Abstract
Vascular smooth muscle cells (VSMC) are dynamic cells exposed to fluctuating concentrations of nutrients on a daily basis. Non-esterified fatty acids (NEFA) have been indicted as potential mediators of atherosclerosis and exaggerated VSMC remodeling observed in diabetes, and in vitro data supports a model of VSMC activation by NEFA. However, recent observations suggest that metabolic stressors such as oxidants and NEFA may also simultaneously induce cytoprotective events as part of a homeostatic “off switch”. Our group has established that the transcription factor, cAMP Response Element Binding protein (CREB), is important for maintenance of VSMC quiescence, differentiation, and survival. We therefore examined whether acute physiological NEFA exposure would regulate CREB in primary cultures of bovine aortic VSMC, and explored the relationship between signaling to the cytoprotective CREB and the activating MAPK pathways. In vitro exposure of VSMC to three classes of polyunsaturated NEFA leads to significant acute, transient, dose-dependent, and repeatedly inducible CREB activation. As expected ERK, P38 MAPK, Akt, JNK, and PKC pathways are also activated by NEFA. Using a battery of pharmacological inhibitors and antioxidants we demonstrate that CREB activation is mediated by a novel PKC isoform and is reactive oxygen species (ROS)-independent, while ERK activation, in contrast, is mediated by ROS and is PKC-independent. These data suggest parallel and mechanistically distinct stimulation of separate stabilizing and activating pathways in VSMC response to acute NEFA-mediated stress. Furthermore, the downregulation of CREB in models of chronic metabolic stress reported in the literature would be expected to disrupt this homeostasis and shift the balance towards VSMC activation, consistent with emerging models of atherosclerosis.
Keywords: CREB, atherosclerosis, signal transduction, fatty acids, reactive oxygen species, vascular smooth muscle cells
INTRODUCTION
Vascular smooth muscle cells (VSMC) are dynamic cells exposed to fluctuating concentrations of nutrients on a daily basis. These cells exhibit phenotypic modulation that permits active response to the local environment. While the switch from a highly differentiated quiescent, contractile phenotype to the “active”, proliferative, migratory phenotype is important in response to injurious stimuli, it also plays a key role in the pathology of atherosclerosis (1). Proliferation and migration of VSMC are important events in the formation of atherosclerotic plaque, while apoptosis plays a role in the plaque instability and rupture associated with acute coronary syndromes (2). Thus the choice between VSMC activation and differentiation must be carefully and constantly regulated. Non-esterified fatty acids (NEFA) have been indicted by epidemiological studies as potential mediators of the atherosclerosis and exaggerated VSMC remodeling observed in diabetes and metabolic syndrome (3). Multiple reports in the literature demonstrate that NEFA can induce VSMC proliferation, migration, and apoptosis in vitro (4-9). Increases in reactive oxygen species (ROS), activation of Protein Kinase C (PKC) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and increases in diacyl glycerol (DAG) levels have all been demonstrated and proposed to contribute to VSMC activation with fatty acid exposure. However, recent observations suggest that metabolic stressors such as oxidants and NEFA may also simultaneously induce antioxidant cytoprotective events as part of a homeostatic “off switch”.For instance, addition of NEFA to growth medium has been shown to protect endothelial cells from the cytotoxic effects of hydrogen peroxide-generated oxidative stress (10). Others have demonstrated similar protection of rat hepatocytes and found concurrent increases in manganese superoxide dismutase (MnSOD) (11). In general, induction of cytoprotective antioxidant pathways by NEFA or their oxidation products has been demonstrated in a variety of cell types, including VSMC (12-16). Furthermore, VSMC lacking MnSOD exhibit increased MAPK signaling and an exaggerated proliferative response to thrombin (17). Thus the net result of VSMC exposure to NEFA is likely to be a critically regulated balance between induction of separate activating (mitogenic, promigratory, and apoptotic) and cytoprotective (antioxidant, differentiating, and prosurvival) pathways.
Our group has demonstrated that the transcription factor, cAMP response element binding protein (CREB), is important for maintenance of VSMC quiescence and differentiation and for survival of many differentiated cell types, including VSMC (18-23). Furthermore, under conditions of chronic metabolic stress, such as hyperglycemia, cytokine exposure, hydrogen peroxide exposure, and hypoxia, CREB function is acutely increased (a presumed cytoprotective response) and then chronically downregulated (a presumed pathological response) in VSMC culture and in vivo (24-28). The response of VSMC CREB to NEFA exposure has not been investigated. We therefore examined whether NEFA would regulate CREB in primary culture bovine aortic VSMC (BoASMC) and explored the relationship between signaling to CREB versus activation of the MAPK pathways.
We report the novel observation that all major classes of unsaturated NEFA acutely and transiently stimulate (i.e. phosphorylate) CREB in VSMC. In contrast to the NEFA-mediated phosphorylation of ERK, an established mitogenic signal, CREB phosphorylation is independent of ROS/ERK and dependent upon PKC.
MATERIALS AND METHODS
Materials
fatty acid free bovine serum albumin (BSA) and all fatty acids are from Sigma (St Louis, MO). Antibodies to CREB, PCREB, P38 MAPK, pP38 MAPK, ERK, pERK, JNK, pJNK, Akt, and pAkt are from Cell Signaling (Danvers, MA). The PCREB ELISA assay was purchased from Invitrogen Biosource (Carlsbad, CA). Bisindolyl maleimide (BIS) is from Calbiochem (San Diego, CA), and N-acetyl cysteine is purchased from Sigma (St. Louis, MO). Other kinase inhibitors (H89, Go, HBD, and Rottlerin) were purchased from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, PA). Fetal bovine serum, glutamine, and penicillin/streptomycin were purchased from Gemini Bio Products, Inc (Sacramento, CA). Minimal Essential Eagle’s medium and all other reagents were purchased from Sigma (St. Louis, MO).
NEFA preparation
NEFA are prepared as 10mM stock solutions in PBS with 10% fatty acid free BSA. Unsaturated fatty acids are liquid at room temperature and are directly added to prewarmed PBS with 10% FA-free BSA. BSA is used as NEFA exist in vivo complexed with serum albumin. Solutions are prepared fresh and stored for up to one week at −20°C
VSMC culture
Experiments were performed in bovine aortic VSMC (BoASMC). These primary culture cells are prepared at this institution from fresh bovine aortic arches as previously described (29). Cultures are propagated and maintained in Modified Eagle’s medium (MEM) supplemented with 200 units/ml penicillin, 0.2 mg/ml streptomycin, and 10% fetal bovine serum (FBS) as described by this lab (25). For low serum conditions, cells were transferred to supplemented MEM with 0.1% FBS.
Inhibitor and NEFA treatment
For experiments, BoASMCs are seeded at 1.5-3×105 cells/well on 6-well plates and incubated overnight at 37°C under 5% CO2. Cells are then transferred to 2 mls/well MEM with 0.1% FBS and incubated for 48-72 hours to allow cells to become quiescent and differentiated. Inhibitors are added for 30 minute pretreatment prior to NEFA addition at the following concentrations: 1μM or 4μM BIS, 30μM HBDDE, 10μM Go6976, 10μM Rottlerin, 10μM H89, and 30mM NAc. NEFA, prepared at 10 mM in prewarmed PBS with 10%BSA, are then added at the indicated concentrations for the indicated times with or without additional BSA to bring the total concentration of BSA to 0.8 mg/dL (approximate physiological concentration in interstitial fluid (30)). At the appropriate times medium is removed and cells are washed twice with ice cold PBS and harvested by scraping in 100μl of 1x Laemmli sample buffer. Extracts are then sonicated briefly to decrease viscosity, boiled, spun, and stored at −20°C.
Western Blot Analysis
Equal protein samples are fractionated on 12% SDS-polyacrylamide gels. Proteins are electrophoretically transferred to nylon membranes and equivalence of protein loading is assessed by Ponseau stain. Membranes are blocked with TBSTween with 5% milk and probed overnight at 4°C with the appropriate protein-specific primary antisera in PBS with 5% BSA. Immunologically-identified proteins are recognized using alkaline phosphatase-conjugated species-specific IgG and CDP-Star Enhanced Chemiluminescence (New England Biolabs, Beverly, MA). Autoradiographic results are quanitified densitometrically using the fluor-S MultiImager and Quantity One software (Bio-Rad).
Statistical Analysis
Results are represented as mean ± SEM. The Graphpad PRISM software was used for statistical analysis. Multiple group comparisons were done by one way ANOVA followed by Dunnett’s post test to determine differences between individual time points and controls.
RESULTS
NEFA induce a dose-dependent increase in CREB phosphorylation
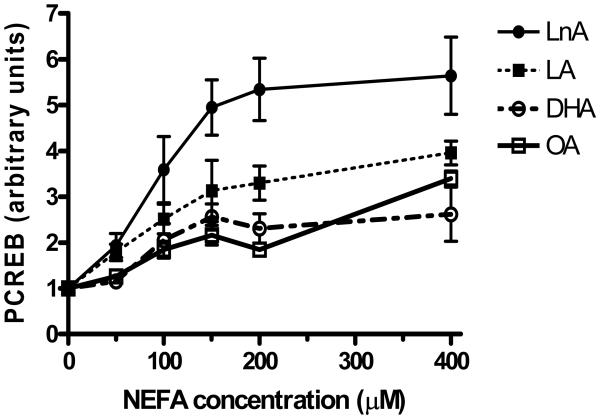
As NEFA elevation is one aspect of the metabolic stress of diabetes that has been shown to have cytotoxic and activating effects on VSMC, we investigated whether exposure of BoASMC to physiological levels of NEFA of different classes affected the phosphorylation state of CREB. Oleic (OA) and linoleic (LA) acids are the most studied and the most abundant monounsaturated fatty acid (MUFA) and omega-6 polyunsaturated fatty acid (PUFA), respectively (31). The most abundant plant and cold water fish n-3-PUFA are α-linolenic acid (LnA) and docosahexaenoic acid (DHA), respectively. Treatment of quiescent, serum-starved BoASMC with each of these NEFA, complexed with BSA to reproduce the physiological albumin complex, results in a rapid dose-dependent increase in CREB phosphorylation (Figure 1). The exact dose dependence varies with different NEFA and different NEFA batches, but LnA is generally the most potent activator of CREB. Results shown are in the presence of low concentrations of BSA contributed by the NEFA solutions only. Similar results are seen when additional BSA is added to bring the total albumin level to the lower end of presumed physiological levels in interstitial fluid of 0.8-1.0 mg/dL (about 20% of normal serum albumin (30)). However, at this high albumin level, basal CREB activation is increased, presumably by contaminating activating serum components, thus blunting the CREB response to NEFA (data not shown).
Figure 1. NEFA exhibit dose-dependent stimulation of CREB phoshorylation.
50% confluent BoASMC were transferred to DMEM/0.1% FBS for 48 hours and then exposed to varying concentrations of OA, LA, LnA, or DHA for 20 minutes. Extracts were prepared either in nondenaturing mammalian cell lysis buffer (MLB) with protease and phosphatase inhibitors for analysis by PCREB ELISA or in denaturing sample buffer for analysis by immunoblot with anti-PCREB antibodies. In all cases samples with equal total protein were subjected to analysis. Results are combined from 2-3 separate experiments and plotted as a ratio of NEFA-stimulated to untreated control PCREB concentration.
NEFA-stimulation of CREB phosphorylation is acute, transient, and increases with repetitive exposure
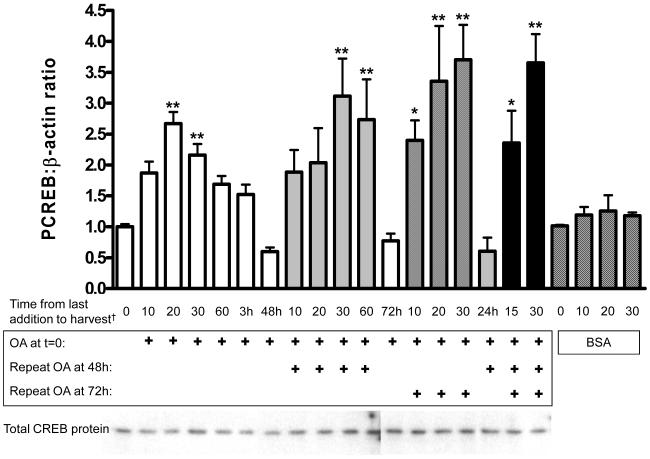
Time courses of NEFA activation of CREB at the high physiological concentration of 150 μM reveal a rapid, transient 2-4-fold stimulation of CREB phosphorylation with a peak at 20 minutes and a return to basal or sub-basal levels within 3-24 hours (OA, Figure 2; LA, OA, LnA short time course, Figure 4). All NEFA tested had a similar time course at this concentration with minor, nonsignificant variations in amplitude and duration of activation. Repeated exposures to OA, mimicking repeated elevations of NEFA with normal feeding and fasting cycles, induced repeated elevations of PCREB with an apparent trend towards a greater amplitude and longer duration of activation. Throughout the time-course total CREB protein levels remained unchanged (Figure 2, western blot). Addition of equal volumes of PBS with 10% BSA alone did not induce CREB activation (Figure 2, checkered bars).
Figure 2. Oleic acid induction of CREB phosphorylation is acute, transient, and increased on repetitive stimulation.
50% confluent BoASMC were made quiescent for 48 hours in DMEM with 0.1% FBS and then exposed to 150 μM oleic acid for the times indicated (in minutes except where indicated). OA was added either once at time 0 (white bars), twice at times 0 and 48 hours (diagonal stripes), twice at times 0 and 72 hours (crosshatched), or three times at times 0, 48, and 72 hours (black). For the control time course BSA was added at the same concentration as in OA additions (checkered bars). Extracts were either prepared in MLB and analyzed by PCREB ELISA or in denaturing sample buffer and analyzed by immunoblot as in Figure 1. Plots are combined from 2 (multiple NEFA exposures)-4 (single exposure) independent experiments. Total CREB protein is shown in the representative blots below the graph. † Time from last OA addition to harvest in minutes except where indicated by an “h” for hours; *P<0.05 vs. untreated; **P<0.01 vs. untreated. Direct comparison of peak values for each acute OA exposure shows no significant difference (p=0.38).
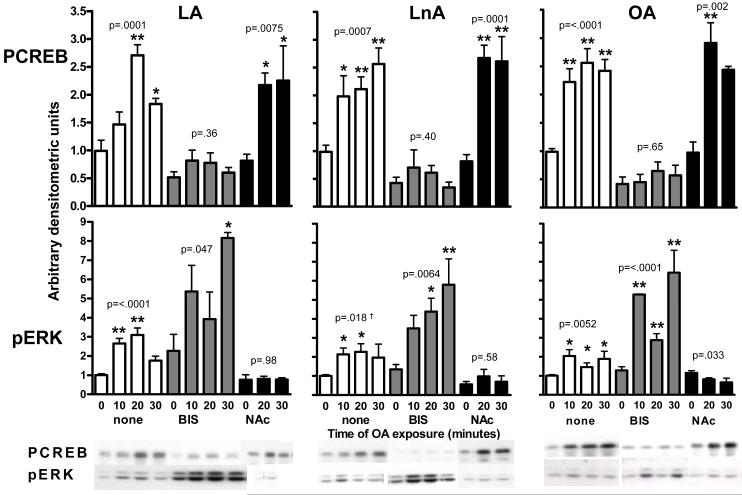
Figure 4. CREB and ERK activation upon NEFA exposure is mediated by different signaling pathways.
50% confluent BoASMC were made quiescent for 48 hours in DMEM with 0.1% FBS, pretreated for 30 min with no inhibitor (white), 4μM BIS (crosshatched), or 30 mM NAc (black) and then exposed to 100μM LA (left panels), LnA (central panels), or OA (right panels) for the times indicated. Extracts were prepared in denaturing sample buffer, and equal protein samples were subjected to SDS-PAGE and western blot analysis with antibodies to PCREB (top panels) and pERK (bottom panels). Blots were developed and quantitated as in Figure 3. Representative blots are shown below the graphs. *P<0.05, **P<0.01 vs. matched no NEFA sample. P values for the trend in each series are shown. † 30 min time point omitted from ANOVA because of large variance.
NEFA activate multiple intracellular signaling pathways in VSMC
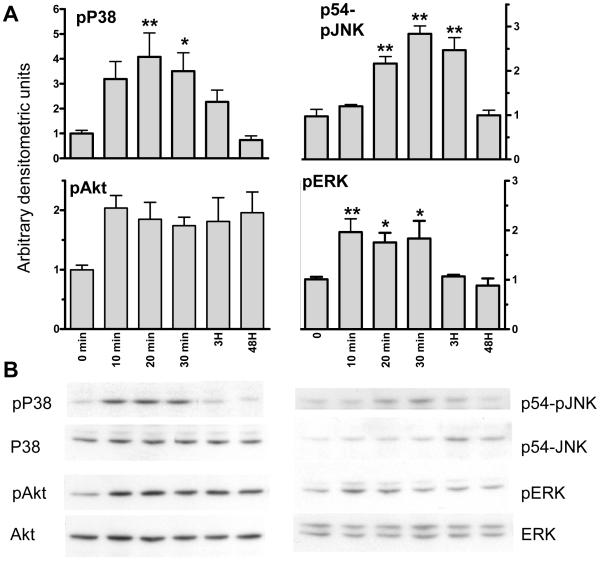
Multiple pathways including PI3K, ERK, and PKC have been implicated in phenotypic modulation in VSMC in response to different stimuli (8, 31, and 32). Other pathways including P38 MAPK and JNK have been implicated in control of apoptosis (33, 34). We investigated the effects of the three classes of unsaturated NEFA on these intracellular signaling pathways. Exposure to all 3 classes of unsaturated NEFA at physiological concentrations of 75-150 μM induce rapid, acute, and transient activation of P38, ERK, and JNK MAPK and a trend towards activation of the PI3K substrate, Akt. For simplicity only OA is shown as all 4 NEFA induce virtually identical activation (Figure 3). During this time course total levels of ERK, P38, Akt, and JNK protein remain constant with the exception of a small increase in total JNK at 3 hours (Figure 3B). Phosphorylation returns to control levels within 3-24 hours for P38 MAPK, ERK, and JNK. In contrast, Akt appears to remain persistently phosphorylated after initial exposure to NEFA.
Figure 3. NEFA induce acute, transient activation of multiple intracellular signaling pathways.
50% confluent BoASMC were made quiescent for 48 hours in DMEM with 0.1% FBS and then exposed to 150 μM oleic acid for the times indicated. Extracts were prepared in denaturing sample buffer and equal protein samples were subjected to SDS-PAGE and western blot analysis with antibodies to phosphorylated forms of p38, ERK, JNK, and Akt. A. Films were scanned, values normalized to T0=1, and plotted. B. Representative western blots of both the phosphorylated and unphosphorylated forms are shown. *P<0.05 vs. untreated; **P<0.01 vs. untreated. P value for trend= 0.003, <0.0001, 0.267, and 0.003 for pP38, pJNK, pAkt, and pERK, respectively.
NEFA-mediated activation of CREB and ERK occur by divergent pathways
Oxidative stress, PKC, and ERK have all been implicated in the phenotypic activation of VSMC in response to NEFA (6, 8, and 32). ERK is an established signaling pathway contributing to VSMC activation. We set out to determine whether CREB and ERK activation by FFA are mediated by the same pathway. We employed a panel of pharmacological inhibitors of signaling pathways and an antioxidant to address this question. Figure 4 illustrates the effect on CREB and ERK activation of exposure to 150 μM OA, LA or LnA in the presence and absence of a generic PKC inhibitor (4μM bisindolyl maleimide, BIS, aka GF109203X) and the antioxidant, N-acetyl cysteine (NAc, 30mM). NEFA-induced phosphorylation of CREB is largely blocked by generic PKC inhibition and unaffected by antioxidant. In contrast, ERK activation is variably stimulated by PKC inhibition and completely blocked by NAc. These data suggest that separate signaling events contribute to CREB and ERK activation.
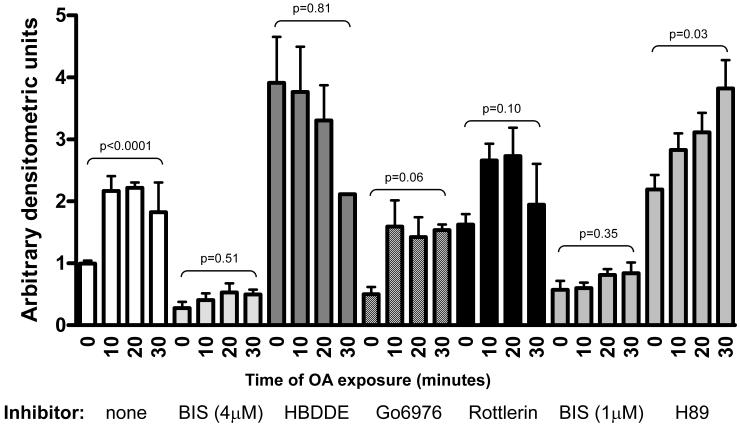
NEFA-mediated activation of CREB is mediated by a “novel” PKC isoform
The identity of the primary CREB activating kinase was further investigated using a panel of isoform-specific pharmacological kinase inhibitors HBDDE (PKCα and γ), Go6976 (PKCα and β), and Rottlerin (PKC δ), as well as BIS at 1μM, a concentration reported to inhibit conventional (α and β) and novel (δ, ε, η, θ, or μ), but not atypical (tau, lambda, and zeta) PKC isoforms. In order to address concerns that BIS may also exert some PKA inhibitory activity at the original concentration, H89, a PKA-specific inhibitor, and low concentration BIS were used. Although H89 increased basal PCREB, significant OA-mediated activation was still demonstrated (Figure 5). Together with the fact that the lower BIS concentration still showed full inhibition of CREB activation, this demonstrates that PKA is not primarily responsible for OA-mediated CREB activation. Full inhibition with 1μM BIS also suggests that an atypical PKC isoform is not responsible. Activation was also not fully inhibited by any of the isoform-specific typical PKC inhibitors used. Though activation in the presence of Go6976 and Rottlerin did not achieve statistical significance, there is a strong trend towards OA-mediated CREB activation in the setting of PKCα/β inhibition with Go6976 and a weaker trend with Rottlerin-mediated PKCδ inhibition suggesting that these isoforms are not fully responsible for OA-mediated activation. No activation was seen after pretreatment with HBDDE but this inhibitor alone induced a dramatic NEFA-independent activation of CREB suggesting that PKCγ has a basal inhibitory effect on CREB phosphorylation. Though it is impossible to rule out complex multi-isoform effects on CREB, these experiments suggest, but do not prove, that a “novel” PKC isoform(s), possibly epsilon, is responsible for OA-mediated CREB activation. This is consistent with the literature suggesting NEFA-mediated activation of atypical and/or novel PKC isoforms in VSMC (8, 35).
Figure 5. NEFA-mediated CREB activation is independent of typical PKC isoforms.
50% confluent BoASMC were made quiescent for 48 hours in DMEM with 0.1% FBS, pretreated for 30 min without inhibitor (white bars) or with the indicated inhibitor and then exposed to 150μM OA for the times indicated. Extracts were prepared in denaturing sample buffer, and equal protein samples were subjected to SDS-PAGE and western blot analysis with antibodies to PCREB. Blots were developed and quantitated as in Figure 3. P values for the trend in each series are shown.
Discussion
VSMC are dynamic cells that are able to undergo a transition to an actively proliferating state in response to injury. This transition, while important for healing, can also be pathological and contributes to atherosclerosis. A large body of literature supports the expectation that this important transition is a carefully regulated process and that injurious environmental factors induce opposing cytotoxic and cytoprotective pathways (see introduction). CREB is known to have cytoprotective, anti-proliferative, and differentiating effects on multiple cell types, including VSMC (18-23). As dedifferentiation, proliferation, and death of VSMC are involved in lesions in cardiovascular disease, we have investigated the effects of NEFA exposure on CREB activation in VSMC. We have made the novel observation that multiple classes of NEFA, previously known to activate mitogenic signaling pathways, also acutely activate CREB. This activation is dose dependent, with different NEFA varying slightly in their dose dependence. We also observe that exposure to a panel of NEFA acutely and transiently activates ERK MAPK, P38 MAPK, JNK MAPK, and PI3K pathways as expected from previous literature. Most intriguingly, activation of CREB by NEFA is regulated by signaling events that are completely distinct from ROS generation and ERK activation. To our knowledge this is the first report of NEFA-mediated induction of a pathway with anti-proliferative, anti-apoptotic, prodifferentiation potential and of distinct, alternate signaling to differentiating and activating pathways in vascular cells.
In a free living setting in insulin sensitive individuals, circulating concentrations of NEFA (or lipoprotein-delivered concentrations of NEFA to the vessel wall) are highly variable and dependent upon fed vs. fasted states. Normal physiological NEFA levels are highest in the fasted state (about 300-600 μM) when lipolysis is activated and suppressed to <100 μM in the fed state (36). This is in contrast to conditions such as insulin resistance or type 2 diabetes where NEFA delivery is chronically high with fasting levels as high as 1500 μM and incomplete suppression in the fed state (37). We were intrigued to observe that with a single exposure to NEFA, activation of CREB and other signaling pathways is transient. Recurrent and possibly higher magnitude of NEFA activation of these pathways occurs with repetitive exposure as might be seen in vivo with feeding and fasting cycles leading to fluctuating NEFA delivery (high during fasting and low postprandially). Thus, downstream effects of these signaling pathways and of increased CREB activity may tend to accumulate with time. The ultimate phenotypic outcome in terms of a balance between VSMC activation/toxicity vs. differentiation/normal function will necessarily depend on the balance of pathways activated. This balance will also affect the context in which cells respond to other stimuli, thus providing a mechanism, for example, for NEFA affects on VSMC response to angiotensin II (7, 32). In diabetic and insulin resistant individuals where NEFA levels tend to remain high, the balance of activating and protective pathways may be quite different. As is seen in other environmental responses (eg beta-1 and beta-2 adrenergic response and insulin signaling (38, 39)), chronic stimulation of these pathways may lead to differential down-regulation of pathway components. Previous work from our laboratory demonstrates that CREB protein is, in fact, down-regulated in VSMC in vitro in response to the chronic metabolic or oxidative stress (25, 26, and 29) and in the vascular media of several different rodent models of vascular disease and metabolic stress including insulin resistance, diabetes, obesity, hypoxia, aging (24, 25) and high fat fed LDL receptor deficient mice (unpublished results). This loss of the differentiating pathway under chronic stress may shift the balance towards VSMC activation in conditions of chronic NEFA elevation such as diabetes and metabolic syndrome and may contribute to the increased atherosclerosis associated with these conditions.
Finally we find that CREB activation by NEFA exposure occurs by a mechanism distinct from that of the NEFA-mediated ERK activation implicated in proliferation. CREB phosphorylation in response to NEFA is unaffected by the antioxidant, NAc, whereas ERK activation is completely blocked. Thus, CREB activation occurs independently of ROS while ERK activation is entirely dependent on ROS. The novel observation that CREB activation is not affected by antioxidants appears to implicate parallel and distinct cytoprotective and cytotoxic post-receptor responses to NEFA exposure.
Our observations using pharmacological inhibitors of PKC and PKA demonstrate that PKC is essential for NEFA-mediated CREB phosphorylation. Interestingly, this pathway does not appear to be important for NEFA-mediated ERK phosphorylation. To better delineate the specific PKC isoform contributing to CREB activation by NEFA, we used a battery of isoform-specific pharmacological inhibitors. The many PKC isoforms identified to date have been grouped into three categories: conventional, novel, and atypical. 1μM BIS, reported to inhibit conventional and novel, but not atypical PKC isoforms, blocks CREB activation. As specific inhibitors of the conventional PKC isoforms do not appear to inhibit NEFA-mediated CREB activation, our studies implicate a novel PKC (PKCδ, ε, η, θ, or μ) in this process. In fact, VSMC expression and a role in control of proliferation, apoptosis, and migration for PKCδ and ε have been well documented in the literature (for example 40-45).
Studies in the literature implicate ROS in the activating effects of oleic and linoleic acids on VSMC (6, 8, and 32). Lu et al find that ROS are essential for the activation of the ERK pathway and for increased proliferation in response to oleic acid (6). Our results corroborate their observations in that antioxidant does eliminate ERK activation by all classes of FFA. They also find that PKC activation is required for stimulation of total MAPK activity and for increased proliferation (8). Interestingly, we find that PKC inhibition stimulates ERK phosphorylation. This discrepancy could be explained by the detection of MAPkinases other than ERK by their assay which measures 32P incorporation into myelin basic protein. Alternatively, or in addition, the discrepancy could reflect interspecies differences between rat and bovine VSMC, a possibility supported by our preliminary proliferation data (Schauer, unpublished results). The published observation that PKC activation is required for NEFA-stimulated proliferation is not necessarily at odds with our results. Activation of CREB via PKC would be expected to have anti-apoptotic effects which could appear to enhance the proliferative effect seen in their studies.
Dietary studies have implicated diets rich in certain NEFA as instrumental in increasing CVD risk (n-6 PUFA) and others as protective (n-3 PUFA, MUFA) (46-48). A priori one might have expected that beneficial NEFA would either be less toxic and thus induce less CREB phosphorylation or would exert their beneficial effects by inducing more CREB activation and cytoprotection. This does not appear to be the case in vitro. LnA, the most potent activator of CREB phosphorylation, is the most abundant plant n-3 PUFA and, therefore, the most abundant dietary n-3 PUFA in a typical diet, but is not highly represented in serum fatty acids (consisting of both NEFA and lipoprotein bound, triglyceride-associated fatty acids). In 6 subjects in a German study saturated fatty acids are the most abundant at 54% of total (PA 27%, stearic at 22%), OA next at 24%, LA at 10%, n3-PUFA at 1% (predominately eicosapentaenoic acid, EPA) (49). An analysis of skeletal muscle FA reveals that LnA is also not abundant in skeletal muscle cell membranes. LA at about 30% of total membrane FA is the most abundant, with OA, PA, and arachidonic acid next at about 12-15% each. DHA is the dominant membrane n-3 PUFA at 2.3% (50). With the exception of DHA, our data would suggest that the potency of CREB activation by a NEFA is more closely related to its usual abundance in the serum than with its apparent CVD association. The lower degree of activation of CREB by DHA could reflect decreased VSMC toxicity of DHA.
In summary we find that VSMC respond to NEFA exposure by inducing both activating and cytoprotective pathways, and interestingly that induction of these pathways occurs via distinct signaling pathways. These studies and the existing literature areconsistent with the hypothesis that elevated NEFA levels of diabetes and metabolic syndrome impact VSMC phenotype. More studies are needed to understand fully the complex effects of NEFA on intracellular signaling and gene regulation in VSMC.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH T32 and NIH R01- DK064741-01), the Department of Veterans Affairs (VA Merit), an NIH P01 vascular core grant, and the American Heart Association. We appreciate the technical assistance of Jody Gunter, Dr. Peter Watson, and Matthew Hockin, as well as the careful reading and advice of Dr. Boris Draznin.
Abbreviations
- VSMC
vascular smooth muscle cells
- BoASMC
bovine aortic smooth muscle cells
- NEFA
non-esterified fatty acid(s)
- ROS
reactive oxygen species
- CREB
cAMP response element binding protein
- CVD
cardiovascular disease
- PKC
protein kinase C
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- DAG
diacyl glycerol
- NAc
N-acetyl cysteine
- BIS
Bisindolyl maleimide
- JNK
Jun N-terminal kinase
- BSA
bovine serum albumin
- OA
oleic acid
- LA
linoleic acid
- LnA
linolenic acid
- MUFA
monounsaturated fatty acid
- PUFA
polyunsaturated fatty acid
- DHA
docosahexaenoic acid
- MnSOD
manganese superoxide dismutase
- HO-1
heme oxygenase-1
References
- 1.Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003 Feb 6;91(3A):3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson M, Wessman Y, Almgren P, Groop L. High levels of nonesterified fatty acids are associated with increased familial risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1588–94. doi: 10.1161/01.atv.20.6.1588. [DOI] [PubMed] [Google Scholar]
- 4.Oram JF, Bornfeldt KE. Direct effects of long-chain non-esterified fatty acids on vascular cells and their relevance to macrovascular complications of diabetes. Front Biosci. 2004 May 1;9:1240–53. doi: 10.2741/1300. [DOI] [PubMed] [Google Scholar]
- 5.Askari B, Renard CB, Bornfeldt KE. Regulation of smooth muscle cell accumulation in diabetes-accelerated atherosclerosis. Histol Histopathol. 2002 Oct;17(4):1317–28. doi: 10.14670/HH-17.1317. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, Greene EL, Nagai T, Egan BM. Reactive oxygen species are critical in the oleic acid-mediated mitogenic signaling pathway in vascular smooth muscle cells. Hypertension. 1998 Dec;32(6):1003–10. doi: 10.1161/01.hyp.32.6.1003. [DOI] [PubMed] [Google Scholar]
- 7.Lu G, Meier KE, Jaffa AA, Rosenzweig SA, Egan BM. Oleic acid and angiotensin II induce a synergistic mitogenic response in vascular smooth muscle cells. Hypertension. 1998 Apr;31(4):978–85. doi: 10.1161/01.hyp.31.4.978. [DOI] [PubMed] [Google Scholar]
- 8.Lu G, Morinelli TA, Meier KE, Rosenzweig SA, Egan BM. Oleic acid-induced mitogenic signaling in vascular smooth muscle cells. A role for protein kinase C. Circ Res. 1996 Sep;79(3):611–8. doi: 10.1161/01.res.79.3.611. [DOI] [PubMed] [Google Scholar]
- 9.Rao GN, Alexander RW, Runge MS. Linoleic acid and its metabolites, hydroperoxyoctadecadienoic acids, stimulate c-Fos, c-Jun, and c-Myc mRNA expression, mitogen-activated protein kinase activation, and growth in rat aortic smooth muscle cells. J Clin Invest. 1995 Aug;96(2):842–7. doi: 10.1172/JCI118130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karman RJ, Gupta MP, Garcia JG, Hart CM. Exogenous fatty acids modulate the functional and cytotoxic responses of cultured pulmonary artery endothelial cells to oxidant stress. J Lab Clin Med. 1997 May;129(5):548–56. doi: 10.1016/s0022-2143(97)90009-3. [DOI] [PubMed] [Google Scholar]
- 11.Sohma R, Takahashi M, Takada H, Kuwayama H. Protective effect of n-3 polyunsaturated fatty acid on primary culture of rat hepatocytes. J Gastroenterol Hepatol. 2007 Nov;22(11):1965–70. doi: 10.1111/j.1440-1746.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Shiraishi F, Visner GA, Nick HS. Linoleyl hydroperoxide transcriptionally upregulates heme oxygenase-1 gene expression in human renal epithelial and aortic endothelial cells. J Am Soc Nephrol. 1998 Nov;9(11):1990–7. doi: 10.1681/ASN.V9111990. [DOI] [PubMed] [Google Scholar]
- 13.Kuratko CN, Constante BJ. Linoleic acid and tumor necrosis factor-alpha increase manganese superoxide dismutase activity in intestinal cells. Cancer Lett. 1998 Aug 14;130(1-2):191–6. doi: 10.1016/s0304-3835(98)00136-0. [DOI] [PubMed] [Google Scholar]
- 14.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4299–304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meilhac O, Zhou M, Santanam N, Parthasarathy S. Lipid peroxides induce expression of catalase in cultured vascular cells. J Lipid Res. 2000 Aug;41(8):1205–13. [PubMed] [Google Scholar]
- 16.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, et al. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003 Dec 19;278(51):51006–14. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 17.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, et al. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol. 2005 May;25(5):950–6. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 18.Reusch JE, Watson PA. Loss of CREB regulation of vascular smooth muscle cell quiescence in diabetes. Rev Endocr Metab Disord. 2004 Aug;5(3):209–19. doi: 10.1023/B:REMD.0000032409.13963.bc. [DOI] [PubMed] [Google Scholar]
- 19.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, et al. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003 Jul 1;17(13):1575–80. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reusch JE, Klemm DJ. Inhibition of cAMP-response element-binding protein activity decreases protein kinase B/Akt expression in 3T3-L1 adipocytes and induces apoptosis. J Biol Chem. 2002 Jan 11;277(2):1426–32. doi: 10.1074/jbc.M107923200. [DOI] [PubMed] [Google Scholar]
- 21.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000 Jan;25(1):11–4. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 22.Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, et al. Insulin- like growth factor-I induces bcl-2 promoter through the transcription factor cAMP- response element-binding protein. J Biol Chem. 1999 Sep 24;274(39):27529–35. doi: 10.1074/jbc.274.39.27529. [DOI] [PubMed] [Google Scholar]
- 23.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000 Apr 14;275(15):10761–6. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 24.Watson PA, Vinson C, Nesterova A, Reusch JE. Content and activity of cAMP response element-binding protein regulate platelet-derived growth factor receptor- alpha content in vascular smooth muscles. Endocrinology. 2002 Aug;143(8):2922–9. doi: 10.1210/endo.143.8.8959. [DOI] [PubMed] [Google Scholar]
- 25.Watson PA, Nesterova A, Burant CF, Klemm DJ, Reusch JE. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J Biol Chem. 2001 Dec 7;276(49):46142–50. doi: 10.1074/jbc.M104770200. [DOI] [PubMed] [Google Scholar]
- 26.Garat CV, Fankell D, Erickson PF, Reusch JE, Bauer NN, McMurtry IF, et al. Platelet-derived growth factor BB induces nuclear export and proteasomal degradation of CREB via phosphatidylinositol 3-kinase/Akt signaling in pulmonary artery smooth muscle cells. Mol Cell Biol. 2006 Jul;26(13):4934–48. doi: 10.1128/MCB.02477-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jambal P, Masterson S, Nesterova A, Bouchard R, Bergman B, Hutton JC, et al. Cytokine-mediated down-regulation of the transcription factor cAMP-response element-binding protein in pancreatic beta-cells. J Biol Chem. 2003 Jun 20;278(25):23055–65. doi: 10.1074/jbc.M212450200. [DOI] [PubMed] [Google Scholar]
- 28.Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, et al. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem. 2003 Mar;84(5):982–96. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 29.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, et al. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem. 2001 Dec 7;276(49):46132–41. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 30.Ellmerer M, Schaupp L, Brunner GA, Sendlhofer G, Wutte A, Wach P, et al. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am J Physiol Endocrinol Metab. 2000 Feb;278(2):E352–6. doi: 10.1152/ajpendo.2000.278.2.E352. [DOI] [PubMed] [Google Scholar]
- 31.Askari B, Carroll MA, Capparelli M, Kramer F, Gerrity RG, Bornfeldt KE. Oleate and linoleate enhance the growth-promoting effects of insulin-like growth factor-I through a phospholipase D-dependent pathway in arterial smooth muscle cells. J Biol Chem. 2002 Sep 27;277(39):36338–44. doi: 10.1074/jbc.M205112200. [DOI] [PubMed] [Google Scholar]
- 32.Greene EL, Lu G, Zhang D, Egan BM. Signaling events mediating the additive effects of oleic acid and angiotensin II on vascular smooth muscle cell migration. Hypertension. 2001 Feb;37(2):308–12. doi: 10.1161/01.hyp.37.2.308. [DOI] [PubMed] [Google Scholar]
- 33.Kanda H, Miura M. Regulatory roles of JNK in programmed cell death. J Biochem (Tokyo) 2004 Jul;136(1):1–6. doi: 10.1093/jb/mvh098. [DOI] [PubMed] [Google Scholar]
- 34.Diep QN, Touyz RM, Schiffrin EL. Docosahexaenoic acid, a peroxisome proliferator- activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 2000 Nov;36(5):851–5. doi: 10.1161/01.hyp.36.5.851. [DOI] [PubMed] [Google Scholar]
- 35.Parmentier JH, Zhang C, Estes A, Schaefer S, Malik KU. Essential role of PKC-zeta in normal and angiotensin II-accelerated neointimal growth after vascular injury. Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1602–13. doi: 10.1152/ajpheart.01363.2005. [DOI] [PubMed] [Google Scholar]
- 36.Raben A, Holst JJ, Madsen J, Astrup A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Am J Clin Nutr. 2001 Feb;73(2):177–89. doi: 10.1093/ajcn/73.2.177. [DOI] [PubMed] [Google Scholar]
- 37.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988 Aug;37(8):1020–4. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 38.Yuan L, Ziegler R, Hamann A. Chronic hyperinsulinism induced down-regulation of insulin post-receptor signaling transduction in Hep G2 cells. J Huazhong Univ Sci Technolog Med Sci. 2002;22(4):313–6. doi: 10.1007/BF02896773. [DOI] [PubMed] [Google Scholar]
- 39.Wallukat G. The beta-adrenergic receptors. Herz. 2002 Nov;27(7):683–90. doi: 10.1007/s00059-002-2434-z. [DOI] [PubMed] [Google Scholar]
- 40.Allen TR, Krueger KD, Hunter WJ, 3rd, Agrawal DK. Evidence that insulin-like growth factor-1 requires protein kinase C-epsilon, PI3-kinase and mitogen-activated protein kinase pathways to protect human vascular smooth muscle cells from apoptosis. Immunol Cell Biol. 2005 Dec;83(6):651–67. doi: 10.1111/j.1440-1711.2005.01387.x. [DOI] [PubMed] [Google Scholar]
- 41.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, et al. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest. 2001 Nov;108(10):1505–12. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li PF, Maasch C, Haller H, Dietz R, von Harsdorf R. Requirement for protein kinase C in reactive oxygen species-induced apoptosis of vascular smooth muscle cells. Circulation. 1999 Aug 31;100(9):967–73. doi: 10.1161/01.cir.100.9.967. [DOI] [PubMed] [Google Scholar]
- 43.Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, et al. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem. 2005 Oct 21;280(42):35310–7. doi: 10.1074/jbc.M507187200. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi H, Igarashi M, Hirata A, Sugae N, Tsuchiya H, Jimbu Y, et al. Altered PDGF-BB-induced p38 MAP kinase activation in diabetic vascular smooth muscle cells: roles of protein kinase C-delta. Arterioscler Thromb Vasc Biol. 2004 Nov;24(11):2095–101. doi: 10.1161/01.ATV.0000144009.35400.65. [DOI] [PubMed] [Google Scholar]
- 45.Fan CY, Katsuyama M, Yabe-Nishimura C. PKCdelta mediates up-regulation of NOX1, a catalytic subunit of NADPH oxidase, via transactivation of the EGF receptor: possible involvement of PKCdelta in vascular hypertrophy. Biochem J. 2005 Sep 15;390(Pt 3):761–7. doi: 10.1042/BJ20050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Jama. 2004 Sep 22;292(12):1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 47.Hirafuji M, Machida T, Hamaue N, Minami M. Cardiovascular protective effects of n-3 polyunsaturated fatty acids with special emphasis on docosahexaenoic acid. J Pharmacol Sci. 2003 Aug;92(4):308–16. doi: 10.1254/jphs.92.308. [DOI] [PubMed] [Google Scholar]
- 48.Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem. 2004 Aug;263(1-2):217–25. doi: 10.1023/B:MCBI.0000041863.11248.8d. [DOI] [PubMed] [Google Scholar]
- 49.Stefan N, Wahl HG, Fritsche A, Haring H, Stumvoll M. Effect of the pattern of elevated free fatty acids on insulin sensitivity and insulin secretion in healthy humans. Horm Metab Res. 2001 Jul;33(7):432–8. doi: 10.1055/s-2001-16231. [DOI] [PubMed] [Google Scholar]
- 50.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993 Jan 28;328(4):238–44. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]