Abstract
To investigate the association between physical activity and health, we need accurate and detailed free-living physical activity measurements. The determination of energy expenditure of activity (EEACT) may also be useful in the treatment and maintenance of nutritional diseases such as diabetes mellitus. Minute-to-minute energy expenditure during a 24-h period was measured in 60 sedentary normal female volunteers (35.4 ± 9.0 years, body mass index 30.0 ± 5.9 kg/m2), using a state-of-the-art whole-room indirect calorimeter. The activities ranged from sedentary deskwork to walking and stepping at different intensities. Body movements were simultaneously measured using a hip-worn triaxial accelerometer (Tritrac-R3D, Hemokentics, Inc., Madison, Wisconsin) and a wrist-worn uniaxial accelerometer (ActiWatch AW64, MiniMitter Co., Sunriver, Oregon) on the dominant arm. Movement data from the accelerometers were used to develop nonlinear prediction models (separately and combined) to estimate EEACT and compared for accuracy. In a subgroup (n = 12), a second 24-h study period was repeated for cross-validation of the combined model. The combined model, using Tritrac-R3D and ActiWatch, accurately estimated total EEACT (97.7 ± 3.2% of the measured values, p = 0.781), as compared with using ActiWatch (86.0 ± 4.7%, p < 0.001) or Tritrac-R3D (90.0 ± 4.6%, p < 0.001) alone. This model was also accurate for all intensity categories during various physical activities. The subgroup cross-validation also showed accurate and reproducible predictions by the combination model. In this study, we demonstrated that movement measured using accelerometers at the hip and wrist could be used to accurately predict EEACT of various types and intensity of activities. This concept can be extended to develop valid models for the accurate measurement of free-living energy metabolism in clinical populations.
Introduction
Physical activity has been known to be have beneficial effects on overall health, particularly in decreasing the incidence of morbidity/mortality associated with common chronic diseases such as coronary heart disease, hypertension, and type II diabetes.1,2 However, little quantitative evidence has yielded causal relationships,3,4 mostly because of the large individual and time variations in the characteristics of the parameters of activity and health, and the lack of ability to accurately quantify physical activity. For example, both observational studies and clinical trials in a variety of populations have supported the hypothesis that physical activity plays a significant role in the prevention and treatment of type II diabetes, but what is less clear is how much physical activity is needed.5,6 Objective and accurate measurements of physical activity and energy expenditure (EE) are crucial in the treatment and maintenance of such chronic diseases.
EE of activity (EEACT) varies within and among individuals, and contributes the largest variability to total EE in humans.7 This contribution has significant consequences on overall energy balance, which determines the long-term body weight outcome. The current standard in objective measuring methods for EE are doubly labeled water8,9 and indirect calorimetry.10,11 The doubly labeled water method provides a mean value of EE for the entire measurement period, usually around 10–14 days, and does not allow one to calculate the day-to-day variation in EE. The other disadvantages of the technique are its high cost and the limited availability of 18O. The indirect calorimeter is the best method to measure the components of daily EE [resting EE (REE), thermic effect of food, and EEACT]. It is relatively simple, and can be used either with a ventilated hood system (for a resting subject) or in a respiratory chamber for a longer period of time.12 A major advantage of indirect calorimetry is the immediate response of oxygen consumption. Another advantage of indirect calorimetry in comparison with other methods is the possibility of assessing nutrient oxidation rates. However, it can only measure EE accurately under laboratory conditions.
Portable accelerometers, developed to objectively measure body movements and record detailed data for an extensive period, have been adopted to assess physical activities and EEACT.13–20 We previously showed that EE estimated by a hip-worn triaxial accelerometer (Tritrac-R3D, Hemokentics, Inc., Madison, WI) significantly underestimated EEACT as compared with EEACT measured by a whole-room indirect calorimeter.15 We then developed and validated a nonlinear model that used the acceleration components from the Tritrac-R3D for the estimation of EEACT. Although the estimation was accurate for the group, individual variation in EEACT prediction still existed, potentially because of undetected upper body movements. Since small errors over time can be significantly contributed to overall energy balance, our mission is to minimize individual errors. Therefore, we hypothesize that by adding an upper-body acceleration component (measured by ActiWatch AW64, MiniMitter Co., Sunriver, OR) to our previous hip-worn accelerometer model, the overall estimation accuracy of EEACT would be improved, compared with using each individual monitor alone. This investigation was also to demonstrate the process of using a whole-room indirect calorimeter to develop subject-specific EEACT predictive equations from portable accelerometers in humans.
Subjects and Methods
Subjects
The data were part of a prospective study looking at possible seasonal variations in physical activity in sedentary women. Normal healthy women (n = 60) of heterogeneous characteristics and sedentary by self-report were recruited from local areas. Signed informed consent approved by the Institutional Review Board at Vanderbilt University was obtained before their participation in the study. Women were eligible for participation if they were apparently healthy, with no evidence of past or present metabolic diseases (e.g., thyroid disorders and type II diabetes), were not pregnant as determined by a serum pregnancy test, did not use drugs known to affect energy metabolism, were eating a balanced diet, and were non-smokers. All participants were studied between days 3 and 12 after the onset of menses (follicular phase) to eliminate the influence of menstrual function on energy expenditure.21 Study participants were compensated for taking part in the study. During the 2 weeks prior to the study, all participants were encouraged to maintain their normal pattern of activity. To cross-validate the models developed from this study, a randomly selected subgroup of subjects were asked to volunteer to repeat the protocol under identical conditions within 4 days of the first study. Characteristics of all study participants are shown in Table 1.
Table 1. Subject Physical Characteristics.
| All (n = 60) | Subgroup (n = 12) | |||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Body mass (kg) | 70.7 ± 16.4 | 45.0–131.1 | 65.9 ± 9.4 | 54.4–83.5 |
| Height (cm) | 164.9 ± 7.3 | 151.0–184.0 | 164.9 ± 8.3 | 156.0–184.0 |
| Age (yrs) | 35.4 ± 9.0 | 20.0–52.0 | 27.6 ± 5.1a | 22.0–38.0 |
| BMI (kg m−2) | 30.0 ± 5.9 | 16.7–47.0 | 24.2 ± 2.3 | 21.4–30.3 |
BMI, body mass index.
Significantly different compared with the rest of the subjects (n = 48), p = 0.004.
Experimental procedures
All participants reported to the General Clinical Research Center (GCRC) after a 10-h overnight fast. The 24-h study protocol involved spontaneous daily activities and an exercise protocol that was similar to the manual work and leisure activities that participants would perform in daily life. Specifically, the exercise protocol consisted of three 10-min walking periods with average speeds of 0.6 m/s, 0.9 m/s, and 1.2 m/s across the room and three 10-min stepping periods with average speed of 12 steps/10 s, 18 steps/10 s, and 24 steps/min, respectively, all with at least 10-min resting periods between each exercise. During the walking and stepping segments, subjects followed the appropriate exercise cadence set by a metronome. The spontaneous physical activities included various types and intensities, such as sitting, TV viewing, deskwork, walking around the room, and even some voluntary exercises using the provided treadmill and stepper. Meals designed by the registered dietitian to maintain approximate energy balance were prepared at the Vanderbilt University GCRC metabolic kitchen and provided to the subject at 8:30 a.m., 12:30 p.m., and 5:00 p.m. The participants were asked to go to bed from 9:30 p.m. until 6:00 a.m.
Measurement of physical activity
The Tritrac-R3D monitor (weighing 170 g and measuring 11.1 × 6.7 × 3.2 cm) was placed in a nylon pouch secured to the belt at the waistline on the right hip to measure body acceleration in three dimensions (x or anteroposterior, y or vertical, and z or medial–lateral axis). The ActiWatch (weighing 17 g and measuring 2.8 × 2.7 × 1 cm), a uniaxial accelerometer, was worn at the wrist of the dominant hand to assess arm movements. The ActiWatch was worn during the entire study period, while the Tritrac-R3D was not worn during sleep for better comfort. Both monitors were set to record data at 1-min intervals.
Measurement of EE
The rate of EE was measured minute-by-minute in a whole-room indirect calorimeter (Fig. 1), an airtight environmental room that is temperature and humidity controlled. To provide facilities for daily living and to bridge the difference between laboratory and free-living environments, the room is equipped with a desk, chair, outside window, toilet, sink, telephone, TV/VCR, audio system/alarm clock, and fold-down mattress. It has been validated as a highly accurate system for determining detailed EE and physical activity.11,22 Oxygen consumption (VO2) and carbon dioxide production (VCO2) are used to calculate minute-by-minute EE with a system error of less than 1%.11 This accuracy is critical for validation and model development of EE.
FIG. 1.
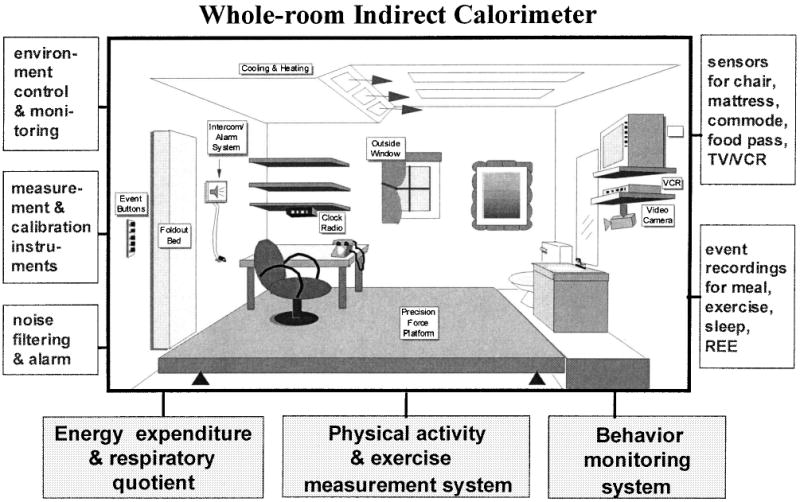
Schematic diagram of the whole-room indirect calorimetry chamber at Vanderbilt University.
Anthropometry
Body weight was measured to the nearest 0.05 kg with a digital scale. Height was measured to the nearest 0.5 cm with a stadiometer.
Model development
The model development algorithm was similar to our previous studies.15,16 The body accelerations ascertained from the Tritrac-R3D and the ActiWatch were used to fit the measured EEACT (EE – REE), first in separate models, and then combined in one model. REE was calculated during the 30-min resting supine posture while awake and immediately following overnight fasting and sleeping. A nonlinear model was previously proven to be superior compared with linear models,7 and thus was also adopted for the current study. After synchronizing the acceleration outputs with the measured EE, the acceleration counts from the Tritrac-R3D were stratified into the horizontal component (denoted H, where ), and the vertical component (denoted V, V = y). Each component was modeled by nonlinear power parameters to model individual EEACT as the following:
| (1) |
| (2) |
| (3) |
where EÊACT(k) represents the estimated EEACT at the kth minute, and parameters such as a, b, c, p, p1, p2, and p3 were optimized to predict EEACT that had the best fit compared with the measured EEACT.
Statistical analysis
Descriptive data were expressed in mean ± 1 standard deviation (SD). Optimization was performed using the least sum of squared error algorithm with universal minimum. Correlation coefficient (Pearson's r) and standard errors of estimation (SEE) were used as the evaluation criteria:
| (4) |
where EEestimated represents the estimated EE value by each model, and EEmeasured represents the EE measured by the indirect calorimeter for each study participant. The MATLAB software package (for Windows, version 6.1, Math-Works, Inc., Natick, MA) was used for the model development and evaluation of final predictions. Differences were compared by analysis of variance (ANOVA, Tukey's test) using SPSS for Windows (for Windows, version 11.0, SPSS, Inc., Chicago, IL); 95% confidence interval and p < 0.05 were used to identify statistical significance. Bland–Altman23 plots, which express the difference with respect to the mean of the two measurements in a scatter graph, were used to explore differences between modeled and measured total EEACT across the study population.
To further evaluate the accuracy of the models for various activity intensities, the time periods of the study day were categorized according to the intensity. We stratified the non-sleeping activities into four categories: 1–2.5, 2.5–4.0, 4.0–6.0, and >6.0 times the REE (METs, including EEACT and REE), using measured EE as the standard. The estimated EE from the prediction models within the same time periods of these intensities was also categorized and compared with the measured EE using ANOVA.
Results
Table 1 presents descriptive data for the 60 study participants and for the cross-validation subgroup (n = 12).
The 24-h EE was 2,132 ± 335 kcal, total EEACT was 821 ± 167 kcal, and REE was 1,403 ± 233 kcal for the entire group. Physical activity, measured in counts per minute for each individual by the Tritrac-R3D (vector magnitude) and ActiWatch, was significantly correlated with measured EEACT (R = 0.825 ± 0.046 and 0.646 ± 0.093, respectively, p < 0.001). The estimated EEACT yielded from the predictive models (Eqs. 1–3) was significantly (p < 0.001) correlated with measured EEACT, and was higher (p < 0.001) than the correlation between the raw counts and measured EEACT (Table 2). However, compared with the EEACT measured in the room calorimeter, models using ActiWatch (Eq. 2) and Tritrac-R3D (Eq. 1) individually significantly underestimated total EEACT: −113 (−189, −38) kcal (p < 0.001) and −85 (−161, −10) kcal (p = 0.019), respectively. The total EEACT predicted using the Tritrac+ActiWatch model (Eq. 3) was not statistically different from the measured EEACT: −28 (−103, 48) kcal (p = 0.781). The degrees of agreement between estimated EEACT using each of these three predictive models and EEACT measured in the room calorimeter are presented by the Bland–Altman plots in Figure 2. These plots show that the main differences among the three predictive models were the magnitude of mean error and the range of deviations in estimating total EEACT. This analysis demonstrates that the combination of two accelerometers improved both parameters over the two individual models.
Table 2. Minute-by-Minute R and SEE Between EEACT Measured by the Indirect Calorimeter and Estimated by the Models.
| Model | R (estimated vs. measured EE) | SEE (kcal/min) |
|---|---|---|
| Tritrac-R3D (Eq. 1) | ||
| Mean ± SD | 0.90 ± 0.03 | 0.364 ± 0.088 |
| Range | 0.81–0.95 | 0.234–0.570 |
| ActiWatch (Eq. 2) | ||
| Mean ± SD | 0.73 ± 0.08a | 0.575 ± 0.142a |
| Range | 0.53–0.90 | 0.356–0.862 |
| Tritrac ± ActiWatch (Eq. 3) | ||
| Mean ± SD | 0.92 ± 0.03 | 0.334 ± 0.087 |
| Range | 0.81–0.96 | 0.212–0.546 |
Significantly different from the Tritrac-R3D model and the Tritrac + ActiWatch model, all p < 0.001.
FIG. 2.
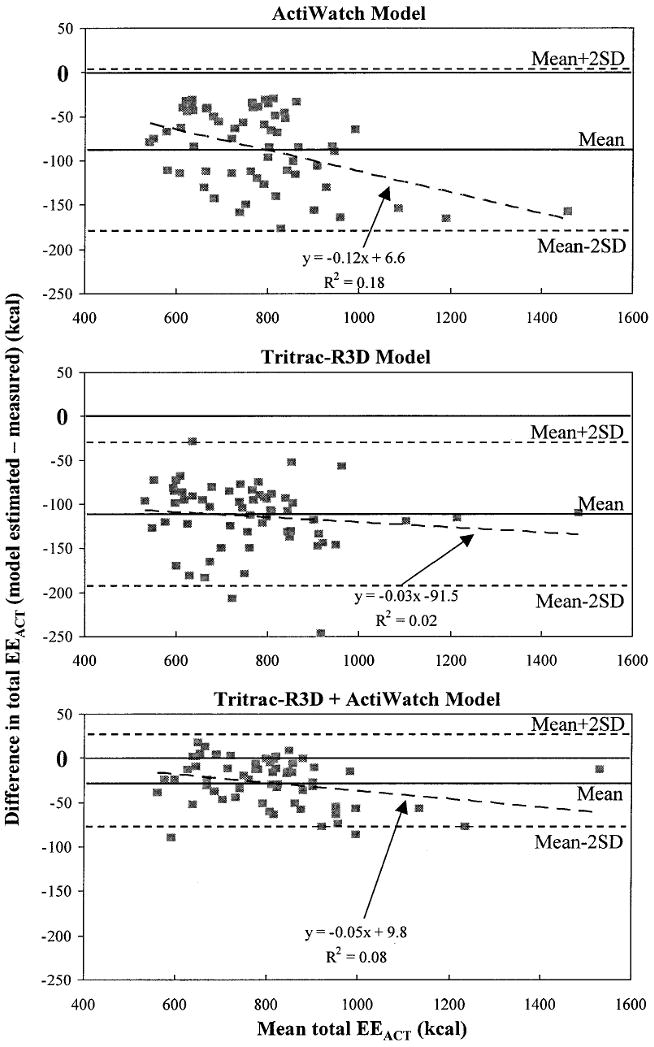
Mean ± 2 SD of the difference between the measured (calorimeter) and estimated 24-h EEACT using prediction models (ActiWatch, Tritrac-R3D, and Tritrac-R3D+ActiWatch, in Eqs. 2, 1, and 3, respectively), with respect to the mean of the EEACT values.
Furthermore, both the Tritrac-R3D model (Eq. 1) and the Tritrac + ActiWatch model (Eq. 3) significantly (all p < 0.001) increased correlation coefficient (R) values and reduced SEE compared with the ActiWatch model (Eq. 2). The improvements from the Tritrac-R3D model to the combination model, although all in positive directions, did not reach statistical significance in terms of R (p = 0.278) or SEE (p = 0.218). The summary results of the fitting parameters of the models for Tritrac-R3D, ActiWatch, and the two monitors combined (Eqs. 1–3) are summarized in Table 2. When compared among different intensity categories, we found the performance from the three predictive models varied (Fig. 3). The ActiWatch model underestimated the EE when intensity exceeded 4 METs (p < 0.001). Only the combination model was able to produce nonsignificant differences in EEACT values in all physical activity intensities.
FIG. 3.
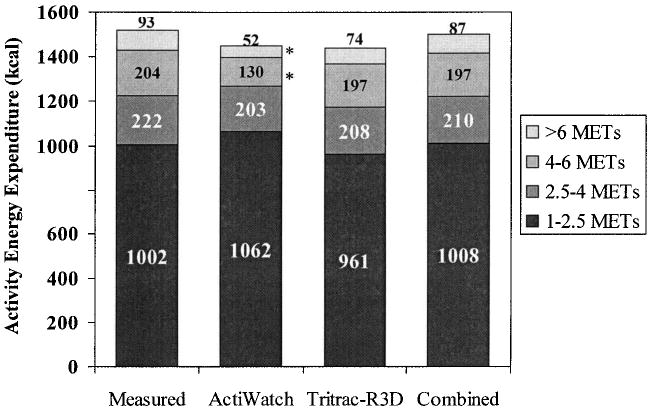
Group mean EEACT in different intensity categories as measured by the calorimeter, modeled by the ActiWatch, Tritrac-R3D, and combined (Tritrac-R3D+ActiWatch) models. *p < 0.05 compared with the measured values.
In the smaller cross-validation subgroup, 12 subjects (characteristics summarized in Table 1) who repeated the 24-h measurements expended 2,008 ± 260 kcal/day and 2,000 ± 295 kcal/day for the first and second study day (p = 0.840), respectively. EEACT was also comparable between the two days (820 ± 199 kcal/day and 761 ± 201 kcal/day, p = 0.763). One of the two study days was selected randomly for model development. The fitting parameters in the combined model (Eq. 3) for each of these individuals were then applied to the acceleration output from the other study day, thus deriving the predicted EEACT. The comparison between the predicted and measured EEACT from the room calorimeter would then yield the cross-validity of the models. Total EEACT during the study period used for modeling was 710.4 ± 96.7 kcal and 665.2 ± 92.1 kcal for measured and fitted (6.4 ± 2.3%, p = 0.014), respectively. Total EEACT during the cross-validation study day was 724.5 ± 108.3 kcal and 684.2 ± 104.2 kcal for measured and predicted (4.3 ± 4.8%, p = 0.140), respectively. The scatter plot for the measured versus predicted EEACT (Fig. 4) further illustrates that the model appears stable and is able to accurately reproduce total EEACT for the majority of these subjects.
FIG. 4.
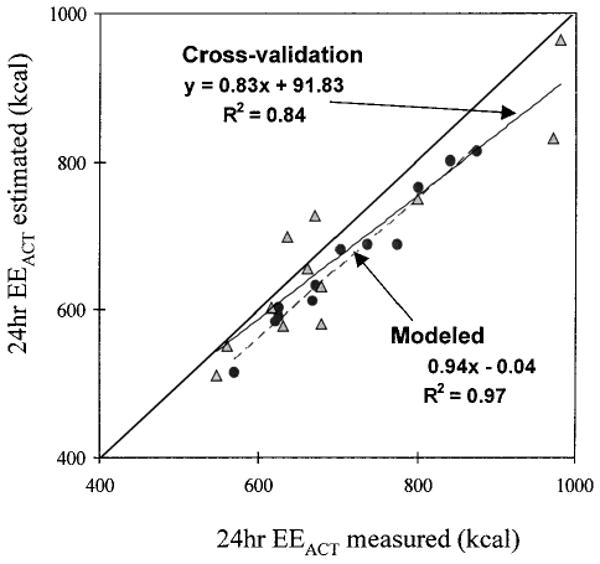
Estimated total EEACT from the modeled day (dotted with dashed trend line) and the cross-validation day (triangle with solid trend line), versus the measured EEACT in the subgroup (n = 12). The line of identity is also shown for theoretical ″perfect″ fit.
Discussion
The need for accurate assessment of physical activity and its associated EE under free-living conditions is underscored by the rising toll of chronic diseases, such as obesity, type II diabetes, and cardiovascular disease. While considerable evidence supports a relationship between physical inactivity and type II diabetes,5,6 the appropriate amount of physical activity needed to aid in the prevention or amelioration of this disease epidemic is somewhat speculative, largely because of the lack of accurate and validated methodology for measuring free-living physical activity and its associated EE. Portable accelerometers have been recognized as a reliable and objective technique for measuring physical activities under free-living conditions.18,19 However, their ability to estimate EEACT has been an area that needs great improvement. Results of this study have shown that EEACT could be accurately assessed using these noninvasive movement monitors.
Estimation of EE by accelerometers has been studied under laboratory and free-living conditions. In earlier studies in which uniaxial accelerometers were attached at different anatomic sites, vertical acceleration at the hip had the highest correlation coefficient with measured EE during common activities such as walking and stepping. 13,24,25 Subsequent studies have demonstrated improvements of the EE–acceleration associations with three-dimensional (triaxial) accelerometers in walking, running, and step exercise under laboratory conditions.26,27 The current prediction models adopted by the Tritrac-R3D use the linear regression approach and only the vector magnitude of counts from all three axes. The performance of this model was shown to be acceptable for level walking and jogging (40–70% VO2max on a treadmill) for a small group of young and fit individuals in one study,28 but both overestimations19,20,29,30 and underestimations17,31 have been reported in walking and other free-living physical activities. Previously, we also found a significant underestimation of total EEACT by 50–70% using the Tritrac-R3D linear regression model.15
Furthermore, we developed an approach to model EEACT using a hip-worn triaxial accelerometer (Tritrac-R3D) by separating horizontal and vertical components and using a nonlinear power model to associate acceleration and EEACT over the broad intensity range of daily physical activities. Both approaches significantly (p < 0.01) improved the estimation accuracy of EEACT.15,16 However, the Tritrac-R3D monitor was mainly sensitive to body movements at the center of mass, and thus could not adequately measure changes in EE due to physical activities performed by the upper body, which are a major part of many sedentary activities.
Swartz et al.32 reported a bivariate regression model that combined hip and wrist acceleration data (uniaxial) and significantly improved prediction of EE in free-living physical activities. In our current study, we added a wrist-worn uniaxial accelerometer to our previously tested hip-worn triaxial accelerometer model, and further advanced this modeling concept using nonlinear modeling. One major advantage of this type of nonlinear modeling was that if a better fitting could be achieved with a linear model, then the power parameters (p values in Eqs. 1–3) would then be equal or very close to 1. In all three models, all power parameters were significantly less than 1 (all p < 0.01). This was in agreement with our previous findings.15
By comparing the results from different models, the estimated EEACT using Tritrac-R3D (Eq. 1) was significantly better than using ActiWatch (Eq. 2), in terms of SEE and R (Table 2). This indicated stronger associations between EEACT and the acceleration components measured at or close to the center of body mass rather than at the wrist, logically reflecting higher energy costs due to weight-bearing movements. Movements from the arm can often be quick even with little force exertion, thus leading to the slight overestimation of EEACT (6.2 ± 4.7%, p = 0.121) during low-intensity activities (1–2.5 METs) by the ActiWatch model (Fig. 3). In contrast, the ActiWatch model significantly underestimated EEACT during activities of higher intensity (>4.0 METs) because of less upper body motion during walking and stepping. The Tritrac-R3D model in Eq. 1 slightly underestimated the lower-intensity physical activities (−4.3 ± 6.9% during 1–2.5 METs, p = 0.459), very likely because of the lack of signals picked up by the monitor during upper body movements, which was consistent with our previous study.15 By adding the ActiWatch, the combination model slightly improved SEE and R values, compared with the Tritrac-R3D model (Table 2). Although neither achieved statistical significance, this model was the only one that showed nondifferential estimation of EEACT during all non-sleeping physical activity intensity categories. As a result, total EEACT was predicted most accurately by the combined model (97.7 ± 3.2%), compared with ActiWatch (86.0 ± 4.7%) and Tritrac-R3D (90.0 ± 4.6%) models. The contributions to the total estimated EEACT by the combination model were 67 ± 23% and 33 ± 18% from the acceleration components from the Tritrac-R3D and the ActiWatch, respectively. In line with our hypothesis, these findings collectively suggest that the combination model used Tritrac-R3D data for most of the weight-bearing activities when EE intensities were relatively high, and used the ActiWatch data for upper body activities during more sedentary activities when the intensities were lower.
This study had some limitations. First, it only included healthy normal adult females (11 African Americans, two Asian American, and 47 Caucasians), which may limit the generalizability of these model parameters. Unlike our previous study, we did not derive the generalized parameters for our models, mainly because this was a fairly homogeneous population (middle-aged sedentary females) that lacked the spectrum of variations for generalization. However, the concept of using activity monitors to assess EEACT has been proven valid and feasible. Furthermore, we concentrated on a sedentary population with relatively high body mass index. This approach, however, allows us to establish a baseline for future studies on energy metabolism and physical activity in obesity and type II diabetes.
In conclusion, this investigation evaluated several EEACT prediction models using a hip-worn triaxial accelerometer (Tritrac-R3D) and a wrist-worn uniaxial accelerometer (ActiWatch) in a group of healthy women under close to free-living conditions in a whole-room indirect calorimeter. We found that a combined model using both monitors better estimated EEACT across all intensities compared with any single monitor model. In our study group, it has a 96.5% chance to detect total EEACT within ± 75 kcal, which may be clinically significant in exercise or diet prescription. This model was further validated for its predictive accuracy and stability in a subgroup. It is possible that the concept of developing predictive models for healthy individuals described in this study can be extended and validated in disease populations, such as type II diabetes. This may facilitate development of physical activity guidelines used as adjuvant therapy for the prevention and treatment of type II diabetes. Currently, we are using this approach to conduct a prospective study in assessing physical activity levels and EE in type II diabetic patients under various intensities of disease management protocols.
Acknowledgments
The authors wish to dedicate this paper to the memory of Mr. Jin Feng Chai for his assistance in data collection and technical expertise. This work was supported by grants RR00095, DK26657, DK02973, and DK46084 from the National Institutes of Health and grant DAMD17-02-1-0716 from the Department of Defense.
References
- 1.Blair SN, Kohl HW, Paffenbarger RS, Clark PG, Cooper CH, Gibbons LK. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 2.Paffenbarger RS, Hyde RT, Wing AL, Hsieh C. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 3.Ainsworth BE, Basset DR, Strath SJ, Swartz AM, O'Brien WL, Thompson RW, Jones DA, Macera CA, Kimsey CD. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc. 2000;32(Suppl):S457–S464. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 4.Paffenbarger RS, Blair S, Lee I, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kriska A. Physical activity and the prevention of type 2 diabetes mellitus. Sports Med. 2000;29:147–151. doi: 10.2165/00007256-200029030-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mullooly C. Cardiovascular fitness and type 2 diabetes. Curr Diabetes Rep. 2002;2:441–447. doi: 10.1007/s11892-002-0109-z. [DOI] [PubMed] [Google Scholar]
- 7.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man: methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyman MB, Fuss P, Young VR, Evans WJ, Roberts SB. Prediction of total energy expenditure using the Caltrac activity monitor [abstract] Int J Obesity. 1991;15(Suppl 1):23. [Google Scholar]
- 9.Shulz S, Westerterp KR, Bruck K. Comparison of energy expenditure by the doubly labeled water technique with energy intake, heart rate, and activity recording in man. Am J Clin Nutr. 1989;49:1146–1154. doi: 10.1093/ajcn/49.6.1146. [DOI] [PubMed] [Google Scholar]
- 10.Spurr GB, Prentice AM, Murgatroyd PR, Goldberg GR, Reina JC, Christman NT. Energy expenditure from minute-by-minute heart-rate recordings: comparison with indirect calorimetry. Am J Clin Nutr. 1988;48:552–559. doi: 10.1093/ajcn/48.3.552. [DOI] [PubMed] [Google Scholar]
- 11.Sun M, Reed GW, Hill JO. Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure. J Appl Physiol. 1994;76:2686–2691. doi: 10.1152/jappl.1994.76.6.2686. [DOI] [PubMed] [Google Scholar]
- 12.Jéquier E. Direct and indirect calorimetry in man. In: Garow JS, Halliday D, editors. Substrate and Energy Metabolism. London: J. Libbey; 1985. pp. 82–92. [Google Scholar]
- 13.Bouten CV, Westerterp KR, Verduin M, Janssen JD. Assessment of energy expenditure for physical activity using a triaxial accelerometer. Med Sci Sports Exerc. 1994;12:1516–1523. [PubMed] [Google Scholar]
- 14.Bouten CV, Verboeket-van de Venne WP, Westerterp KR, Verduin M, Janssen JD. Physical activity assessment: comparison between movement registration and doubly labeled water. J Appl Physiol. 1996;81:1019–1026. doi: 10.1152/jappl.1996.81.2.1019. [DOI] [PubMed] [Google Scholar]
- 15.Chen KY, Sun M. Improving energy expenditure estimation by using a triaxial accelerometer. J Appl Physiol. 1997;83:2112–2122. doi: 10.1152/jappl.1997.83.6.2112. [DOI] [PubMed] [Google Scholar]
- 16.Chen KY, Sun M, Butler MG, Thompson T, Carlson MG. Development and validation of a measurement system for assessment of energy expenditure and physical activity in Prader-Willi syndrome. Obes Res. 1999;7:387–394. doi: 10.1002/j.1550-8528.1999.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakicic JM, Winters C, Lagally K, Ho J, Robertson RJ, Wing RR. The accuracy of the Tritrac-R3D accelerometer to estimate energy expenditure. Med Sci Sports Exerc. 1999;31:747–754. doi: 10.1097/00005768-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Westerterp KR. Assessment of physical activity level in relation to obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31(Suppl):S522–S525. doi: 10.1097/00005768-199911001-00006. [DOI] [PubMed] [Google Scholar]
- 19.Campbell CL, Crocker PRE, McKenzie DC. Field evaluation of energy expenditure in women using Tritrac accelerometers. Med Sci Sports Exerc. 2002;34:1667–1674. doi: 10.1097/00005768-200210000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Leenders NYJM, Nelson TE, Sherman WM. Ability of different physical activity monitors to detect movement during treadmill walking. Int J Sports Med. 2003;24:43–50. doi: 10.1055/s-2003-37196. [DOI] [PubMed] [Google Scholar]
- 21.Webb P. 24-hour energy expenditure and the menstrual cycle. Am J Clin Nutr. 1986;44:616–619. doi: 10.1093/ajcn/44.5.614. [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Hill JO. A method for measuring mechanical work and work efficiency during human activities. J Biomech. 1993;26:229–241. doi: 10.1016/0021-9290(93)90361-h. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 24.Haymes EM, Byrnes WC. Walking and running energy expenditure estimated by Caltrac and indirect calorimetry. Med Sci Sports Exerc. 1993;25:1365–1369. [PubMed] [Google Scholar]
- 25.Wong TC, Webster JG, Montoye HJ, Washburn R. Portable accelerometer device for measuring human energy expenditure. IEEE Trans Biomed Eng. 1981;28:467–471. doi: 10.1109/TBME.1981.324820. [DOI] [PubMed] [Google Scholar]
- 26.Ayen TG, Montoye HJ. Estimation of energy expenditure with a simulated three-dimensional accelerometer. J Ambulatory Monit. 1988;1:293–301. [Google Scholar]
- 27.Meijer GA, Westerterp KR, Verhoeven MH, Koper BM, Hoor F. Methods to assess physical activity with special reference to motion sensors and accelerometers. IEEE Trans Biomed Eng. 1991;38:221–228. doi: 10.1109/10.133202. [DOI] [PubMed] [Google Scholar]
- 28.Sherman WM, Morris DM, Kirby TE, Petosa RA, Smith BA, Frid DJ, Leenders N. Evaluation of a commercial accelerometer (Tritrac-R3D) to measure energy expenditure during ambulation. Int J Sports Med. 1998;19:43–47. doi: 10.1055/s-2007-971878. [DOI] [PubMed] [Google Scholar]
- 29.Nichols JF, Morgan CG, Sarkin JA, Sallis JF, Calfas KJ. Validity, reliability, and calibration of the Tritrac accelerometer as a measure of physical activity. Med Sci Sports Exerc. 1999;31:908–912. doi: 10.1097/00005768-199906000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Welk GJ, Blair SN, Wood K, Jones S, Thompson RW. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc. 2000;32(Suppl):S489–S497. doi: 10.1097/00005768-200009001-00008. [DOI] [PubMed] [Google Scholar]
- 31.Bassett DR, Ainsworth BE, Swartz AM, Strath SJ, O'Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32(Suppl):S471–S480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 32.Swartz AM, Strath SJ, Bassett DR, O'Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(Suppl):S450–S456. doi: 10.1097/00005768-200009001-00003. [DOI] [PubMed] [Google Scholar]


