Abstract
Background
Curcumin, a natural polyphenol product of the plant Curcuma longa, has been shown to inhibit the growth and progression of colorectal cancer; however, the anticancer mechanism of curcumin remains to be elucidated.
Materials and Methods
Colorectal cancer cells were treated with curcumin and changes in proliferation, protein and mRNA levels were analyzed.
Results
Curcumin inhibited proliferation of colorectal cancer cells. This effect was mediated by inhibition of mammalian target of rapamycin (mTOR) signaling as evidenced by decreased phosphorylation of downstream effectors of mTOR complex 1 (mTORC1), p70S6K and 4E-BP1. Curcumin decreased total expression of mTOR, Raptor and Rictor protein and mRNA levels. Surprisingly, curcumin induced phosphorylation of Akt(Ser 473); this effect may be attributed to a decrease in levels of the PHLPP1 phosphatase, an inhibitor of Akt.
Conclusion
Our data suggest that curcumin, a natural compound, may exert its antiproliferative effects and inhibition of mTOR signalling and thus may represent a novel class of mTOR inhibitor.
Keywords: Curcumin, colorectal cancer, mTOR, Raptor, Rictor, Akt, PHLPP
Curcumin (diferuloylmethane), a component of the spice turmeric derived from the roots of the plant Curcuma longa, has been used through the ages for its antioxidant, anti-inflammatory, chemopreventive and chemotherapeutic effects (1). Curcumin can suppress tumor initiation (by reducing proliferation and inducing apoptosis), promotion and metastasis in a variety of malignant cells (2, 3). We have previously shown that one mechanism for the chemotherapeutic effects of curcumin on colorectal cancer (CRC) is through inhibition of neurotensin-induced expression and secretion of the chemokine, interleukin-8 (IL-8), subsequently reducing cell migration (4).
The phosphatidylinositol-3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway is a key regulator of growth and survival in response to cellular stress (5). Activation of Class I PI3K, by activating mutations or loss of the tumor suppressor protein phosphatase and tensin homologue (PTEN), is associated with growth and progression of a number of types of cancer including CRC (6, 7). Our laboratory has shown that inhibition of PI3K/Akt pathway components reduces growth, increases apoptosis and sensitivity to chemotherapy, and reduces the metastatic capability of CRC (8-12).
The mTOR kinase lies downstream of Akt and positively regulates phosphorylation of p70 S6 kinase (S6K) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) (5). Rapamycin treatment results in hypo-phosphorylation of S6K and 4E-BP1, thus causing blockade of cap-dependent mRNA translation (5). Raptor and Rictor are components of mTOR complexes 1 and 2 (mTORC1 and 2) respectively, both exhibiting distinct cellular functions. The activation status of Akt regulates mTORC1 activity, thus placing it downstream in the PI3K signaling cascade. Inhibition of mTORC1 by rapamycin leads to activation of a negative feedback loop via S6K and insulin-like growth factor-1 receptor, which results in feedback activation of Akt (5, 13). In contrast, mTORC2 directly phosphorylates Ser 473 in the C-terminal hydrophobic motif of Akt, which is necessary for full activation of the latter (5). Thus, mTOR complexes act both upstream and downstream of Akt. Moreover, the PHLPP (PH domain leucine-rich repeats protein phosphatase) family of Ser/Thr protein phosphatases have recently been identified as the phosphatases responsible for dephosphorylating Akt directly (14, 15). Recent evidence indicates that PHLPP 1 and 2 act as tumor suppressors in CRC; their expression is lost or reduced in 78% and 80% of CRCs respectively (16).
Curcumin inhibited Akt phosphorylation in certain cancer cells (17, 18) and has also recently been shown to inhibit mTOR signaling at relatively low concentrations in rhabdomyosarcoma, prostate and breast cancer cells (19, 20). It has previously been shown that curcumin inhibited proliferation of CRCs (21), and we have previously shown that curcumin reduced neurotensin-mediated migration of CRCs (4). In our current study, we investigated whether curcumin inhibits mTOR signaling in CRC since mTOR functions as a central regulator of proliferation and migration.
Materials and Methods
Cell culture
HCT116 CRC cells, obtained from the American Type Culture Collection (Manassas, VA, USA), were maintained in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS). KM20 CRC cells, obtained from Dr. Isaiah Fidler (M. D. Anderson Cancer Center, Houston, TX, USA), were maintained in minimum essential media (MEM) with 10% FBS. Curcumin was purchased from Sigma (St. Louis, MO, USA); a 25 mM stock solution in ethanol was maintained at −20°C.
Cell proliferation assays
A cell proliferation assay measuring total DNA content was performed using crystal violet dye. Briefly, cells were plated in 96-well plates and allowed to adhere overnight, then treated with different doses (0-100 μM) of curcumin or vehicle (70% ethanol). After 24, 48, and 72 h, the media were removed and cells were fixed and stained with a solution containing 20% methanol and 0.5% crystal violet. After rinsing and drying, the dye was solubilized with 15% acetic acid and the absorbance was read at 570 nm.
Western blotting
Western blotting was performed as described previously (8). In Fig. 2, HCT116 cells were serum starved overnight and treated with 25 μM curcumin for 2 hours, followed by stimulation with 20 ng/ml EGF for 45 minutes, prior to cell lysis. Antibodies used were β-Actin (Sigma, St. Louis, MO, USA); p-mTOR (Ser 2448), mTOR, Raptor, Rictor, p-p70S6K (Thr389), p70S6K, pAkt (Ser 473), Akt, p-4E-BP1 (Thr 37/46) and 4E-BP1 (Cell Signaling, Danvers, MA, USA); PHLPP1 (Bethyl Laboratories, Montgomery, TX, USA). Data are representative of three independent experiments with similar results.
Figure 2. Curcumin inhibits EGF-stimulated PI3K-Akt-mTOR signaling.
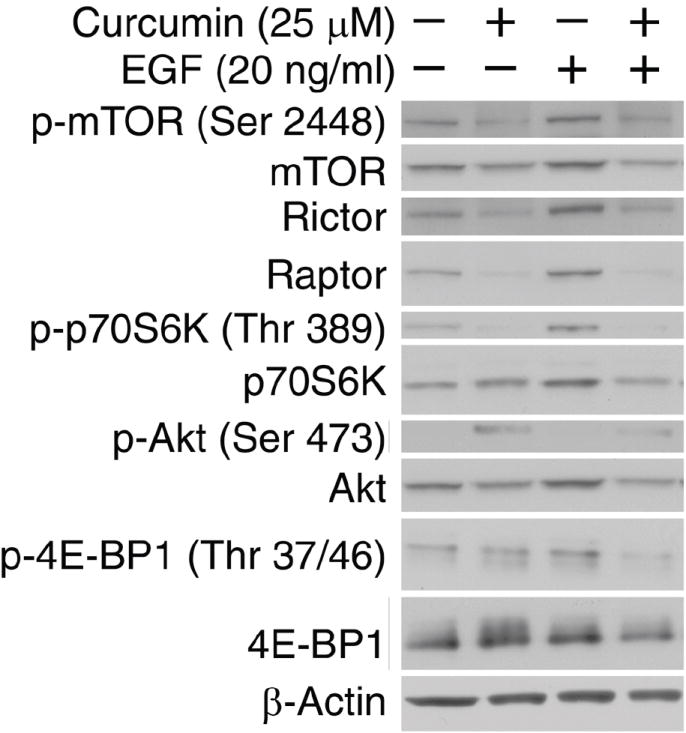
HCT116 cells were serum starved overnight and treated with 25 μM curcumin for 2 hours, followed by stimulation with 20 ng/ml EGF for 45 minutes, prior to cell lysis. Western blotting was performed for rictor, raptor, and the phosphorylated and total forms of mTOR, p70S6K (Thr389), Akt (Ser473) and 4E-BP1 (Thr37/46), with β-Actin serving as a loading control.
RNA isolation and quantitative RT-PCR
HCT116 cells were treated with curcumin (0-100 μM) for 6 h and RNA was isolated using RNEasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Real-time PCR for mTOR (FRAP1), Rictor, Raptor, and 18S was performed as described elsewhere (22). Separate tubes (singleplex) one-step RT-PCR was performed with 80 ng RNA for all target genes and endogenous control. The reagent used was TaqMan one-step RT-PCR master mix reagent kit (Foster City, CA, USA) the cycling parameters for one-step RT-PCR were: reverse transcription 48°C for 30 min, AmpliTaq activation 95°C for 10 min, denaturation 95°C for 15 s and annealing/extension 60°C for 1 min (repeat 40 times) on Applied Biosystems PRISM 7000 (Applied Biosystems, Foster City, CA). Duplicate CT values were analyzed in Microsoft Excel using the comparative CT(ΔΔCT) method as described by the manufacturer. The amount of target (2-ΔΔCT) was obtained by normalizing to endogenous reference (18S) and relative to a calibrator (one of the experimental samples).
Statistical analysis
Dose effect of curcumin on HCT116 and HT29 cell proliferation (Fig. 1) and on mRNA (Fig. 3) were analyzed, separately for each incubation time and each kind of cancer cells (Fig. 1) or each kind of protein (Fig. 3), using one-way classification analysis of variance. The dose was assessed at the 0.05 level of significance. Comparisons of each dose with the control (no curcumin) were conducted using Fisher’s least significant different procedure with Bonferroni adjustment for the number of comparisons. Statistical computations were carried out using PROC GLM in SAS®, Release9.1 [R1].
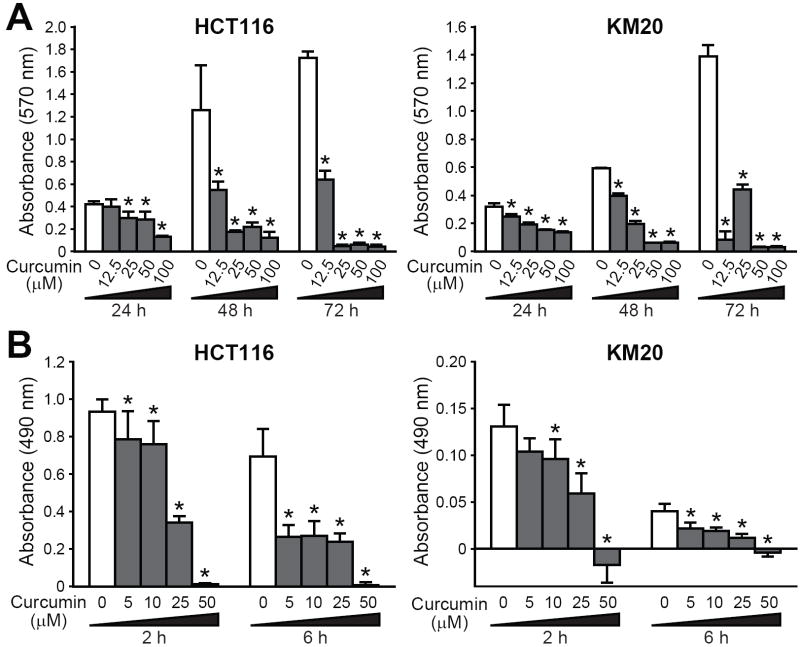
Figure 1. Curcumin inhibits colorectal cancer cell proliferation.
HCT116 and KM20 CRC cells were treated with 0-100 μM curcumin for different time periods. A, Cell proliferation as assessed by crystal violet proliferation assay. B, Neutral red-based cytotoxicity assays were performed (*p<0.05 compared to the control).
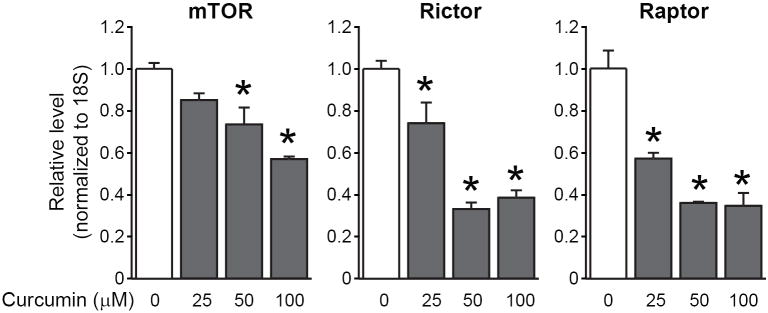
Figure 3. Curcumin reduces mTOR, Raptor and Rictor mRNA levels in a dose-dependent fashion.
HCT116 cells were treated with curcumin at the indicated doses for 6 hours. RNA was extracted for analysis using quantitative RT-PCR. Messenger RNA levels of mTOR, Raptor and Rictor after curcumin treatment are shown after normalization to 18S mRNA levels (*p<0.05 compared to the control).
Results
Curcumin inhibits the growth of human CRC cells in a time and dose-dependent manner
Curcumin inhibits growth of a number of types of cancer (2, 3). Based on these studies, we first determined the effects of curcumin on the proliferation of CRC cells. The doses of curcumin used were comparable to these in previous studies. HCT116 and KM20 cells were treated with curcumin (0-100 μM) for 72 h. As shown in Figure 1A, curcumin significantly reduced proliferation of both cell lines, although with different effects. Curcumin (12.5 μM) significantly reduced cell numbers as early as 24 h. It is interesting to note that this dose iwas cytostatic in KM20 cells, while being cytotoxic in HCT116 cells as approximately 90% of the cells were dead by 72 h. High-dose curcumin (50-100 μM) was clearly cytotoxic in both CRC cell lines, with a significant decrease in cell number at the earliest time point (24 h).
To further confirm the effects of curcumin on CRC proliferation, we utilized a neutral red (NR)-based cytotoxicity assay, which assesses the ability of viable cells to uptake NR, a cationic dye that penetrates cell membranes and accumulates in lysosomes. As shown in Figure 1B, the effects of curcumin were evident as early as 2 h after treatment with a low dose (5 mM) of curcumin.
Curcumin inhibits EGF-stimulated phosphorylation of S6K, 4E-BP1 and mTOR
Recent evidence has implicated mTOR as a central regulator of proliferation in various normal and malignant cells (5). Several proteins regulated by mTOR such as cyclin D1, ornithine decarboxylase, NF-κB, Akt and PKC, are also targeted by curcumin (1, 2). Thus, we hypothesized that curcumin may reduce proliferation by inhibiting the mTOR signaling pathway. After an overnight serum starvation, HCT116 cells pre-treated with curcumin for 2h were treated with EGF for 45 min to stimulate the PI3K/Akt/mTOR pathway. As shown in Figure 2, curcumin inhibited EGF-stimulated phosphorylation of mTOR (Ser 2448; a site regulated by Akt), as well as S6K (Thr 389; a site regulated by mTOR) and 4E-BP1 (Thr 37/46; both sites regulated by mTOR), two well-characterized downstream effector molecules of mTORC1. Interestingly, total protein levels of both Rictor and Raptor significantly increased with EGF treatment. Curcumin reduced total protein levels of mTOR, Raptor and Rictor. The latter effect was noted both in the presence and absence of EGF. Surprisingly, curcumin induced phosphorylation of Akt (Ser 473), while reducing the total protein levels of Akt. Therefore, we conclude that curcumin inhibits downstream components of the mTOR signaling pathway, while inducing Akt phosphorylation.
Curcumin suppresses mTOR, Rictor and Raptor protein and mRNA levels, but induces Akt (Ser 473) phosphorylation due to a decrease in PHLPP1 protein level
In order to confirm the effects of curcumin on mTORC1 and mTORC2 expression in CRC cells, we treated HCT116 cells with increasing doses of curcumin (0-100 μM) and performed Western blotting and quantitative RT-PCR analysis. As shown in Figure 3, mTOR, Raptor and Rictor mRNA levels decreased in a dose-dependent manner with curcumin treatment. Similarly, curcumin also reduced mTOR, Raptor and Rictor protein expression in a dose-dependent manner (Figure 4); this effect was noted as early as 2 h (data not shown). Similar results were noted with curcumin treatment of KM20 cells (data not shown).
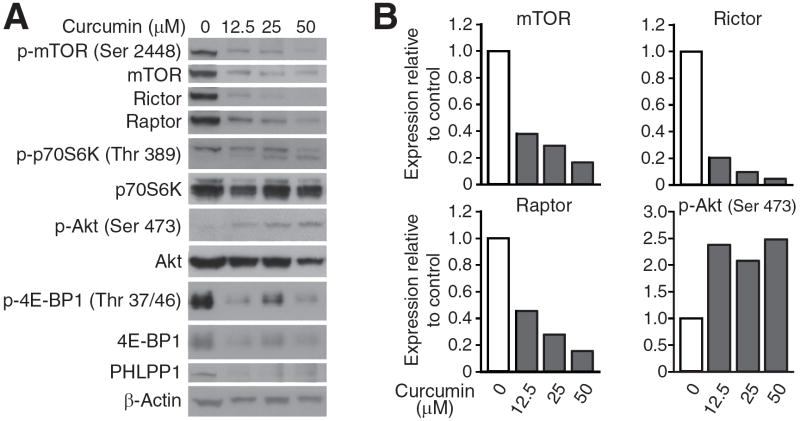
Figure 4. Curcumin reduces mTOR, Raptor and Rictor protein expression in a dose-dependent fashion.
HCT116 cells were treated with curcumin at the indicated doses for 6 hours and cells were lysed. Proteins were analyzed by western blotting. A, Representative Western blots of mTOR components and downstream signals. B, Densitometry graphs showing relative changes in protein expression normalized to β-actin.
To determine downstream effectors of curcumin-mediated inhibiton of mTOR complex components, we next examined levels of phosphorylated and total p70S6K, 4E-BP1 and Akt. As shown in Figure 4, activation of S6K and 4E-BP1 was inhibited by curcumin, while total levels of both proteins were essentially unchanged. Surprisingly, curcumin induced Akt (Ser 473) phosphorylation in a dose-dependent manner despite a significant decrease in Rictor expression. Total Akt levels also decreased upon treatment with moderate doses (50 μM) of curcumin, which is consistent with effects noted in other types of cancer cells (23-25). One possible mechanism for an increase in pAkt(Ser 473) is through inhibition of the constitutively active Akt phosphatase, PHLPP. In this regard, we found that PHLPP1 protein levels were reduced by low doses of curcumin. Therefore, curcumin produced a dose-dependent decrease of mTOR, Raptor and Rictor mRNA and protein levels. However, curcumin resulted in a dose-dependent induction of Akt(Ser 473) phosphorylation; this effect may be attributed to a decrease in PHLPP1 protein levels.
Discussion
mTOR has been recognized as a key therapeutic target for the treatment of several types of cancer since it is a central hub for regulation of cellular processes that are critical for growth (such as proliferation) and progression (such as migration) of cancer (5). We showed that curcumin reduced neurotensin-mediated migration of CRCs (4), and here we show that curcumin reduced proliferation of CRC cells in a dose- and time-dependent manner. This prompted us to further study the effect of curcumin on mTOR signaling since the anticancer mechanism of curcumin remains to be fully elucidated. To this end, we found that curcumin reduced expression of mTOR, Raptor and Rictor protein and mRNA levels, as well as downstream mTORC1-mediated phosphorylation of p70S6K and 4E-BP1. Therefore, the antiproliferative effects of curcumin in CRC may be mediated by inhibiton of the mTOR signaling pathway.
In support of our findings, several recent studies have shown that curcumin targets the mTOR pathway and represents a powerful therapeutic tool for use against cancer dependent on elevated PI3K signaling (3, 26-29). Curcumin down-regulates expression of the p53 ubiquitin E3 ligase, MDM2, partially through inhibition of the mTOR pathway in prostate cancer (26). Furthermore, curcumin induces autophagy in gliomas via inhibition of mTOR and ERK signaling (27, 28). It was recently shown that curcumin inhibits mTORC1 signaling by directly inhibiting the kinase activity of mTORC1 due to disruption of the mTOR-Raptor interaction in rhabdomyosarcoma (29). Taken together with our findings, this suggests that curcumin may exert its inhibitory effect on mTOR signaling by reducing total expression of mTOR complex components as well as preventing association of the components from forming stable complexes. As compared to rapamycin, which is a specific inhibitor of mTORC1 in most cells, our findings suggest that curcumin is a novel class of mTOR inhibitor that inhibits both mTORC1 and mTORC2 in CRC cells.
Rictor is a critical component of mTORC2 that is required for assembly and activity of the PDK-2 kinase (mTORC2). Therefore, lower Rictor levels would be expected to reduce the phosphorylation of Akt (Ser 473). Surprisingly, curcumin induced Akt (Ser 473) phosphorylation in a dose-dependent manner despite a significant decrease in Rictor levels. Total Akt levels also decreased slightly upon treatment with moderate doses (50 μM) of curcumin, which is consistent with other published studies (16-18). One possible mechanism for an increase in pAkt (Ser 473) could be through inhibition of the constitutively active Akt phosphatase, PHLPP1. PHLPP1 protein levels were found to be reduced by curcumin even at low doses. There are no studies showing the effects of curcumin on PHLPP, although it has been shown to have variable effects on other types of phosphatases. Another factor contributing to the increased Akt (Ser 473) phosphorylation may be feedback activation of Akt via the S6K-IGF-1R-mediated negative feedback loop upon mTORC1 inhibition by curcumin. Therefore, curcumin may induce Akt (Ser 473) phosphorylation via a novel PHLPP1 down-regulation mechanism despite a significant decrease in levels of the mTORC2 kinase component, Rictor.
Most chemotherapeutic agents not only induce apoptosis in cancer cells, but also severely damage normal host cells. Curcumin, a natural compound that is part of daily diets in several countries, has been proven to be a safe product since it preferentially induces apoptosis in highly proliferating tumor cells rather than normal ones (1). Doses up to 8-10 g may be administered daily to patients for 3 months without toxicity (2). In fact, consumption of curcumin as part of the daily diet in several Asian countries has been suggested to be the reason for their lower rate of CRC compared to developed countries (1, 2). Moreover, curcumin undergoes intestinal metabolism, particularly glucuronidation and sulphation, in addition to first-pass metabolism in the liver. Therefore, the gastrointestinsl tract could be a preferential target for this compound due to greater exposure to unmetabolized curcumin upon oral administration (30). Due to its high potency and efficacy against cancer both in vitro and in vivo, as well as its safety for use in humans, curcumin is currently being evaluated in phase III clinical trial as part of a combination treatment for metastatic CRC.
In summary, we show that the antiproliferative effect of curcumin may be mediated by inhibition of mTOR signaling. Curcumin produces a dose-dependent decrease of mTOR, Raptor and Rictor mRNA and protein levels. Surprisingly, curcumin results in a dose-dependent induction of Akt (Ser 473) phosphorylation; this effect may be attributed to a dose-dependent decrease in PHLPP1 protein levels. Finally, curcumin represents a new class of mTOR inhibitor that can inhibit both mTORC1 and mTORC2.
Acknowledgments
The authors thank Jing Li for critical review of the manuscript and Karen Martin for manuscript preparation. This work was supported by grants RO1CA104748, RO1DK48498, PO1DK35608 and K01 CA10209 (to TG) from the National Institutes of Health and a Sealy Center for Cancer Cell Biology Fellowship (PG).
References
- 1.Sa G, Das T. Anticancer effects of curcumin: cycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 3.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006;12:5346–5355. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3’-kinase p85α gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- 7.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 8.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rychahou PG, Murillo CA, Evers BM. Targeted RNA interference of PI3K pathway components sensitizes colon cancer cells to TNF-related apoptosis-inducing ligand (TRAIL) Surgery. 2005;138:391–397. doi: 10.1016/j.surg.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Li N, Wang X, Kim MM, Evers BM. Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3’-kinase inhibition in the KM20 human colon cancer cell line. Clin Cancer Res. 2002;8:1940–1947. [PubMed] [Google Scholar]
- 12.Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, Chung DH, Evers BM. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci USA. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 14.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 18.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 19.Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer. 2006;119:757–764. doi: 10.1002/ijc.21932. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7:2609–2620. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695–1706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- 22.Chen LA, Li J, Silva SR, Jackson LN, Zhou Y, Watanabe H, Ives KL, Hellmich MR, Evers BM. PKD3 is the predominant protein kinase D isoform in mouse exocrine pancreas and promotes hormone-induced amylase secretion. J Biol Chem. 2009;284:2459–2471. doi: 10.1074/jbc.M801697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel H, Polanco-Echeverry G, Segditsas S, Volikos E, McCart A, Lai C, Guenther T, Zaitoun A, Sieber O, Ilyas M, Northover J, Silver A. Activation of AKT and nuclear accumulation of wild type TP53 and MDM2 in anal squamous cell carcinoma. Int J Cancer. 2007;121:2668–2673. doi: 10.1002/ijc.23028. [DOI] [PubMed] [Google Scholar]
- 24.Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, Majumdar AP. Mechanisms of curcumin- and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutr Cancer. 2006;55:185–194. doi: 10.1207/s15327914nc5502_10. [DOI] [PubMed] [Google Scholar]
- 25.Bava SV, Puliappadamba VT, Deepti A, Nair A, Karunagaran D, Anto RJ. Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-kappaB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J Biol Chem. 2005;280:6301–6308. doi: 10.1074/jbc.M410647200. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 27.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 28.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 29.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69:1000–1008. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]