Abstract
The persistence of seemingly maladaptive genes in organisms challenges evolutionary biological thought. In Xiphophorus fishes, certain melanin patterns form malignant melanomas due to a cancer-causing gene (Xiphophorus melanoma receptor kinase; Xmrk), which arose several millions years ago from unequal meiotic recombination. Xiphophorus melanomas are male biased and induced by androgens however male behavior and Xmrk genotype has not been investigated. This study found that male X. cortezi with the spotted caudal (Sc) pattern, from which melanomas originate, displayed increased aggression in mirror image trials. Furthermore, Xmrk males (regardless of Sc phenotype) bit and performed more agonistic displays than Xmrk deficient males. Male aggressive response decreased when males viewed their Sc image as compared to their non-Sc image. Collectively, these results indicate that Xmrk males experience a competitive advantage over wild-type males and that intrasexual selection could be an important component in the evolutionary maintenance of this oncogene within Xiphophorus.
Keywords: sexual selection, Xiphophorus, Xmrk, melanoma, aggression, behavior, genetic linkage
Introduction
Initial investigations of cancer focused on the proximate causes of the disease (e.g., progression, susceptibility); however, more recent studies have addressed the ultimate causes behind the persistence of cancer (for discussion see Greaves, 2007). This more evolutionary approach has led to our understanding that the maintenance of genes with oncogenetic potential can result from factors such as genomic conflict (‘selfish’ genes: Summers et al., 2002; Kleene, 2005), antagonistic coevolution (maternal-fetal interactions: Lala et al., 2002; Summers & Crespi, 2005), antagonistic pleiotropy (Summers & Crespi, 2008), and sexual selection (Fernandez & Morris, 2008); establishing evolutionary trade-offs as a central theme underlying the evolutionary biology of cancer (Graham, 1992; Greaves, 2000; Crespi & Summers, 2006). For example, the tumor suppression gene TP53 is not only effective at suppressing cancer, but also stem cells that replenish worn out tissues (i.e., antagonistic pleiotropy; for review Rodier et al., 2007). Thus, expression of TP53 represents a trade-off because it functions to decrease tumor susceptibility but also accelerates senescence (Tyner et al., 2002; Weinstein & Ciszek, 2002; Campisi, 2005; Krtolica, 2005).
Cancer-causing genes (oncogenes) that are encoded on the sex chromosomes pass directly from parent to offspring through the germ line. Such oncogenes can persist across many generations and therefore should be influenced by selection. In the Xiphophorus melanoma model, a germline oncogene (Xiphophorus melanoma receptor kinase, Xmrk) that originated from a tandem gene duplication of the Xiphophorus epidermal growth factor receptor (Egfr) gene has remained functional despite being deleterious (Schartl et al., 1995; Weis & Schartl, 1998) and located in an extremely unstable genomic region (Volff et al., 2003). The event that led to the creation of Xmrk is believed to predate the divergence of Xiphophorus fishes (i.e., platyfishes and swordtails) with Xmrk being subsequently lost in numerous species (Kazianis & Borowsky, 1995; Weis & Schartl, 1998). Xmrk is the predominant gene underlying disease progression and melanomas do not occur if this gene is disrupted (Schartl et al., 1999). Although the initial discovery of Xiphophorus melanoma susceptibility was induced through hybrid crosses (Gordon, 1927; Kosswig, 1928), it is now clear that Xiphophorus fishes are susceptible to spontaneous melanomas in the absence of hybridization (Kallman, 1971; Borowsky, 1973; Fernandez & Bowser, 2008). In both hybrid and non-hybrid melanomas, the pathway leading to transformed phenotype results from the overexpression of the Xmrk oncogene within the Ras/Raf/MAPK signaling cascade with subsequent tumors originating from species-specific macromelanophore patterns (Schartl et al., 1995; Meierjohann & Schartl, 2006). Such malignancies are costly due to the invasion of underlying muscle tissue, which ultimately impairs swimming ability (Schartl et al., 1995;Fernandez & Bowser, 2008). Given this, explaining how the Xmrk oncogene has been passed down through the germline for million of years has remained a considerable challenge for evolutionary biologists (Meierjohann & Schartl, 2006).
In addition to being a model organism in which to study cancer, work on Xiphophorus fishes has been instrumental in developing several concepts of sexual selection. These include the evolution of female mate choice (Basolo, 1990, 1995), genetic determinants of size at sexual maturity and sex determination (Kallman et al., 1973), as well as alternative reproductive strategies (Ryan & Causey, 1989). Xiphophorus (Poeciliidae, Cyprinodontiformes) is a morphologically diverse group of fishes that uses visual cues to not only compete for but also select potential mates (body size: Ryan et al., 1990; Morris et al., 1992; Fernandez et al., 2008; sword size: Basolo, 1990; Benson & Basolo, 2006). Several behavioral studies have highlighted the specific importance of melanin patterns in sexual selection (Morris, 1998; Basolo & Trainor, 2002; Morris et al., 2003; Moretz & Morris, 2006) and found that the same phenotype can play a role in both mate choice and male-male competition. For example, the vertical body bars in numerous species are attractive to females and males can vary the expression of their vertical bars to deter rival males during agonistic encounters (Morris et al., 1995). However, the vast majority of Xiphophorus behavioral studies have focused on melanin patterns comprised of micromelanophores, which are smaller (up to 100 μM) and relatively more evenly spaced pigment cells than the larger (300–500 μM) overlapping macromelanophores that can be associated with the Xmrk oncogene (Weis & Schartl, 1998). This dearth of investigation is surprising given that the preservation of Xmrk has only occurred in those Xiphophorus that possess macromelanophore patterns (M patterns) suggesting these M patterns may play a key role in the evolutionary maintenance of Xmrk (Meierjohann & Schartl, 2006; Fernandez & Morris, 2008).
Recently, positive selection was demonstrated for the Xmrk oncogene in Xiphophorus cortezi providing the first empirical evidence that sexual selection plays a role in the continued maintenance of this deleterious gene. Fernandez and Morris (2008) found that females prefer males with the spotted caudal (Sc) M pattern that is associated with the presence of the Xmrk genotype to wildtype males without the Sc phenotype (and Xmrk genotype). In X. cortezi, melanomas are male biased and their incidence is greatest in males 9–12 months old (Schartl et al., 1995) and, therefore, Xmrk has the potential to substantially decrease the reproductive lifespan of males (Fernandez & Morris, 2008). Whether or not the increased attractiveness of Sc patterned males or the possible employment of different mating strategies (e.g., terminal effort signaling; Polak & Starmer, 1998; Hunt et al., 2004) compensates for the potentially reduced reproductive lifespan of Xmrk males remains to be elucidated. Similarly, male aggression and the Xmrk oncogene has not been explored in Xiphophorus despite the fact that Schartl and colleagues (1995) state anecdotally that melanomas in X. cortezi occur most frequently in ‘sexually active males of high social rank’. This suggests that there may be a relationship between dominance hierarchy (i.e., male-male competition) and the Xmrk oncogene.
The primary goal of this study was to determine if male aggression in the Northern swordtail X. cortezi is correlated with the presence of the Sc M pattern or the oncogene Xmrk. Xiphophorus cortezi is polymorphic for the Xmrk oncogene, which is located on the X and/or Y chromosomes. Xmrk is an essential component for the phenotypic expression of Sc (Schartl et al., 1995; Weis & Schartl, 1998). Unlike Xmrk, the precise genomic location of Sc is not known although it is hypothesized to be autosomally determined based upon breeding experiments (Kallman, 1971). Sc is an extremely asymmetrical pattern that typically consists of one or more irregular elongations that commence at the base of the caudal fin and extend roughly one-third of the length of the caudal fin (Fig. 1; Kallman, 1971; Schartl et al., 1995). All individuals with the Sc pattern have Xmrk (Schartl et al., 1995; Weis & Schartl, 1998); however, this phenotype has incomplete penetrance (Kallman, 1971). Therefore, it is feasible that an individual who lacks phenotypic expression of Sc but has the Sc genotype can possess the associated Xmrk genotype. This study will use molecular biology techniques and male mirror image stimulation tests to specifically address the following questions: 1) Can individuals without the phenotypic expression of Sc possess the Xmrk genotype? 2) Are males with the naturally occurring Sc pattern more aggressive than wild type males who lack the Sc phenotype but have it artificially applied? 3) Are males with the Xmrk genotype more aggressive than wild type males without the Xmrk genotype? and 4) Does male aggressive response differ based upon Sc phenotype?
Figure 1.
These X. cortezi males (Panels A–D) were all collected on the same day from the Conchita collection site (San Luís Potosí, Mexico). This amount of variation in pattern size and saturation is typical across X. cortezi populations. Scale bar: 5 mm.
Methods
Specimen collection and housing
Xiphophorus cortezi males used in this study were collected from five natural populations within the Rio Panuco basin: Arroyo Tanute N 21 39 123, W 99 02 127; Arroyo Chalpuhuacanita N 21 12 364, W 98 40 153; Rio San Martin N 21 22 173, W 98 39 543; Arroyo Tecolutlo N 21 07 270, W 98 28 075; and Arroyo Conchita N 21 33 5, W 98 59 320 (Hidalgo and San Luis Potosi provinces; Mexico). All males were collected as adults during two field seasons: December 2005 (Mean ± σ, Conchita: SL= 39.3 ± 3.78 mm, n = 30; Tanute: SL = 33.87 ± 4.71 mm, n = 15) and April 2006 (Mean ± σ, Chalpuhuacanita: SL = 38.7 ± 3.9 mm, n = 23; San Martin: SL = 38.08 ± 3.81 mm, n = 26; Tecolutlo: SL = 39.76 ± 3.02 mm, n = 13). Standard length (SL) was defined as the distance from the tip of the snout to the base of the caudal peduncle. In the laboratory, males from each population were housed in communal tanks with females from the same locality. All fish were maintained under standard laboratory conditions throughout the experiment consisting of 12L:12D cycle, daily feeding (Tetramin® flakes), and a constant temperature of 22° C (±1° C).
DNA isolation, PCR, and Xmrk genotyping
Males were anesthetized with tricane methanesulphonate (MS-222) and fin clipped after their last MIS test (see below) and DNA was extracted using DNeasy® tissue kit (Qiagen Inc.) following the manufacturer’s instructions. Total elution volume was 100 μl. The presence of Xmrk was determined by cross-referencing the polymerase chain reaction (PCR) products of two newly developed primers sets. These primers were designed from published Xiphophorus montezumae sequences in GenBank (Accession #s AY298857, AY298858). The published sequences in Genbank are derived from Xmrk specific clones (Volff et al., 2003), however there are regions of these sequences that are shared by both the Xmrk oncogene and the proto-oncogene (Efgr ortholog). The following primer set was used to screen for the presence of the Xmrk genotype: “Montoncoup” sense primer 5′-GGGTCATAAATCACTCATCCATC located in the promoter region at nt 21–43 (nt numbering according to AY298858; Volff et al., 2003) and “Dwnmont2” antisense primer 5′-ACAAGTTTGTGGAAATAAACCTGAACTC located in Intron 1 at nt 688–715 (nt numbering according to AY298858; Volff et al., 2003). Because the Montoncoup primer corresponds to a region that is specific to the Xmrk oncogene, this primer set amplifies a single ~ 700 bp fragment if the individual male has the Xmrk oncogene (Xmrk deficient = no band). For the amplification of oncogene and proto-oncogene products, the following primers were developed: “Montoncoup5” sense primer 5′-GATGTTACTTTAGTTCTGGAGTC located at nt 2956–2978 (nt numbering according to AY298857; Volff et al., 2003) and “Montoncodwn1” the antisense primer 5′-TCAGTTTGTTGGATCAGAGATG located at nt 266–287 (nt numbering according to AY298858; Volff et al., 2003). The Montoncoup5 primer corresponds to a sequence found in both the oncogene and protooncogene, therefore the second primer set (Montoncoup5/Montoncodwn1) produces bands for both the proto-oncogene and the oncogene. The use of this second primer set enabled 1) verification of the presence of amplifiable DNA 2) validation of the findings of the first PCR screening (i.e., Montoncoup/Dwnmont2). The final concentration of the primers was 100 nM.
The total reaction volume of all PCR amplifications was 10 μl. 1 μl of DNA template was used per reaction. PCR amplification was done under different conditions for each primer set used. For the Montoncoup/Dwnmont2 primer set, initial denaturation was at 94 °C for 3 min, then 29 cycles of denaturation at 95 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 45 s, followed by a final extension at 72 °C for 5 min. For the Montoncoup5/Montoncodwn1 primer set, initial denaturation was at 94 °C for 3 min, then 29 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s, and extension at 72 °C for 75 s, followed by a final extension at 72 °C for 5 min. A 5 μl aliquot of the amplification products were fractionated by electrophoresis on an 1.0% agarose gel in 1X TAE (48 mM Tris-acetate, 1 mM EDTA) buffer and visualized after staining with ethidium bromide (0.5μg/ml TAE) and UV transillumination. The molecular marker used was Promega 1 KB (Madison, WI). The gel image was taken with a Gel Logic100 system (Kodak). A total of five DNA samples could not be amplified and these males were removed from all analyses involving male Xmrk genotype (Table 1). The penetrance (complete/incomplete) of the Sc phenotype was determined by comparing the Sc phenotype of each individual male against his Xmrk genotype from these PCR screening results.
Table 1.
The frequency of the spotted caudal (Sc) phenotype and the Xmrk genotype in X. cortezi males from the five populations in this study. Note the increase in the frequency of Xmrk males compared to Sc males in populations due to the incomplete penetrance of the Sc pattern.
| Population | Total | Sc males | Non-Sc males | Xmrk | No Xmrk |
|---|---|---|---|---|---|
| Chalpuhuacanita | 23 | 7 | 16 | 9 | 14 |
| Conchita | 30 | 10 | 20 | 23 | 7 |
| Tanute | 15 | 3 | 12 | 8a | 3a |
| Tecolutlo | 13 | 0 | 13 | 0b | 12b |
| San Martín | 26 | 0 | 26 | 1 | 25 |
Non-viable DNA for four males
Non-viable DNA for one male
Mirror image stimulation
Standard mirror image stimulation (MIS) tests were used to determine if the Sc phenotype (analysis 1) or Xmrk genotype (analysis 2) was correlated with male aggression and to investigate whether the Sc pattern is perceived as a signal in agonistic encounters (analysis 3). Male MIS tests were only conducted once for each treatment because the results of these behavioral assays have been shown to be highly repeatable in X. cortezi and numerous other Xiphophorus species (Franck et al., 1985; Moretz & Morris, 2003). Males with naturally occurring spotted caudal (Sc) were tested in19L experimental aquaria with a line drawn at one end of the tank delineating the 10 cm interaction zone. Each experimental tank had a small plastic plant placed outside of the interaction zone for refugia. Individual males were placed in the 19 L experimental tanks and visually isolated from one another. During this transfer, all males were anesthetized with tricane methanesulphonate (MS-222) in order to accurately measure them (SL) and collect information about the individual’s phenotype. Spotted caudal (Sc) pigment pattern and vertical body bars were scored, the latter of which has previously been shown to influence male aggression in X. cortezi (Moretz, 2005). All males were in isolation for a minimum of 2 days (Mean = 8.26; Min = 2, Max = 34) before the start of the test, which allowed the males to establish residency in the experimental tank (Moretz & Morris, 2003). The testing procedure consisted of placing a mirror at one end of an individual’s tank and recording the number of displays and bites directed at the mirror image over a five minute trial period. The mirror was placed thirty minutes after the painting manipulations (either mock water or dye). The five minute trial period began as soon as the individual entered the interaction zone and directly faced his image. Interaction time was recorded and defined as the time that an individual spent within the 10 cm interaction zone either displaying, biting, swimming back and forth in front of his image, or simply facing his mirror image. Two displays were recorded: lateral display, a lateral orientation of the body while quivering (Ryan & Causey, 1989); vertical headstand, head tilts downward until body is at ~45° angle with the substrate (Lyons & Morris, 2008). Both of these displays are common in X. cortezi male agonistic confrontations (Moretz & Morris, 2003). Finally, the number of bites directed at the mirror image was also recorded during the trial period.
It was necessary that the males collected in the field without naturally occurring Sc saw both a stimulus with the Sc pattern and a stimulus without the Sc pattern. Therefore, in one treatment, a temporary Sc pattern was applied to these males lacking the Sc phenotype using Dr. Naylor’s Blue-Kote antiseptic dye (H. W. Naylor Co., Inc., Morris, NY; Hoefler & Morris, 1999) to approximate the average size of the Sc pattern for that male’s population. In the case of Tecolutlo, in which all individuals collected lacked Sc (Table 1), an average sized Sc phenotype was painted based upon the other four populations. In trials where males were not to receive a dye painting of Sc, these males were painted with water to control for handling effects. Care was taken to ensure that handling time (~ 40 seconds) was consistent between treatments (painting vs. mock painting) and across individuals. Previous work has demonstrated that this technique neither harms the fishes nor alters their behavior (Hoefler & Morris, 1999) and that X. cortezi did not differ in their response to phenotypes painted with this dye and the natural phenotypes (Hoefler & Morris, 1999). The two tests conducted on males without the Sc phenotype, one test as ‘painted Sc’ phenotype and the other as ‘no Sc’ phenotype, were performed using the same methodology as performed on naturally occurring Sc males described above. Trials in which males without Sc were either painted or mock painted were conducted at least two weeks apart in order to reduce the influence of the first trial experience. Two weeks has been determined to be sufficient to reduce the effects of prior encounters (Moretz & Morris, 2003). Treatment order was randomized.
Statistical analyses
The possible influence of the male’s resident population on the four recorded aggression variables was assessed using Kruskal-Wallis ANOVAs. However, because this question concerns differences between populations and not between treatments, there were three data sets for this initial analysis only (natural Sc, painted Sc, and male response to Sc). In other words, analyses were conducted within treatment (phenotype) groups and not across them. For all three of these data sets, the four aggression metrics (i.e., interaction time, headstand displays, lateral displays, and bites) were analyzed in separate ANOVAs with population as a fixed factor. Because in the male response to Sc comparison (analysis 3) each male was tested twice and Kruskal-Wallis ANOVAs are univariate, I used the difference in the numbers of displays/bites performed in the natural state (No Sc) versus the painted Sc state as the dependent variable for the Kruskal-Wallis ANOVAs.
Analysis 1: Aggression and the Sc phenotype
In the assessment of male aggression using mirror image techniques (analyses 1 and 2), it is critical to ensure that males are given the same stimulus. Therefore, in the two analyses of male aggression, males always saw an Sc patterned male when looking at the mirror (either naturally occurring Sc or artificially applied Sc) and their differences in aggression to that stimulus could be quantified. To determine if there was a correlation between the natural expression of the Sc phenotype and male aggression, the results of the mirror image trials for naturally occurring Sc males were compared against the results of painted Sc males (males that did not have natural Sc expression). Nonparametric Mann Whitney U tests were used to compare the mean aggression levels of naturally occurring Sc males to males without the Sc phenotype.
Analysis 2: Aggression and the Xmrk genotype
Because the Sc pattern has incomplete penetrance (Kallman, 1971), it is possible for the painted Sc males in analysis 1, which lacked natural Sc expression, to have the Xmrk genotype (see Table 1). Given a goal of this study was to determine if there was a correlation between the Xmrk oncogene and male aggression, these same data were also analyzed according to whether or not males had the Xmrk genotype (i.e., irrespective of whether they had a natural Sc phenotype or a painted Sc phenotype). Nonparametric Mann Whitney U tests were used to compare the mean aggression levels of males with the Xmrk genotype to wildtype (Xmrk deficient) males.
Analysis 3: Male response to the Sc phenotype
To determine if the Sc phenotype is a visual signal used in male agonistic encounters one must account for individual variations in male aggression. Therefore, X. cortezi males without naturally occurring Sc were tested twice using mirror image stimulation, once with a temporarily applied Sc pattern and a second time in their natural non-Sc state after receiving a mock water painting. Thus, individual males served as their own controls. Each male’s aggression towards their artificially painted Sc image and their natural non-Sc image were compared to determine if males alter their response when viewing an Sc patterned male. Wilcoxon signed ranks test were calculated to determine if there is a difference in the aggressive responses of males when viewing either their painted Sc image or their natural non-Sc image.
All statistical analyses were conducted using SPSS 16.0.1.
Results
Kruskal-Wallis ANOVAs found that there was no effect of population on any of the four dependent variables recorded for either the naturally occurring Sc males (headstands: X22 = 2.832, P = 0.243; lateral displays: X22 = 2.809, P = 0.245; bites: X22 = 1.49, P = 0.475; interaction time: X22 = 3.817, P = 0.148) or the painted Sc males (headstands: X22= 4.764, P = 0.092; lateral displays: X22 = 0.079, P = 0.961; bites: X22= 1.177, P = 0.555; interaction time: X22 = 0.562, P = 0.755). In the response to Sc comparison, males from different populations again did not differ in their aggressive responses for all four variables measured (headstands: X24 = 3.799, P = 0.434; lateral displays: X24 = 0.769, P = 0.943; bites: X24= 7.961, P = 0.093; interaction time: X24= 4.873, P = 0.301). Thus, the amount of aggression for each treatment was consistent across populations. Because male aggression did not vary across the five populations sampled, the data were pooled for each of the four variables in analyses 1, 2, and 3.
Penetrance of the Sc phenotype
X. cortezi males without phenotypic expression of Sc can have the associated Xmrk oncogene (i.e., incomplete penetrance; Table 1). Incomplete penetrance of Sc was observed in four of the five populations and therefore appears to be widespread within the geographic distribution of X. cortezi.
Analysis 1: Aggression and the Sc phenotype
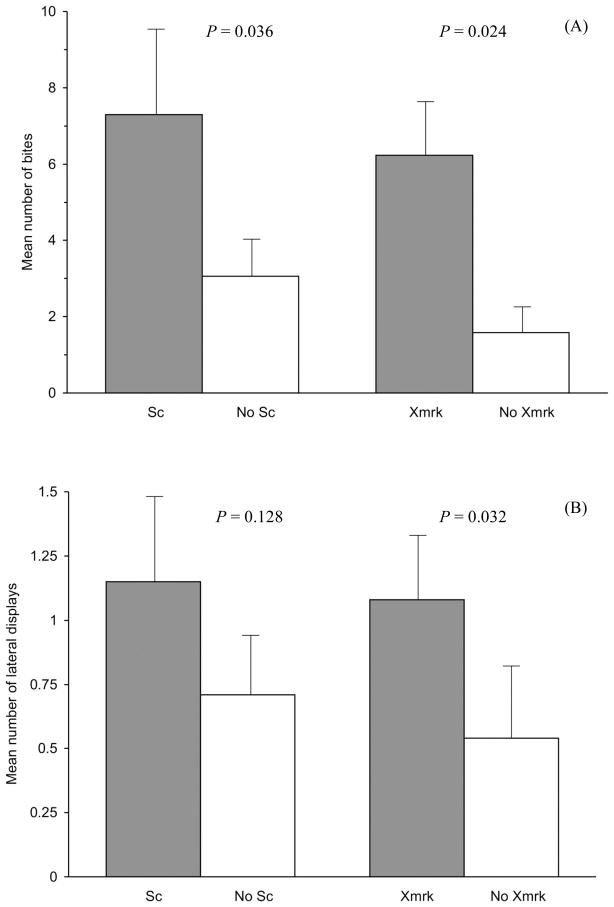
Naturally occurring Sc males spent significantly more time interacting with their mirror image than non-Sc males painted with the Sc pattern (Table 2; Z = −2.341, P = 0.019). Males with naturally occurring Sc also bite more at their image than painted Sc males (Fig. 2A; mean ± SE, natural Sc males = 7.3 ± 2.28, painted Sc males = 3.06 ± 1.03; Z = −2.095, P = 0.036). There was no difference in the number of displays performed by natural Sc males and painted Sc males (mean ± SE; headstand displays: natural Sc males = 1.2 ± 0.53, painted Sc males = 1.77 ± 0.49; Z = −0.242, P = 0.808; Fig. 2B, lateral displays: natural Sc males = 1.15 ± 0.37, painted Sc males = 0.71 ± 0.22; Z = −1.523, P = 0.128).
Table 2.
The amount of time males spent interacting with their mirror images in the MIS trials. Data are mean values ± SE.
| Analysis | Male condition | Interaction time (sec) | Probability |
|---|---|---|---|
| 1 | Natural Sc Painted Sc |
219.7±23.1 141.0±18.0 |
P = 0.019 |
| 2 |
Xmrk No Xmrk |
198.5±19.1 110.8±21.6 |
P = 0.002 |
| 3 | No Sc Painted Sc |
188.8±12.4 162.5±12.7 |
P = 0.077 |
Figure 2.
The number of bites (A) and lateral displays (B) performed by X. cortezi males during the five minute MIS trials. The results of analysis 1 (aggression and Sc phenotype) are presented on the left side of each bar graph (Sc/no Sc) and the results of analysis 2 (aggression and the Xmrk genotype) are presented on the right side of each graphic (Xmrk/no Xmrk). As a reminder in both analyses 1 and 2, males were responding to the same stimulus, their mirror image with the Sc phenotype (i.e., no Sc males had the Sc phenotype painted on). Bars represent the mean ± SE.
Analysis 2: Aggression and the Xmrk genotype
Xmrk males spent more time interacting with their image than wildtype (i.e., Xmrk deficient) males (Table 2; Z = −3.06, P = 0.002). Males with the Xmrk genotype also bit more (Fig. 2A; mean ± SE, Xmrk males = 6.23 ± 1.59, wildtype males = 1.58 ± 0.74; Z = −2.264, P = 0.024) and performed more lateral displays (Fig. 2B ; mean ± SE, Xmrk males = 1.08 ± 0.26, wildtype males = 0.54 ± 0.31; Z = −2.146, P = 0.032) at their image than wildtype males. There was no difference in the number of headstands performed by Xmrk and wildtype males (mean ± SE, Xmrk males = 2.03 ± 0.52, wildtype males = 1.17 ± 0.61; Z = −1.351, P = 0.177). It is noteworthy that there was no significance difference in the aggression levels of Xmrk males with naturally occurring Sc and Xmrk males who did not naturally express Sc but were painted with Sc (Mann-Whitney U test: headstands: Z = −0.903, P = 0.367; lateral displays: Z = −0.362, P = 0.717; bites: Z = −0.887, P = 0.398; interaction time: Z = −0.746, P = 0.455). This suggests that the increased aggression of males with the Xmrk genotype is not due to the process responsible for the physical expression of Sc. The relationship between male aggression and Xmrk also cannot be explained by the presence or absence of vertical body bars in X. cortezi males, which has previously been shown to influence aggression (Moretz & Morris, 2003; Moretz, 2005). Males with vertical bars did interact more than barless males (Mann-Whitney U test: Z = −2.290, P = 0.022; data not shown) however there was no significant difference in the three aggression variables measured between the barred and barless morphs (Mann-Whitney U test: headstands: Z = −1.364, P = 0.172; lateral displays: Z = −0.147, P = 0.883; bites: Z = −1.820, P = 0.069). Furthermore, the Xmrk genotype and the vertical body phenotype were not correlated within individuals (Pearson correlation test: r64= −0.017, P = 0.894).
Analysis 3: Male response to the Sc phenotype
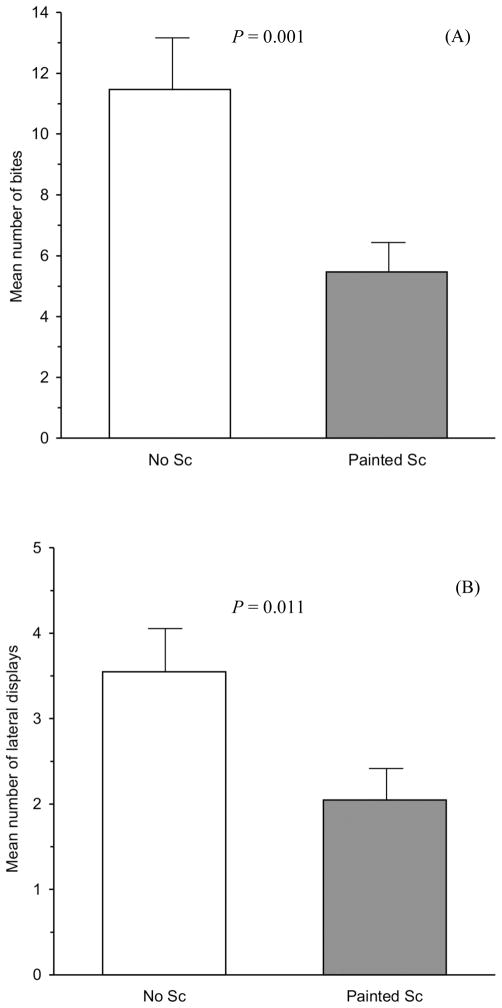
In the examination of male response to Sc, X. cortezi males bit more at their natural non-Sc image than at their painted Sc image (Fig. 3A; mean ± SE, non-Sc image = 11.46 ± 1.85, Sc image = 5.46 ± 1.04; Z = −3.368, P = 0.001). Males also performed more lateral displays towards their natural non-Sc image than their painted Sc image (Fig. 3B; mean ± SE, non-Sc image = 3.55 ± 0.54, Sc image = 2.05 ± 0.42; Z = −2.544, P = 0.011). Male response to Sc was not different for the number of headstand displays performed (mean ± SE, Sc image = 3.18 ± 0.54, non-Sc image = 2.48 ± 0.47; Z = −1.080, P = 0.28) or interaction time (Table 2; Z = −1.767, P = 0.077).
Figure 3.
The number of bites (A) and lateral displays (B) performed by X. cortezi males towards either their non-Sc image (white bars) or their painted Sc image (grey bars) during the five minute MIS trials. Bars represent the mean ± SE.
Discussion
This study found that X. cortezi males with naturally occurring Sc bit more and performed more agonistic displays at their image than non-Sc males painted with the Sc pattern. Furthermore, Xmrk males, regardless of whether they naturally expressed the Sc pattern, were also more aggressive than Xmrk deficient males. This result suggests that genetic factors linked to the Xmrk oncogene on the sex chromosomes are underlying the observed male aggression (see below) and not the genomic region surrounding the autosomally encoded Sc phenotype. Differences in male aggressive response towards the Sc pattern indicate that the Sc phenotype does function as a signal in X. cortezi male agonistic encounters. X. cortezi males without naturally occurring Sc, regardless of their Xmrk genotype, performed significantly fewer lateral displays and bites when viewing their painted Sc mirror image as compared to their natural non-Sc image. This result implies that the Sc signal is sufficient to convey information about individual male aggression and that it likely plays a role in determining the outcome and duration of male encounters. Collectively, the findings of this study demonstrate that individual males with the Xmrk genotype experience a competitive advantage in male-male competition at the potential expense of a reduced reproductive lifespan due to melanoma formation. The evolutionary persistence of Xmrk oncogene within X. cortezi likely results from the benefits of increased male attractiveness (Fernandez & Morris, 2008) and aggression associated with genetic factors linked to Xmrk genotype outweighing the potential costs of decreased offspring viability due to genetic incompatibility (Schartl et al., 1998) and a decreased reproductive lifespan.
The Xmrk oncogene encodes for an epidermal growth factor receptor protein (Egfr paralog) that is unlikely to be responsible for the observed increased aggression in X. cortezi males with this genotype. However, the Xmrk oncogene is in close genomic proximity to a number of genes important in sexual selection (Froschauer et al., 2002), including but not limited to the master sex determining gene (SD), the pituitary locus that determines size at sexual maturation (P alleles; Kallman et al., 1973), and the red-yellow locus (RY; Kallman, 1975). For example, RY encodes for red, yellow, brown, and orange coloration on the body and fins of Xiphophorus, and female preferences have been documented for such color morphs (Basolo & Trainor, 2002; Franck et al., 2003). In addition, a recent study found this region also encodes a functional copy of Mc1r (melanocortin type 1 receptor; Selz et al., 2007), which promotes melanogenesis in skin melanocytes (Metz et al., 2006) and has elevated expression levels in Xiphophorus melanomas (Selz et al., 2007). The close proximity of these loci to the Xmrk oncogene combined with the decreased rate of recombination between sex chromosomes (Bergero & Charlesworth, 2008) provides an ideal environment for genetic hitch-hiking (Maynard Smith & Haigh, 1974). Although we known relatively little about melanocortins and their receptors in teleosts fish compared to mammals (Gantz & Fong, 2002; Metz et al., 2006), melanocortins do promote androgen production in the adrenal cortex in mammals (Gantz & Fong 2002) and it is believed that up to seven copies of Mcrs are found within the immediate vicinity of Xmrk (Froschauer et al., 2002). Therefore, melanocortin receptors in this region could underlie the increased aggression of males with the Xmrk genotype if linkage disequilibrium occurs between these loci. However, detailed genetic mapping of this region and population genetic studies are necessary in order to elucidate the precise genetic mechanism responsible for the increased aggressiveness of Xmrk males in this study.
The occurrence of melanomas early in the lifespan of X. cortezi males (~7–12 months; Kallman, 1971; Schartl et al., 1995) and later in the lifespan of females is consistent with the seminal theoretical work proposed by Williams (1957) on antagonistic pleiotropy and senescence. Williams (1957) argues that a gene conferring an advantage at one age and a disadvantage at another will depend not only on the magnitude of these effects but also the timing of these effects. Within Xiphophorus, females (unlike males) have indeterminate growth (Reznick & Miles, 1989) and therefore female fecundity and total reproductive potential increases with age (Williams, 1957). However, Williams argues that the potential male reproductive fitness declines with age once sexual maturity is reached in organisms in which males have determinate growth (Williams, 1957). Because of these life history characteristics, selection should act in different ways to maximize the total reproductive probability of each sex (Williams, 1957). The occurrence of melanomas early in females would be costly (more so than in males) and should be selected against. However, selection favors a strategy in males that maximizes mate acquisitions and direct benefits as soon as sexual maturity is reached because thereafter the risk of mortality increases and reproductive fitness declines (Williams, 1957). The mating advantages associated with Sc and Xmrk in X. cortezi males, via mate preferences (Fernandez & Morris, 2008) and male-male competition (this study), can therefore offset the occurrence of malignancies earlier in males as long as sexual maturity is reached. The prevalence of non-hybrid melanomas across species also conforms to the theoretical work proposed by Williams (1957). Under laboratory conditions, melanomas occur in approximately 42% of X. variatus but only occur after 18 months of age (Kazianis & Borowsky, 1995) when selection against senescence is relaxed. However, the frequency of melanomas in X. cortezi in the laboratory and in nature is less than 10 % (laboratory: Kallman, 1971; Schartl et al., 1995; nature: Fernandez & Morris, 2008) due, at least in part, to their occurrence earlier in adulthood.
The status-signaling hypothesis proposes that variation in trait morphology (e.g., coloration) reflects an individual’s ability to win agonistic contests (Rohwer, 1975, 1977). The use of ‘badges of status’ to reliably signal dominance in social arenas is well documented within the literature for a wide variety of taxa (primates: Gerald, 2001; Setchell & Wickings, 2005; birds: Rohwer, 1977; Møller, 1987; lizards: Thompson & Moore, 1991). Frequently, the expression of such traits directly covaries with testosterone levels (Rand, 1992; Sinervo et al., 2000; Setchell & Dixson, 2001; Cox et al., 2005), and it is not surprising that dominance status is often positively correlated with levels of aggression. The costs of producing and carrying such signals (Zahavi, 1975), as well as the potential direct costs associated with being dominant (Hannes et al., 1984; Creel, 2001; Castro et al., 2006), can maintain the honesty of these signals. These costs are then offset by an individual’s greater access to contested resources (food: Maclean & Metcalfe, 2001; mates: Morris et al., 1992; Rantala & Kortet, 2004). Because Sc is associated with increased aggression in X. cortezi males, and males differentially respond to this signal, the Sc phenotype might serve as a badge of status according to the status-signaling hypothesis. However, one important criterion for the status signaling hypothesis is that variation in a signal can accurately convey information about dominance, thereby allowing subordinate individuals to use this signal to avoid potentially costly agonistic encounters (Rohwer, 1982; Saner & Camerino, 1998). Although it is clear that Sc varies dramatically in size not only within but also between populations (Fig. 1; Kallman, 1971), the relationship between the extent of Sc expression and dominance and/or aggression has not been formally established. The size of Sc (and other M patterns within Xiphophorus) is determined by the expression levels of Xmrk (Weis & Schartl, 1998; Meierjohann & Schartl, 2006) and M patterns do increase in size after exposing Xmrk individuals to elevated levels of testosterone (Schartl et al., 1981; Schartl et al., 1982; Schartl et al., 1995). Lastly, although aggression is some taxa is not correlated with testosterone levels (Steklis et al., 1985; Silverin et al., 2004), Hannes (1986) demonstrated in the swordtail X. helleri that the number of bites and sigmoid threat displays in male agonistic encounters were positively correlated with the testosterone concentrations in blood and whole body tissues. Therefore, it is not unreasonable to suggest a positive relationship exists between the increased aggression levels of Sc and/or Xmrk males detected in this study and the degree of Sc expression; however, future hormonal research needs to verify this in order to confirm the Sc M pattern is a badge of status in X. cortezi.
The use of male mirror image stimulation (as opposed to dyadic contests) to assess dominance has received some criticism because the outcomes of these different behavioral assays are not always consistent (Meliska et al., 1980; Ruzzante, 1992; although see Holtby, 1992). For example, individual Betta splendens that differentially responded during mirror image tests became indistinguishable in their aggression measures when using dyadic contests (Meliska et al., 1980). However, recent behavioral research conducted on X. cortezi, which focused on the vertical bar phenotype, found that X. cortezi males are consistent in their aggression measures across dyadic contests and male mirror image stimulation (Moretz & Morris, 2003; Moretz, 2005). Most importantly for the implications of this study was the finding that the number of bites (an aggression metric used in the current study) was the best predictor of winners in X. cortezi dyadic contests (Moretz, 2005). Furthermore, male MIS is the only method suitable to assess individual aggression levels (Franck et al., 1985; Rowland, 1999). This is because MIS, unlike simultaneous male contests or dyads, provides instantaneous feedback that is not dependent on intermale behavioral interactions while controlling for potential confounds such as body size and coloration (Rowland, 1999). This methodology also permits experimental manipulations and sequential testing in order to assess behavioral responses to a signal of interest while concurrently controlling for individual variation (e.g., male response to Sc in this study).
Xiphophorus has proven to be an ideal system in which to study sexual selection theory. In addition, this genus has served as the premiere animal model for studying the genetic basis to skin cancer for more than 80 years. The findings of this study indicate that merging these fields of scientific investigation can be very insightful and establishes Xiphophorus as an excellent system to study the evolutionary biology of cancer and life history trade-offs as they relate to the Xmrk genotype. Furthermore, bridging this gap is essential in order to further our understanding of the evolutionary relationship between the Xmrk genotype and the associated phenotype in this animal model.
Acknowledgments
I am grateful to the numerous people in the laboratories of Molly R. Morris and Soichi Tanda for their helpful suggestions in reviewing this manuscript and their continual support. I am thankful for the many useful comments on this manuscript provided by Lorie Fernandez and two anonymous reviewers. I would also like to thank Hiro Tanda, Katie Meadows, Lauren Toth, Jennifer Merzweiler, and Paul Crites for their assistant in collecting data. This research was funded by a National Institutes of Health NRSA pre-doctoral fellowship to A. A. Fernandez (GM077096-01), a National Science Foundation grant to Molly R. Morris (IBN-0316687), and an Ohio University Student Enhancement Award to A. A. Fernandez. All experiments comply with laws of the United States and with the Animal Care Guidelines of Ohio University (Animal Care and Use Approval L01-01).
References
- Basolo AL. Female preference for male sword length in the green swordtail, Xiphophorus helleri (Pisces: Poeciliidae) Anim Behav. 1990;40:332–338. [Google Scholar]
- Basolo AL. A further examination of a pre-existing bias favouring a sword in the genus Xiphophorus. Anim Behav. 1995;50:365–75. [Google Scholar]
- Basolo AL, Trainor BC. The conformation of a female preference for a composite male trait in green swordtails. Anim Behav. 2002;63:469–474. [Google Scholar]
- Benson KE, Basolo AL. Male-male competition and the sword in male swordtails, Xiphophorus helleri. Anim Behav. 2006;71:129–134. [Google Scholar]
- Bergero R, Charlesworth D. The evolution of restricted recombination in sex chromosomes. Trends Ecol Evol. 2008;24:94–102. doi: 10.1016/j.tree.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Borowsky R. Melanomas in Xiphophorus variatus (Pisces: Poeciliidae) in the absence of hybridization. Experientia. 1973;29:1431–1433. doi: 10.1007/BF01922860. [DOI] [PubMed] [Google Scholar]
- Campisi J. Aging, tumor suppression and cancer: high wire act! Mech. Ageing Dev. 2005;126:51–58. doi: 10.1016/j.mad.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Castro N, Ros AFH, Becker K, Oliveira RF. Metabolic costs of aggressive behaviour in the Siamese fighting fish, Betta splendens. Aggress Behav. 2006;32:474–480. [Google Scholar]
- Cox RM, Skelly SL, Leo A, John-Alder HB. Testosterone regulates sexually dimorphic coloration in the eastern fence lizard, Sceloporus undulatus. Copeia. 2005;3:597–608. [Google Scholar]
- Creel S. Social dominance and stress hormones. Trends Ecol Evol. 2001;16:491–497. [Google Scholar]
- Crespi B, Summers K. Positive selection in the evolution of cancer. Biol Rev. 2006;81:407–424. doi: 10.1017/S1464793106007056. [DOI] [PubMed] [Google Scholar]
- Fernandez AA, Morris MR. Mate choice for more melanin as a mechanism to maintain a functional oncogene. Proc Natl Acad Sci USA. 2008;105:13503–13507. doi: 10.1073/pnas.0803851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AA, Fernandez LR, Toth L. Head over heels: An examination of a possible mating signal in female swordtails, Xiphophorus cortezi. Anim Behav. 2008;76:1073–1081. doi: 10.1016/j.anbehav.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck D, Hannes RP, Lanffermann H, Ribowski A. Effects of social isolation on aggressiveness in fish with special reference to the swordtail (Xiphophorus helleri) Behav Processes. 1985;10:415–427. doi: 10.1016/0376-6357(85)90041-5. [DOI] [PubMed] [Google Scholar]
- Franck D, Muller A, Rogmann N. A colour and size dimorphism in the green swordtail (population Jalapa): female mate choice, male-male competition, and male mating strategies. Acta ethol. 2003;5:75–79. [Google Scholar]
- Froschauer A, Korting C, Katagiri T, Aoki T, Asakawa S, Shimizu N, Schartl M, Volff JN. Construction and initial analysis of bacterial artificial chromosome (BAC) contigs from the sex-determining region of the platyfish Xiphophorus maculatus. Gene. 2002;295:247–254. doi: 10.1016/s0378-1119(02)00684-4. [DOI] [PubMed] [Google Scholar]
- Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2002;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- Gerald MS. Primate colour predicts social status and aggressive outcome. Anim Behav. 2001;61:559–66. [Google Scholar]
- Graham J. Cancer selection: A new theory of evolution. Aculeus Press; Lexington, VA: 1992. [Google Scholar]
- Greaves M. Cancer: The evolutionary legacy. Oxford University Press; Oxford, U. K: 2000. [Google Scholar]
- Greaves M. Darwinian medicine: a case for cancer. Nat Rev Cancer. 2007;7:213–221. doi: 10.1038/nrc2071. [DOI] [PubMed] [Google Scholar]
- Gordon M. The genetics of viviparous top-minnow Platypoecilus: the inheritance of two kinds of melanophores. Genetics. 1927;12:253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannes RP. Blood and whole-body androgen levels of male swordtails correlated with aggression measures in a standard-opponent test. Aggress Behav. 1986;12:249–254. [Google Scholar]
- Hannes RP, Franck D, Liemann F. Effects of rank order fights on whole body and blood concentrations of androgens and corticosteroids in the male swordtail (Xiphophorus helleri) Z Tierpsychol. 1984;65:53–65. [Google Scholar]
- Hoefler CH, Morris MR. A technique for the temporary application and augmentation of pigment patterns in fish. Ethology. 1999;195:431–438. [Google Scholar]
- Holtby LB. Through a glass darkly: a response to Ruzzante’s reappraisal of mirror image stimulation studies. Can J Fish Aquat Sci. 1992;49:1968–1969. [Google Scholar]
- Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussiere LF. High-quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. [DOI] [PubMed] [Google Scholar]
- Kallman KD. Inheritance of melanophore patterns and sex determination in the Montezuma swordtail, Xiphophorus montezumae cortezi Rosen. Zoologica. 1971;56:77–94. [Google Scholar]
- Kallman KD, Schreibman MP, Borkoski V. Genetic control of gonadotrop differentiation in the platyfish, Xiphophorus maculatus (Poeciliidae) Science. 1973;181:678–680. doi: 10.1126/science.181.4100.678. [DOI] [PubMed] [Google Scholar]
- Kallman KD. The platyfish, Xiphophorus maculatus. In: King RC, editor. Handbook of Genetics. Plenum Press; New York, NY: 1975. pp. 81–132. [Google Scholar]
- Kazianis S, Borowsky R. Stable association of a pigmentation allele with an oncogene: nonhybrid melanomas in Xiphophorus variatus. J Hered. 1995;86:199–203. doi: 10.1093/oxfordjournals.jhered.a111562. [DOI] [PubMed] [Google Scholar]
- Kleene KC. Sexual selection, genetic conflict, selfish genes and the atypical patterns of gene expression in spermatogenetic cells. Dev Biol. 2005;277:16–26. doi: 10.1016/j.ydbio.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Kosswig C. Über bastarde der Teleostier Platyopoecilus und Xiphophorus. Z Indukt Abstamm Vererbungsl. 1928;47:150–158. [Google Scholar]
- Krtolica A. Stem cell: balancing ageing and cancer. Int J Biochem Cell Biol. 2005;37:935–941. doi: 10.1016/j.biocel.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lala PK, Lee BP, Xu G, Chakraborty C. Human placental trophoblast as an in vitro model for tumor progression. Can J Physiol Pharmacol. 2002;80:142–149. doi: 10.1139/y02-006. [DOI] [PubMed] [Google Scholar]
- Lyons S, Morris MR. Headstands: a sexually selected signal in the swordtail fish Xiphophorus nezahualcoyotl. Behaviour. 2008;145:1247–1262. [Google Scholar]
- Maclean A, Metcalfe NB. Social status, access to food, and compensatory growth in juvenile Atlantic salmon. J Fish Biol. 2001;58:1331–1346. [Google Scholar]
- Maynard Smith J, Haigh J. The hitch-hiking effect of a favourable gene. Genet Res. 1974;23:23–35. [PubMed] [Google Scholar]
- Meierjohann S, Schartl M. From Mendelian to molecular genetics: the Xiphophorus melanoma model. Trends Genet. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Meierjohann S, Schartl M, Volff JN. Genetic, biochemical and evolutionary facets of Xmrk-induced melanoma formation in the fish Xiphophorus. Comp Biochem Physiol. 2004;138:281–289. doi: 10.1016/j.cca.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Meliska JA, Peeke HVS. The relationship of mirror-elicited display to combat behaviors in Betta splendens. Behav Neural Biol. 1980;30:207–217. doi: 10.1016/s0163-1047(80)91089-4. [DOI] [PubMed] [Google Scholar]
- Metz JR, Peters JJM, Flik G. Molecular biology and physiology of the melanocortin system in fish: A review. Gen Comp Endocrinol. 2006;148:150–162. doi: 10.1016/j.ygcen.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Møller AP. Variation in badge size in male house sparrows (Passer domesticus): evidence for status signaling. Anim Behav. 1987;35:203–210. [Google Scholar]
- Moretz JA. Aggression and fighting ability are correlated in the swordtail fish Xiphophorus cortezi: the advantage of being barless. Behav Ecol Sociobiol. 2005;59:51–57. [Google Scholar]
- Moretz JA, Morris MR. Phylogenetic analysis of the evolution of a signal of aggressive intent in northern swordtail fishes. Am Nat. 2006;168:336–349. doi: 10.1086/506920. [DOI] [PubMed] [Google Scholar]
- Moretz JA, Morris MR. Evolutionarily labile responses to a signal of aggressive intent. Proc R Soc Lond, B, Biol Sci. 2003;270:2271–2277. doi: 10.1098/rspb.2003.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MR. Female preference for trait symmetry in addition to trait size in swordtail fishes. Proc R Soc Lond, B, Biol Sci. 1998;1399:907–911. [Google Scholar]
- Morris MR, Batra P, Ryan MJ. Male-male competition and access to females in the swordtail Xiphophorus nigrensis. Copeia. 1992;1992:980–986. [Google Scholar]
- Morris MR, Nicoletto PF, Hesselman E. A polymorphism in female preference for a polymorphic male trait in the swordtail fish Xiphophorus cortezi. Anim Behav. 2003;65:45–52. [Google Scholar]
- Morris MR, Mussel M, Ryan MJ. Vertical bars on male Xiphophorus multilineatus: a signal that deters rival males and attracts females. Behav Ecol. 1995;6:274–279. [Google Scholar]
- Polak M, Starmer WT. Parasite-induced risk of mortality elevates reproductive effort in male Drosophila. Proc R Soc Lond, B, Biol Sci. 1998;265:2197–2201. doi: 10.1098/rspb.1998.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MS. Hormonal control of polymorphic and sexually dimorphic coloration in the lizard Sceloporus undulatus erythrocheilus. Gen Comp Endocrinol. 1992;88:461–468. doi: 10.1016/0016-6480(92)90241-b. [DOI] [PubMed] [Google Scholar]
- Rantala MJ, Kortet R. Male dominance and immunocompetence in a field cricket. Behav Ecol. 2004;15:187–191. [Google Scholar]
- Reznick DN, Miles DB. A review of life history patterns in Poeciliid fishes. In: Meffe GK Jr, Snelson FF, editors. Ecology and Evolution of Livebearing Fishes (Poeciliidae) Prentice-Hall; Englewood Cliffs, NJ: 1989. pp. 125–148. [Google Scholar]
- Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer SA. The social significance of avian winter plumage variability. Evolution. 1975;29:593–610. doi: 10.1111/j.1558-5646.1975.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rohwer SA. Status signaling in Harris’ sparrows: some experiments in deception. Behaviour. 1977;61:107–129. [Google Scholar]
- Rohwer SA. The evolution of reliable and unreliable badges of fighting ability. Am Zool. 1982;22:531–546. [Google Scholar]
- Rowland WJ. Studying visual cues in fish behavior: a review of ethological techniques. Environ Biol Fishes. 1999;56:285–305. [Google Scholar]
- Ruzzante DE. Mirror image stimulation, social hierarchies and populational deifferences in agonsitic behaviour: a reappraisal. Can J Fish Aquat Sci. 1992;49:1966–1968. [Google Scholar]
- Ryan MJ, Causey BA. Alternative mating behavior in the swordtails Xiphophorus nigrensis and Xiphophorus pygmaeus (Pisces: Poeciliidae) Behav Ecol Sociobiol. 1989;24:341–348. [Google Scholar]
- Ryan MJ, Hews D, Wagner WE., Jr Sexual selection on alleles that determine body size in the swordtail Xiphophorus nigrensis. Behav Ecol Sociobiol. 1990;26:231–237. [Google Scholar]
- Schartl A, Schartl M, Anders F. Promotion and regression of neoplasia by testosterone-promoted cell differentiation in Xiphophorus and Girardinus. In: Hecker E, Fusenig NE, Kunz W, Marks F, Thielmann HW, editors. Carcinogenesis. Vol. 7. New Raven Press; New York, NY: 1982. pp. 427–434. [PubMed] [Google Scholar]
- Schartl M, Schartl A, Anders F. Phenotypic conversion of malignant melanoma to benign melanoma and vice versa in Xiphophorus. In: Seji M, editor. Phenotypic expression in pigment cells. University of Tokyo Press; Tokyo, Japan: 1981. pp. 507–514. [Google Scholar]
- Schartl A, Malitschek B, Kazianis S, Borowsky R, Schartl M. Spontaneous melanoma formation in nonhybrid Xiphophorus. Cancer Res. 1995;55:159–165. [PubMed] [Google Scholar]
- Schartl M, Wilde B, Hornung U. Triplet repeat variability in the signal peptide sequence of the Xmrk receptor tyrosine kinase gene in Xiphophorus fish. Gene. 1998;224:17–21. doi: 10.1016/s0378-1119(98)00520-4. [DOI] [PubMed] [Google Scholar]
- Schartl M, Hornung U, Gutbrod H, Volff JN, Wittbrodt J. Melanoma loss-of-function mutants in Xiphophorus caused by Xmrk-oncogene deletion and gene disruption by a transposable element. Genetics. 1999;153:1385–1394. doi: 10.1093/genetics/153.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selz Y, Braasch I, Hoffmann C, Schmidt C, Schultheis C, Schartl M, Volff JN. Evolution of melanocortin receptors in teleost fish: The melanocortin type 1 receptor. Gene. 2007;401:114–122. doi: 10.1016/j.gene.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Dixson AF. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Horm Behav. 2001;39:177–184. doi: 10.1006/hbeh.2000.1628. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Wickings EJ. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx) Ethology. 2005;111:25–50. [Google Scholar]
- Senar JC, Camerino M. Status signaling and the ability to recognize dominants: an experiment with siskins (Carduelis spinus) Proc R Soc Lond, B, Biol Sci. 1998;265:1515–1520. [Google Scholar]
- Steklis HD, Brammer GL, Raleigh MJ, McGuire MT. Serum testosterone, male dominance, and aggression in captive groups of vervet monkeys (Cercopithecus aethiops sabaeus) Horm Behav. 1985;19:154–163. doi: 10.1016/0018-506x(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45:225–234. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Miles DB, Frankino WA, Klukowski M, DeNardo DF. Testosterone, endurance, and Darwinian fitness: natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Horm Behav. 2000;38:222–233. doi: 10.1006/hbeh.2000.1622. [DOI] [PubMed] [Google Scholar]
- Summers K, da Silva J, Farwell MA. Intragenomic conflict and cancer. Med Hypotheses. 2002;59:170–179. doi: 10.1016/s0306-9877(02)00249-9. [DOI] [PubMed] [Google Scholar]
- Summers K, Crespi B. Cadherins in maternal-fetal interactions: red queen with a green beard? Proc R Soc Lond, B, Biol Sci. 2005;272:643–649. doi: 10.1098/rspb.2004.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K, Crespi B. The androgen receptor and prostate cancer: a role for sexual selection and sexual conflict? Med Hypotheses. 2008;70:435–443. doi: 10.1016/j.mehy.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park SH, Thompson T, Karsenty G, Bradley A, Donehower LA. P53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Thompson CW, Moore MC. Throat colour reliably signals status in male tree lizards Urosaurus ornatus. Anim Behav. 1991;42:745–754. [Google Scholar]
- Volff JN, Koerting C, Froschauer A, Zhou Q, Wilde B, Schultheis C, Selz Y, Sweeney K, Duschl J, Wichert K, Altschmied J, Schartl M. The Xmrk oncogene can escape nonfunctionalization in a highly unstable subtelomeric region of the genome of the fish Xiphophorus. Genomics. 2003;82:470–479. doi: 10.1016/s0888-7543(03)00168-x. [DOI] [PubMed] [Google Scholar]
- Weinstein BS, Ciszek D. The reserve-capacity hypothesis: evolutionary origins and modern implications of the trade-off between tumor-suppression and tissue-repair. Exp Gerontol. 2002;37:615–627. doi: 10.1016/s0531-5565(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Weis S, Schartl M. The macromelanophore locus and the melanoma oncogene Xmrk are separate genetic entities in the genome of Xiphophorus. Genetics. 1998;149:1909–1920. doi: 10.1093/genetics/149.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Zahavi A. Mate selection - a selection for a handicap. J Theor Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]