacific yew. Biochemistry. 1996;35:2968–2977. doi: 10.1021/bi9526239. [DOI] [PubMed] [Google Scholar]
- Long RM, Croteau R. Preliminary assessment of the C13-side chain 2′-hydroxylase involved in taxol biosynthesis. Biochem Biophys Res Commun. 2005 doi: 10.1016/j.bbrc.2005.08.119. (in press) [DOI] [PubMed] [Google Scholar]
- Lovy Wheeler A, Long RM, Ketchum REB, Rithner CD, Williams RM, Croteau R. Taxol biosynthesis: differential transformation of taxadien-5α-ol and its acetate ester by cytochrome P450 hydroxylases from Taxus suspension cells. Arch Biochem Biophys. 2001;390:265–278. doi: 10.1006/abbi.2001.2377. [DOI] [PubMed] [Google Scholar]
- MacMillan J, Beale MH. Diterpene biosynthesis. In: Cane DE, editor. Comprehensive Natural Products Chemistry, Vol 2, Isoprenoids Including Carotenoids and Steroids. Elsevier Science; Oxford UK: 1999. pp. 217–243. [Google Scholar]
- Mahmoud SS, Croteau R. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA. 2001;98:8915–8920. doi: 10.1073/pnas.141237298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M. Lining up to make a cancer drug. Chem Eng News. 2004;82:12–14. [Google Scholar]
- Menhard B, Eisenreich W, Hylands PJ, Bacher A, Zenk MH. Taxoids from cell cultures of Taxus chinensis. Phytochemistry. 1998;49:113–125. [Google Scholar]
- Menhard B, Zenk MH. Purification of acetyl coenzyme A:10-hydroxytaxane O-acetyltransferase from cell suspension cultures of Taxus chinensis. Phytochemistry. 1999;50:763–774. doi: 10.1016/s0031-9422(98)00674-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Shimizu K, Mishima H, Kurabayashi M. The constituents of the heartwood of Taxus cuspidata Sieb. et Zucc. Chem Pharm Bull (Tokyo) 1968;16:546–548. [Google Scholar]
- Odgen L. Taxus (yews) – a highly toxic plant Vet. Hum Toxicol. 1988;30:563–564. [PubMed] [Google Scholar]
- Ortiz de Montellano PR, De Voss JJ. Substrate oxidation by cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure Mechanism, and Biochemistry. 3. Kluwer Academic/Plenum Press; New York, NY, USA: 2005. pp. 183–245. [Google Scholar]
- Park S-J, Shim WH, Ho DS, Raizner AE, Park S-W, Hong M-K, Lee CW, Choi D, Jang Y, Lam R, Weissman NJ, Mintz GS. A paclitaxel-eluting stent for the prevention of coronary restenosis. N Engl J Med. 2003;348:1537–1545. doi: 10.1056/NEJMoa021007. [DOI] [PubMed] [Google Scholar]
- Pennington JJ, Fett-Neto AG, Nicholson SA, Kingston DG, Dicosmo F. Acetyl CoA-10-deacetylbaccatin-III-O- acetyltransferase activity in leaves and cell suspension cultures of Taxus cuspidata. Phytochemistry. 1998;49:2261–2266. [Google Scholar]
- Pichersky E, Gang DR. Genetics and biochemistry of secondary metabolism in plants: an evolutionary perspective. Trends Plant Sci. 2000;5:439–445. doi: 10.1016/s1360-1385(00)01741-6. [DOI] [PubMed] [Google Scholar]
- Pilger R. Taxaceae. In: Engler A, Prantl K, editors. Das Pflanzenreich. IV.5. Vol. 18. W. Engelmann; Leipzig, Germany: 1903. pp. 1–24. [Google Scholar]
- Platt RV, Opie CT, Haslam E. Plant proanthocyanidins. Part 8 Biosynthesis of flavan-3-ols and other secondary plant products from (2S)-phenylalanine. Phytochemistry. 1984;23:2211–2217. [Google Scholar]
- Polizzi D, Pratesi G, Tortoreta M, Supino R, Riva A, Bombardelli E, Zunino F. A novel taxane with improved tolerability and therapeutic activity in a panel of human tumor xenografts. Cancer Res. 1999;59:1036–1040. [PubMed] [Google Scholar]
- Pompon D, Louerat B, Bronnine A, Urban P. Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 1996;272:51–64. doi: 10.1016/s0076-6879(96)72008-6. [DOI] [PubMed] [Google Scholar]
- Ramos-Valdivia AC, van der Heijden R, Verpoorte R. Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis A review of its biochemistry and function. Nat Prod Rep. 1997;14:591–603. doi: 10.1039/np9971400591. [DOI] [PubMed] [Google Scholar]
- Rikhari HC, Palni LMS, Sharma S, Nandi SK. Himalayan yew: stand structure, canopy damage, regeneration and conservation strategy. Environ Conserv. 1998;25:334–341. [Google Scholar]
- Rohdich F, Hecht S, Gärtner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W. Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc Natl Acad Sci USA. 2002;99:1158–1163. doi: 10.1073/pnas.032658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants Nat. Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents Ann. Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- Rubenstein SM, Williams RM. Studies on the biosynthesis of Taxol: total synthesis of taxa-4(20),11(12)-diene and taxa-4(5),11(12)-diene. The first committed biosynthetic intermediate. J Org Chem. 1995;60:7215–7223. [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schoendorf A, Rithner CD, Williams RM, Croteau R. Molecular cloning of a cytochrome P450 taxane 10β-hydroxylase cDNA from Taxus and functional expression in yeast. Proc Natl Acad Sci USA. 2001;98:1501–1506. doi: 10.1073/pnas.98.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RB. The Organic Chemistry of Enzyme-Catalyzed Reactions. Academic Press; San Diego, CA, USA: 2000. Monooxygenation; pp. 175–226. [Google Scholar]
- Steele CL, Chen Y, Dougherty BA, Hofstead S, Lam KS, Li W, Xing Z. Bristol-Myers Squibb Co; USA: WO 03/066871 A2. Taxus phenylalanine aminomutase and methods to improve Taxol production. 2003:38.
- St-Pierre B, De Luca V. Evolution of acetyltransferase genes: origin and diversification of the BAHD superfamily of acetyltransferases involved in secondary metabolism. Recent Adv Phytochem. 2000;34:285–315. [Google Scholar]
- Straubinger RM. Biopharmaceutics of paclitaxel (Taxol): formulation, activity, and pharmacokinetics. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 237–258. [Google Scholar]
- Suffness M, Wall ME. Discovery and development of Taxol. In: Suffness M, editor. Taxol – Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 3–25. [Google Scholar]
- Tabata H. Paclitaxel production by plant-cell-culture technology. Adv Biochem Engin Biotechnol. 2004;7:1–23. doi: 10.1007/b13538. [DOI] [PubMed] [Google Scholar]
- Takeya K. Plant tissue culture of taxoids. In: Itokawa H, Lee K-H, editors. Taxus – The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 134–150. [Google Scholar]
- vonWachenfeldt C, Johnson EF. Structures of eukaryotic cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 2. Plenum Press; New York, NY, USA: 1995. pp. 183–223. [Google Scholar]
- Walker K, Floss HG. Detection of a phenylalanine aminomutase in cell-free extracts of Taxus brevifolia and preliminary characterization of its reaction. J Am Chem Soc. 1998;120:5333–5334. [Google Scholar]
- Walker K, Croteau R. Taxol biosynthesis: a review of some determinant steps. Recent Adv Phytochem. 1999;33:31–50. [Google Scholar]
- Walker K, Ketchum REB, Hezari M, Gatfield D, Goleniowski M, Barthol A, Croteau R. Partial purification and characterization of acetyl coenzyme A:taxa-4(20),11(12)-dien-5α-ol-O-acetyl transferase that catalyzes the first acylation step of Taxol biosynthesis. Arch Biochem Biophys. 1999;364:273–279. doi: 10.1006/abbi.1999.1125. [DOI] [PubMed] [Google Scholar]
- Walker K, Schoendorf A, Croteau R. Molecular cloning of a taxa-4(20),11(12)-dien-5α-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch Biochem Biophys. 2000;374:371–380. doi: 10.1006/abbi.1999.1609. [DOI] [PubMed] [Google Scholar]
- Walker K, Croteau R. Taxol biosynthesis: molecular cloning of a benzoyl CoA:taxane-2α-O-benzoyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci USA. 2000a;97:13591–13596. doi: 10.1073/pnas.250491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Croteau R. Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci USA. 2000b;97:583–587. doi: 10.1073/pnas.97.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Croteau R. Taxol biosynthetic genes. Phytochemistry. 2001;58:1–7. doi: 10.1016/s0031-9422(01)00160-1. [DOI] [PubMed] [Google Scholar]
- Walker K, Long R, Croteau R. The final acylation step in taxol biosynthesis: cloning of the taxoid C13-side-chain N-benzoyltransferase from Taxus. Proc Nat Acad Sci USA. 2002a;99:9166–9171. doi: 10.1073/pnas.082115799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Fujisaki S, Long R, Croteau R. Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in taxol biosynthesis. Proc Natl Acad Sci USA. 2002b;99:12715–12720. doi: 10.1073/pnas.192463699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Klettke K, Akiyama T, Croteau R. Cloning, heterologous expression and characterization of a phenylalanine aminomutase involved in taxol biosynthesis. J Biol Chem. 2004;279:53947–53954. doi: 10.1074/jbc.M411215200. [DOI] [PubMed] [Google Scholar]
- Wall ME, Wani MC. Paclitaxel: from discovery to clinic. Am Chem Soc Symp Ser. 1995;583:18–30. [Google Scholar]
- Walsh V, Goodman J. From taxol to Taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21:307–336. doi: 10.1080/01459740214074. [DOI] [PubMed] [Google Scholar]
- Wang X, Itokawa H, Lee K-H. Structure–activity relationships of taxoids. In: Itokawa H, Lee K-H, editors. Taxus – The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 298–386. [Google Scholar]
- Wani MC, Taylor HC, Wall ME, Coggan P, McPhail AT. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Wildung MR, Croteau R. A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of Taxol biosynthesis. J Biol Chem. 1996;271:9201–9204. doi: 10.1074/jbc.271.16.9201. [DOI] [PubMed] [Google Scholar]
- Williams DC, Carroll BJ, Jin Q, Rithner C, Lenger SR, Floss HG, Coates RM, Williams RM, Croteau R. Intramolecular proton transfer in the cyclization of geranylgeranyl diphosphate to the taxadiene precursor of Taxol catalyzed by recombinant taxadiene synthase. Chem Biol. 2000a;7:969–977. doi: 10.1016/s1074-5521(00)00046-6. [DOI] [PubMed] [Google Scholar]
- Williams DC, Wildung MR, Jin AQ, Dalal D, Oliver JS, Coates RM, Croteau R. Heterologous expression and characterization of a “pseudomature” form of taxadiene synthase involved in paclitaxel (Taxol) biosynthesis, and evaluation of a potential intermediate and inhibitors of the multistep diterpene cyclization reaction. Arch Biochem Biophys. 2000b;379:137–146. doi: 10.1006/abbi.2000.1865. [DOI] [PubMed] [Google Scholar]
- Woo DDL, Miao SYP, Pelayo JC, Woolf AS. Taxol inhibits progression of congenital polycystic kidney disease. Nature. 1994;368:750–753. doi: 10.1038/368750a0. [DOI] [PubMed] [Google Scholar]
- Wuts PGM. Semisynthesis of Taxol Curr. Opin Drug Disc Devel. 1998;1:329–337. [PubMed] [Google Scholar]
- Xiao Z, Itokawa H, Lee K-H. Total synthesis of taxoids. In: Itokawa H, Lee K-H, editors. Taxus – The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 245–297. [Google Scholar]
- Young DH, Michelotti EL, Swindell CS, Krauss NE. Antifungal properties of Taxol and various analogues. Experientia. 1992;48:882–885. doi: 10.1007/BF02118425. [DOI] [PubMed] [Google Scholar]
- Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VM-Y, Trojanowski JQ. Microtubule-binding drugs offset tau sequestration by stablizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Nat Acad Sci USA. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocher R, Weckwerth W, Hacker C, Kammer B, Hornbogen T, Ewald D. Biosynthesis of Taxol: enzymatic acetylation of 10 deacetylbaccatin III to baccatin III in crude extracts from roots of it Taxus baccata. Biochem Biophys Res Commun. 1996;229:16–20. doi: 10.1006/bbrc.1996.1751. [DOI] [PubMed] [Google Scholar]