Abstract
Dendritic cells are professional antigen-presenting cells that play a key role in the regulation of immune responses. Here we characterize a unique subset of tolerogenic DCs that expressed the chemokine receptor CCR9 and migrated to the CCR9 ligand CCL25, a chemokine implicated in T cell and DC homing to the gut. CCR9+ DCs were of the plasmacytoid DC lineage, possessed an immature phenotype and rapidly downregulated CCR9 in response to maturation-inducing pDC-restricted Toll-like receptor ligands. CCR9+ pDCs were potent inducers of regulatory T cell function and suppressed antigen-specific immune responses both in vitro and in vivo, including inhibition of acute graft-versus-host disease induced by allogeneic CD4+ donor T cells in irradiated recipients. The results identify a highly immunosuppressive population of pDCs present in lymphoid tissues.
Keywords: Plasmacytoid dendritic cells, CCR9, CCL25, tolerance, graft-versus-host disease, toll-like receptor ligands, regulatory T cells
Introduction
Dendritic cells (DCs) constitute a family of bone marrow-derived antigen-presenting cells (APCs) that play a pivotal role in orchestrating immune responses, either by maintaining tolerance to self-antigens, or inducing potent immune responses to infectious agents1. Upon exposure to microbial products, DCs mature by upregulating costimulatory molecules (CD40, CD80 and CD86), and migrate to T cell areas of organized lymphoid tissues where they activate naive T cells and induce effector rather than tolerogenic immune responses. In the absence of such inflammatory or infectious signals, however, DCs present self-antigens in secondary lymphoid tissues for the induction and maintenance of self-tolerance2. The ability of DCs to induce tolerance has led to numerous studies using these cells therapeutically in an effort to control unwanted immune responses in models of allograft rejection, graft-versus-host disease (GVHD) and autoimmune disorders3,4. Most studies have employed myeloid DCs (mDCs) derived from mouse bone marrow or human monocytes cultured in vitro using the cytokines granulocyte-macrophage colony stimulating factor (GM-CSF) in the presence or absence of interleukin 4 (IL-4)4. For example, in vitro derived immature mDCs were able to dampen arthritis in an antigen-driven mouse model5 or prolong allograft survival in a murine transplant model6,7. Since the therapeutic effects have generally been incomplete, some studies have further manipulated mDCs through genetic modification8–10 or exposure to either immunosuppressive agents11, or cytokines such as IL-10 and transforming growth factor-β (TGF-β)12, in an effort to generate more potent tolerogenic mDC populations.
More recent studies, however, suggest that naturally occurring DC subsets may also be effective at inducing peripheral tolerance. Lymphoid-related CD11c+ CD8α+ DCs, mobilized in vivo by the hematopoietic growth factor fms-like tyrosine kinase 3 ligand (Flt3L), were shown to prolong the survival of vascularized heart allografts in rodents13. Plasmacytoid DCs (pDCs) have also been implicated as critical regulators of immune responses14. Plasmacytoid DCs are best known for their high production amounts of type I interferons and subsequent induction of cell-mediated adaptive immune responses after viral activation15. However, freshly isolated pDCs, in the absence of maturation signals, do not induce strong T cell responses; rather, they appear to prime naive CD4+ T cells to differentiate into IL-10 producing regulatory Tr1 cells in vitro in both humans16 and mice17,18. In vivo, unstimulated pDC precursors have been shown to facilitate allogeneic hematopoietic stem cell engraftment19,20 and delay allograft rejection in mice21. Since pDCs have a lower capacity for foreign antigen uptake than their mDC counterparts22, it has been postulated that pDCs present more self-antigen–major histocompatibility complexes (MHC) than mDCs. In addition, the observation that pDCs normally reside in the thymus and in peripheral lymphoid tissues suggests that they may play a greater role in tolerance than other DC subsets23. Thus, resting or immature pDCs may represent a particularly important naturally occurring regulatory DC subset14,15.
In spite of the promise of cellular therapy with DC populations, to date no studies have taken advantage of specific tolerogenic phenotypes (based on DC maturation markers, for example) to sort immunosuppressive from immune activating DCs. Perhaps as a consequence, most DC populations studied have yielded only partial or transient amelioration of autoimmune symptoms or allograft survival [reviewed in 3, 4]. Here we report that the chemokine receptor CCR9 {A000632 http://www.signaling-gateway.org/molecule/query?afcsid=A000632}, previously implicated in T cell homing to the gut24 and T cell precursor homing to the thymus25, is selectively expressed on pDCs of immature phenotype in vivo. CCR9 expression was rapidly downregulated in response to maturational signals and could effectively distinguish endogenous pDCs of immature and mature phenotypes. CCR9+ pDCs constituted a sizeable fraction of the pDC compartment in resting secondary lymphoid tissues; in addition, they are substantially more efficient than CCR9− pDCs at inducing regulatory T cells and they inhibit antigen-specific immune responses both in vitro and in vivo. Finally, sorted and adoptively transferred CCR9+ (but not CCR9−) pDCs effectively prevent acute GVHD, providing long-term suppression of graft versus host responses in an allogeneic T cell transfer model.
Results
DCs express gut-specific T-cell homing receptors
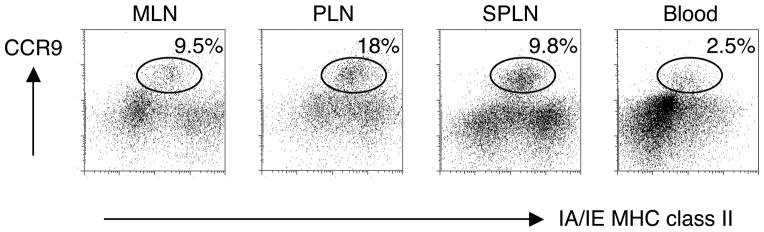
Using a gene expression profiling approach, we evaluated the expression of tissue-specific homing receptor transcripts in DCs from different tissues in an effort to explore the homing patterns of DCs and the impact this might have on tissue-specific immune responses. We characterized expression of selected trafficking receptor transcripts in CD11c+ DCs from mesenteric lymph nodes (MLNs), Peyer’s patches and peripheral lymph nodes (PLNs); and in memory CD4+ T cells from MLNs and the lamina propria of the small intestine for comparison. We focused on the expression of key gut and skin-specific homing receptors since many studies have utilized this known dichotomy between tissue-homing lymphocytes in various inflammatory disorders26,27. The expression of gut-selective homing receptors CCR9 and the β7 integrin (part of theα4β7 heterodimer) was high among gut-associated T cells, whereas their expression of skin-homing receptors CCR4 and CCR10 was low (data not shown), validating the assay. We were surprised however to find high expression levels of CCR9 transcript and protein on DCs from lymphoid tissues, including lymph nodes that drain the gut (MLNs) but also those that do not (PLNs) (Fig. 1 and data not shown). In contrast, transcripts for the skin-homing associated chemokine receptors CCR4 and CCR10 were low in all of the DCs tested here (data not shown). Flow cytometry confirmed high expression of CCR9 protein on immature CD11c+ MHC class IIint cells in lymphoid tissues (Fig. 1), whereas only a small percentage of DCs seen in the blood were CCR9+.
Figure 1. Tissue-specific CCR9 expression profiles of DCs.
CD11c+ DCs were analyzed for surface expression of CCR9 and MHC class II in mesenteric lymph nodes (MLN), peripheral lymph nodes (PLN), spleen (SPLN) and blood of normal BALB/c mice. Cells were gated on CD11c+ DCs. One of two representative experiments is shown.
CCR9 defines an immature population of plasmacytoid DCs
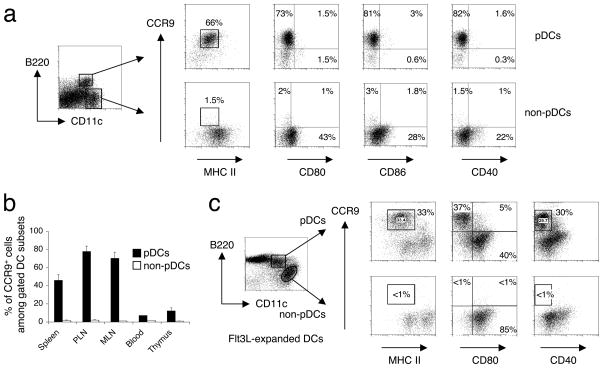
Using flow cytometry we subdivided the DC populations from different lymphoid tissues into pDCs (CD11cintB220+) and non-pDCs (CD11chiB220−). The non-pDC group includes CD11chiB220−CD8α−CD11bhi mDCs and so called CD11chiB220−CD8αhiCD11blo ‘lymphoid’ DCs. Almost all of the CCR9+ DCs in the lymphoid tissues examined resided in the pDC subset (Fig. 2a); and almost all displayed an ‘immature’ phenotype as shown by their low expression of costimulatory molecules CD80, CD86 and CD40, and intermediate expression of MHC class II molecules (Fig. 2a). The predominantly CCR9-deficient mDC (non-pDC) compartment contained DCs with slightly higher expression of costimulatory molecules (Fig. 2a bottom). The pDC compartment in lymphoid tissues contained a sizeable population of CCR9+ pDCs (Fig. 2b), with the highest percentage (~70–80%) of pDCs expressing CCR9 in the PLNs and MLNs. The lowest proportion of CCR9+ DCs among pDCs was in the blood and thymus. As a whole, pDCs are less abundant than mDCs, so that CCR9+ pDCs represent ~12–18% of total CD11c+ DCs in PLNs, ~5–10% of DCs in MLNs and spleen, 2–3% of DCs in blood and <1% in the thymus.
Figure 2. CCR9+ DCs reside in the plasmacytoid DC compartment and have a predominantly immature phenotype.
(a) DCs from pooled peripheral lymph nodes (PLN) were isolated from normal C57BL/6 mice. Cell suspensions were stained and gated on plasmacytoid (CD11cint B220+) and non-plasmacytoid (CD11chi B220−) lineage− DCs (pDCs and non-pDCs respectively). The gated cells were analyzed for expression of MHC class II and costimulatory markers CD80, CD86 and CD40 to assess their maturation state and correlated with CCR9 expression. Gates were set based on isotype controls for MHC class II and costimulatory markers. Representative FACS plots from one out of three mice. (b) Within the gated pDC and non-pDC subsets, the percentage of CCR9+ cells of gated DC subsets was assessed by FACS analysis in blood and various lymphoid tissues indicated (PLN, peripheral lymph nodes; MLN, mesenteric lymph nodes). Error bars represent the SEM from three mice. (c) DCs from pooled PLN expanded by Flt3L-secreting B16 melanoma cells in C57BL/6 mice, were gated on pDCs (CD11cint B220+) and non-pDCs (CD11chi B220−) lineage− DCs. The gated cells were analyzed for expression of MHC class II and costimulatory markers CD80 and CD40 to assess their maturation state and correlated with CCR9 expression. Gates were set based on isotype controls for MHC class II, CD80 and CD40. Representative FACS plots from one out of three mice.
To determine whether additional expansion of pDCs in vivo would alter expression of CCR9 on these cells, we transplanted C57BL/6 mice with a B16 melanoma cell line secreting Flt3L (subsequently referred to as Flt3L-treated B6 mice). This system allows us to expand the pDC population without activation, as Flt3L has been shown to be an important growth and differentiation factor for the development of pDCs from hematopoietic stem cells in mice28. Flt3L treatment in vivo increased the frequency and number of pDCs in lymphoid tissues by almost 10-fold after 10–14 days (data not shown). In addition to the CCR9+ pDC population, a distinct population of CCR9− pDCs was seen with increased expression of costimulatory molecules (Fig. 2c). However, most CCR9+ pDCs remained phenotypically immature even after in vivo expansion with Flt3L. Taken together, the results show that CCR9 defines an immature population of pDCs in peripheral lymphoid tissues, distinguished from most mature pDCs and from mDCs.
CCR9+ pDCs migrate to CCL25
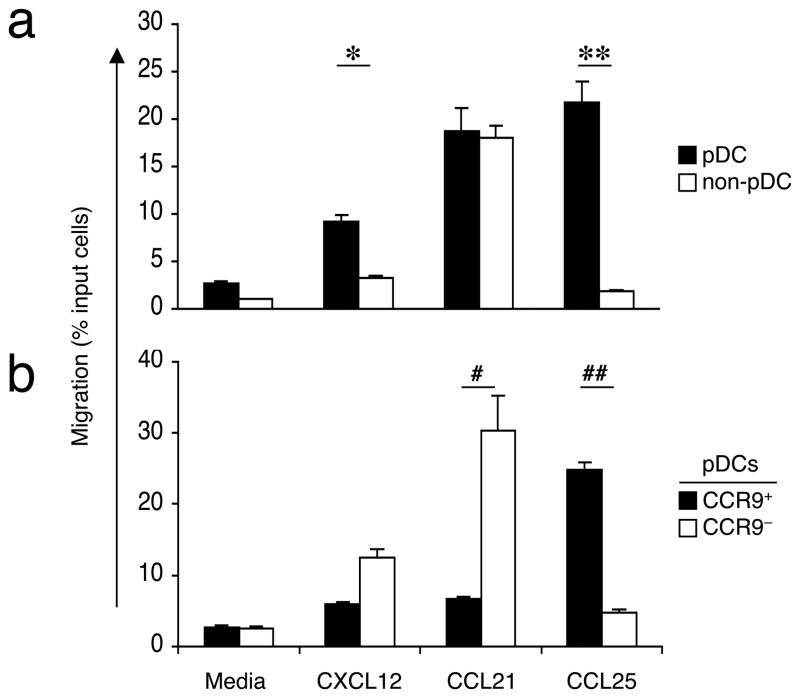
We next asked whether CCR9 on pDCs was functional by assessing the chemotactic responses of different DC subsets to the CCR9 ligand CCL25 (a chemokine formerly called thymus-expressed chemokine or TECK, A002265, http://www.signaling-gateway.org/molecule/query?afcsid=A002265). Because the number of pDCs that can be recovered from normal lymphoid tissues is limiting, we expanded the DC population in Flt3L-treated B6 mice as described above and examined the migration of pooled peripheral lymph node cells in response to various chemokines across a Transwell membrane. As expected, pDCs migrated more efficiently than other DC populations towards CXCL12 (also known as SDF-1, ligand for CXCR4), previously identified as a potent chemoattractant for pDCs (Fig. 3a)29,30. Interestingly, pDCs were the only DC subset to migrate efficiently in response to CCL25, and with a higher chemotactic response compared to CXCL12 (Fig. 3a). Between CCR9+ and CCR9− pDC subsets, only the CCR9+ pDC subset migrated efficiently to CCL25 (Fig. 3b). In contrast, consistent with their immature status, CCR9+ pDCs did not migrate to CCL21 (SLC) (Fig. 3b), a ligand for CCR7 that is upregulated on mature DCs upon activation31.
Figure 3. CCR9 expression allows pDC migration to CCL25.
Chemotactic response of DC subsets from pooled lymph node cells of Flt3L-treated B6 mice toward the CXCR4 ligand CXCL12, CCR7 ligand CCL21, and the CCR9 ligand CCL25. DC subsets were subdivided into pDCs and non-pDCs as described (a) and pDCs were subdivided based on their expression of CCR9 (b). Results are expressed as the mean percentage of total cells migrating towards the chemokine or towards the medium alone. Error bars represent the SEM of triplicate wells with representative results from one out of two experiments. *, P = 0.001, **, P < 0.001 by t-test comparing migration of pDCs versus non-pDCs to CXCL12 or CCL25, respectively. Similarly #, P = 0.008, ##, P < 0.001 comparing migration of CCR9+ versus CCR9− pDCs to CCL21 or CCL25, respectively.
Activated pDCs produce type I interferon and downregulate CCR9
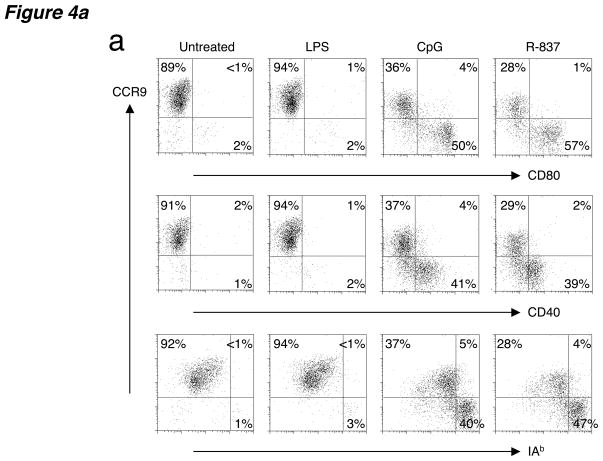
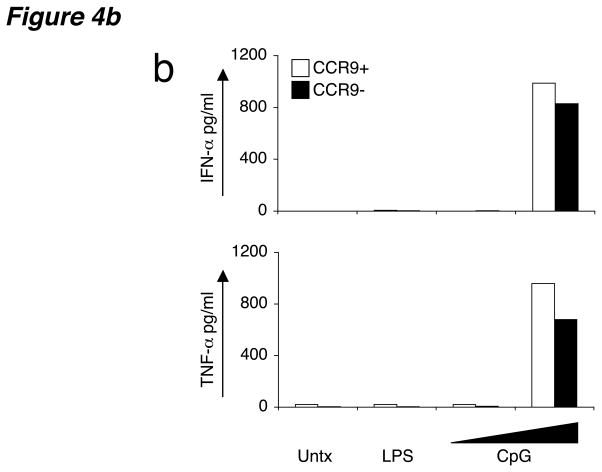
To determine if CCR9 expression is confined to immature pDCs, or instead is maintained on the CCR9+ subset during maturation, we stimulated in vitro sorted CCR9+ pDCs from Flt3L-treated B6 mice using an array of Toll-like receptor (TLR) ligands. Unlike mDCs, pDCs do not express TLR2, TLR4, TLR5 or TLR3, making them unresponsive to bacterial products such as peptidoglycans, lipopolysaccharide (LPS) and flagellin or viral double-stranded RNA mimics, respectively15. However pDCs are equipped with microbial sensors such as TLR7 or TLR9 that detect the presence of single-stranded RNA or microbial DNA, respectively15. As expected, activation of sorted CCR9+ pDCs with LPS induced no DC activation (Fig. 4a) or cytokine production (Fig. 4b), with production of interferon-α (IFN-α) and tumor necrosis factor (TNF-α) as well as expression of MHC class II and costimulatory molecules CD80 and CD40 remaining low and comparable to untreated cells (Fig. 4). However, treatment with pDC-specific TLR ligands R-837 (synthetic TLR7 ligand) or bacterial CpG oligonucleotides (TLR9 ligand) downregulated CCR9 on half or more of the cells, with a concomitant increase in MHC class II, CD80 and CD40 expression on the CCR9-downregulated population (Fig. 4a). In addition, overnight treatment with CpG resulted in a burst of IFN-α and TNF-α production by both CCR9+ and CCR9− pDC subsets (Fig. 4b). These results further support the plasmacytoid identity of CCR9-expressing DCs and define CCR9 as a marker for immature pDCs because CCR9 expression is lost upon TLR-dependent activation of these cells.
Figure 4. CCR9+ DCs downregulate CCR9 after activation with pDC-specific TLR ligands.
(a) Sorted CCR9+ pDCs from pooled PLNs isolated from Flt3L-treated B6 mice were left untreated or activated in the presence of LPS (1 ng/ml), CpG (1 μM) and R-837 (10 μg/ml) for 8–12 h. Cells were stained for CCR9, MHC class II (I-Ab) and the costimulatory ligands CD80 and CD40. Gates were based on isotype controls with control antibody staining yielding <1% of cells in the positive gates. Representative FACS plots shown from one of three experiments with similar results. (b) Sorted CCR9+ and CCR9− pDCs were also activated in the presence of LPS (10 ng/ml) or escalating doses of CpG (2 and 20 μg/ml) overnight for 16 h and supernatants were assayed for IFN-α and TNF–α. Results are expressed as mean concentration (pg/ml) of duplicate cultures. Data presented are from one of two experiments for IFN-α and one of three experiments for TNF-α with similar results.
CCR9+ DCs suppress immune responses and induce regulatory T cells
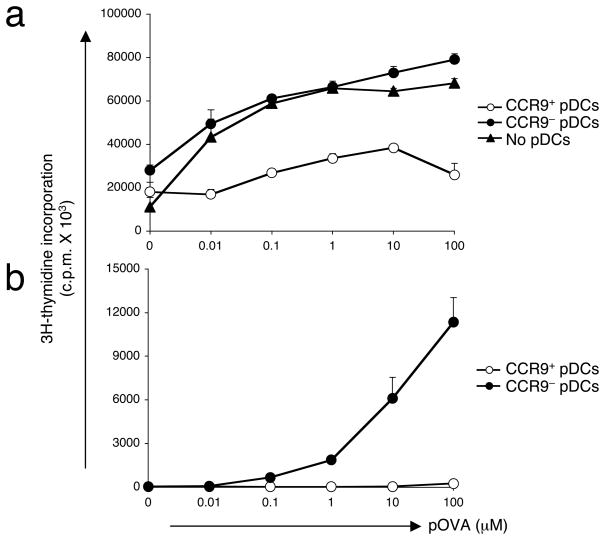
We next sought to determine whether CCR9+ pDCs, with a characteristic immature phenotype, are potent in suppressing immune responses in vitro and in vivo. Using an antigen-specific approach, CCR9+ and CCR9− pDCs were sorted from Flt3L-treated B6 mice and cultured for 2–4 hours with ovalbumin peptide 323–339 (pOVA) prior to i.v. injection into naive B6 mice. Recipient mice were boosted one week later with similar antigen-loaded pDCs and immunized one week after the final boost with pOVA in complete Freund’s adjuvant (CFA). After 10 days, draining lymph nodes were examined for in vitro recall responses to pOVA. Lymphoid populations from mice that initially received CCR9+ pDCs were impaired in their ability to proliferate to pOVA in vitro compared with those mice that had received CCR9− pDCs or no pDCs at all (Fig. 5a).
Figure 5. CCR9+ DCs suppress immune responses in vivo and in vitro.
(a) Sorted CCR9+ or CCR9− pDCs from pooled PLNs isolated from Flt3L-treated B6 mice were pulsed for 2–4 h with OVA peptide prior to i.v. administration of naive C57BL/6 mice. Control mice did not receive pDCs. Recipient mice were boosted 1 week later with the same Ag-loaded pDCs and immunized s.c. one week after the final boost with pOVA emulsified in CFA. After 10 days, cell suspensions from draining lymph nodes were stimulated with a dose range of pOVA for 72 h prior to the addition of 3H-thymidine and subsequent determination of cellular proliferation. Results are expressed as mean c.p.m. and SEM (shown as error bars) of quadruplicate cultures. Data presented are from one of two experiments with similar results. (b) CD4+ T cells MACS-purified from spleens of BALB/c mice were cultured with sorted CCR9+ or CCR9− pDCs from pooled lymph nodes of Flt3L-treated B6 mice at a 5:1 ratio. T cell proliferation to a dose range of pOVA was determined 72 h later by the incorporation of 3H-thymidine for an additional 18 h. Results are expressed as mean cpm of triplicate cultures with error bars denoting the SEM and represent one out of two experiments.
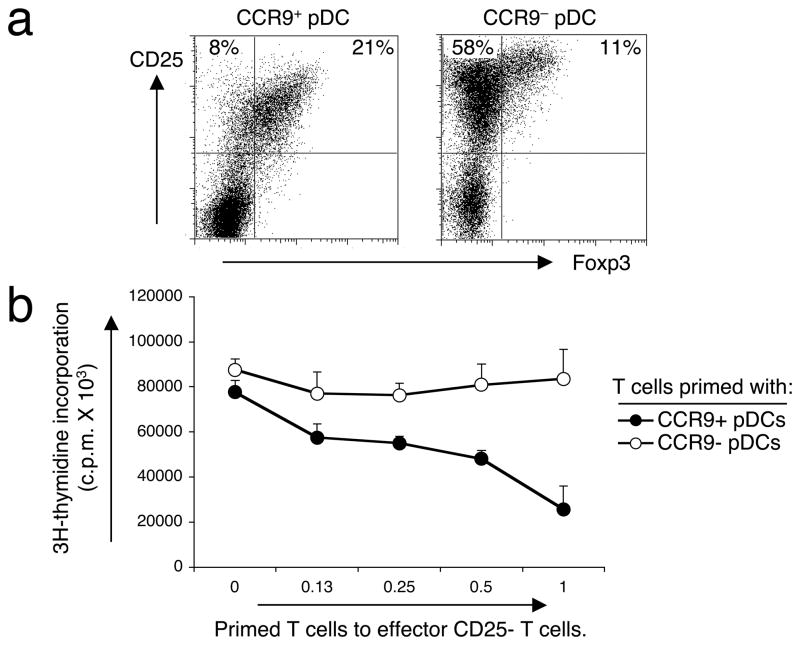
Since pDCs have been shown to play an important role in inducing distinct CD4+ T helper phenotypes15, we wanted to examine the role of CCR9+ DCs in priming T cell responses. We used an in vitro allogeneic stimulation system in which we primed splenic CD4+ T cells from BALB/c mice with sorted CCR9+ and CCR9− pDC subsets from pooled peripheral lymph nodes of Flt3L-treated B6 mice. CCR9+ pDCs failed to support the proliferation of allogeneic T cells (Fig. 5b), in contrast to their CCR9− counterparts. Phenotypic analysis of the cultured T cells showed that CCR9+ pDCs induced fewer activated Foxp3− CD4+ CD25+ T cells than CCR9− pDCs (Fig. 6a). Instead, a higher percentage and a predominant population of Foxp3+ CD4+ CD25+ T cells, which phenotypically resemble regulatory T cells, appeared after 5 days in culture with CCR9+ pDCs compared to cultures with CCR9− pDCs (Fig. 6a). In addition, the CCR9+ pDC-induced T cells suppressed the proliferation of freshly isolated CD4+ CD25− effector T cells in co-culture experiments, whereas T cells primed by the CCR9− DC subset were inefficient at suppressing effector T cell responses (Fig. 6b). Taken together, we propose that the CCR9+ pDC population is the major pDC subset that contributes to T cell tolerance, since these cells induce regulatory T cells, exhibit an immature phenotype and represent almost the entire immature pDC pool in lymphoid tissues.
Figure 6. CCR9+ pDCs are potent inducers of regulatory T cells in vitro.
(a) Splenic CD4+ BALB/c T cells (106) were cultured for 5 days with sorted CCR9+ or CCR9− pDCs (0.2 × 106 cells) from pooled lymph nodes isolated from Flt3L-treated B6 mice. T cell expression of intracellular Foxp3 and cell surface CD25 was determined by flow cytometry. Cells were gated on CD4+ lymphocytes, and the gates were set based on the isotype controls shown for the anti-Foxp3 and anti-CD25 mAbs. One of 2 representative experiments is shown. (b) CD4+ T cells cultured in vitro after 5 days with either CCR9+ pDCs or CCR9− pDCs were added in increasing numbers to 105 freshly isolated BALB/c CD4+ CD25− effector T cells in anti-CD3 and anti-CD28 coated wells. After 48 h, cultures were pulsed with 1 μCi of 3H-thymidine for an additional 18 h, and then harvested. Error bars represent the SEM of triplicate cultures.
CCR9+ DCs suppress acute GVHD
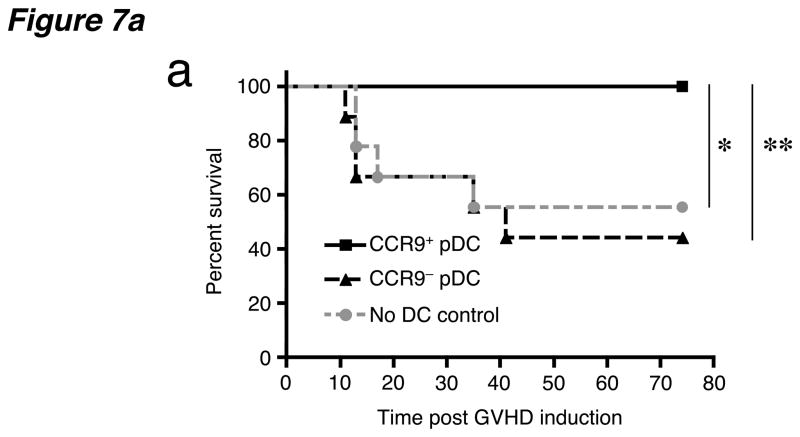
Since CCR9+ pDCs suppress alloresponses in vitro we wanted to examine the effect of CCR9+ DCs in vivo using an animal model of GVHD induced by allogeneic bone marrow transplantation. To determine whether CCR9+ pDCs from Flt3L-treated B6 mice could suppress GVHD induced by CD4+ CD25− BALB/c T cells, we co-injected the two populations at a 1:2 ratio of DCs to T cells along with T-cell depleted BALB/c bone marrow into C57BL/6 hosts within 24 h after lethal total body irradiation (900 rads). All mice that received CD4+ CD25− effector T cells and bone marrow developed clinical signs of GVHD including diarrhea, skin ulcerations and weight loss; approximately 50% died after 5 weeks (Fig. 7a). Comparable results were seen with mice that received CCR9− pDCs together with CD4+ CD25− effector T cells. The addition of CCR9+ pDCs with effector T cells in bone marrow transplanted hosts rescued all the mice from death (100% in two separate experiments: Fig. 7a) and improved clinical symptoms including diarrhea, weight loss and hunched posture.
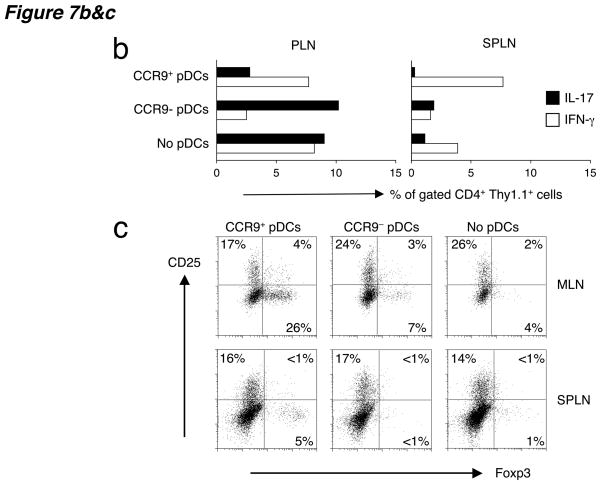
Figure 7. Lethal GVHD of C57BL/6 recipients induced by BALB/c CD4+ CD25− effector T cells can be suppressed by co-injected C57BL/6 CCR9+ DCs.
(a) C57BL/6 mice received 2 × 450 rads of total body irradiation, 2 × 106 BALB/c T-cell depleted bone marrow cells and 0.5–1 × 106 BALB/c splenic CD4+CD25− T cells. Three cohorts of mice received either coinjected sorted CCR9+ pDCs, CCR9+ pDCs or no pDCs at all (no DC control) at 0.2–0.5 × 106 DCs/mouse from pooled peripheral lymph nodes of Flt3L-treated B6 mice. Combined data from two independent experiments are shown, with a total of 9–10 animals per treatment group. The two independent experiments showed similar results with roughly 50% mortality in the absence of protection by CCR9+ pDCs. *, P = 0.027, **, P = 0.010 using the logrank test comparing the survival of allogeneic bone marrow and effector T cell treated mice receiving CCR9+ pDCs versus no pDCs or CCR9+ pDCs versus CCR9− pDCs respectively. (b) Intracellular cytokine staining of splenocytes and peripheral lymph nodes from irradiated C57BL/6 (Thy1.2+) mice three weeks after i.v. transfer of 2 × 106 BALB/c (Thy1.2+) T-cell depleted bone marrow cells, 0.5–1 × 106 splenic Thy1.1+ effector CD4+CD25− T cells from congenic BALB/c.Thy1.1 mice and either no pDCs or sorted CCR9+ or CCR9− pDCs from Flt3L-treated B6 (Thy1.2+) mice. Cells were gated on CD4+ Thy1.1+ effector T cells and each bar represents the percentage of gated cells producing intracellular IL-17 and IFN-γ from 2–3 pooled mice. Data presented are from one of two experiments with similar results. (c) Analysis of the same groups of mice in B for the expression of intracellular foxp3 and surface CD25 from unstimulated splenocytes and MLN cells. Cells were gated on CD4+ Thy1.1+ effector T cells and the gates were set based on the isotype controls for the anti-Foxp3 and anti-CD25 mAbs to include <1% in the positive stained gates. Representative FACS plots from two experiments.
To monitor the effects of CCR9+ DCs on coinjected effector T cells, CD4+ CD25− effector T cells were obtained from congenic BALB/c.Thy1.1 mice. All other mice (that is, irradiated recipients and donor mice for the sorted DC subsets and bone marrow) were Thy1.2+. Three weeks after transfer, Thy1.1+ CD4+ effector T cells from PLNs produced substantial amounts of IL-17 and IFN-γ in GVHD mice that received no pDCs (Fig. 7b) compared to unmanipulated healthy controls (percentages of IL-17- and IFN-γ-producing T cells were <1% in PLNs of untreated controls). Co-injection of CCR9+ pDCs suppressed the frequency of IL-17-producing effector T cells by at least 4-fold, without substantially reducing IFN-γ-producing effector cells (Fig. 7b, left). Since recent studies suggest that the development of TH-17 (IL-17-producing) and TH1 (IFN-γ-producing) cells are antagonistic to each other32,33, co-injected CCR9− pDCs suppressed the appearance of IFN-γ- but not IL-17-producing effector T cells (Fig. 7b, left). IL-17 production in the spleen was less pronounced, but we still observe few IL-17-and many IFN-γ-producing splenic effector T cells after co-transfer of CCR9+ pDCs (Fig. 7b, right). The frequency of cytokine-producing effector T cells was higher in the PLNs and spleen (Fig. 7b) compared to the MLNs (data not shown). Examination of Thy1.1+ CD4+ effector T cells for the regulatory T cell marker Foxp3, revealed an expansion of Foxp3+ CD25− T cells in the MLNs and spleen of recipient mice that received CCR9+ pDCs (Fig. 7c). In contrast, CCR9− pDCs failed to induce Foxp3+ effector T cells similar to the GVHD controls that did not receive DCs. Taken together these results show that CCR9+ DCs are potent suppressors of in vivo alloresponses; reducing the clinical severity of allogeneic GVHD, suppressing effector T cell responses (in particular IL-17 production) and inducing de novo development of Foxp3+ regulatory T cells from effector cells.
Discussion
We have shown that the chemokine receptor CCR9 selectively marks immature pDCs, and that these CCR9+ pDCs are normally present as a resident pDC population in resting secondary lymphoid tissues. CCR9+ DCs underwent maturation by upregulating costimulatory and MHC class II molecules in response to TLR7 and TLR9 but not TLR4 ligands; moreover they produced IFN-α upon TLR activation, confirming their plasmacytoid lineage. Importantly, CCR9 expression was lost upon activation, implying that CCR9 can be used as a reliable marker for immature pDCs. Moreover, we find that the CCR9+, but not CCR9− pDCs potently inhibited immune responses in vivo using an antigen-driven immunization model and an acute GVHD animal model induced by allogeneic bone marrow transplantation. Immune suppression by CCR9+ DCs involved the inhibition of T cell proliferation and inflammatory cytokine production, presumably reflecting the preferential ability of CCR9+ pDC to induce Foxp3+ regulatory T cells. The findings suggest that CCR9 expression defines a physiologically important tolerogenic DC subset, well positioned in lymphoid tissues to participate in homeostatic immune regulation.
What functional relevance might CCR9 have toward the tolerogenic capacity of pDCs? CCR9 expression on pDCs permits their chemotaxis to the CCR9 ligand CCL25, as shown here and in a previous study34. The two principal cell types that express abundant CCL25 in vivo are the small intestinal epithelium and thymic epithelium35. CCR9 mediates migration of intestinal memory T cells and IgA plasma cells to the small intestines, and of T cell precursors to the thymus25,36,37. Thus it is reasonable to postulate that CCR9 might allow tolerogenic DCs to migrate either to the thymus or the gut where they can present peripheral antigens and induce T cell tolerance. Since CCR9 was rapidly downregulated by pDC-specific TLR ligands, activation of CCR9+ pDCs by infectious agents would eliminate their thymic- or gut-specific homing capabilities, ensuring that they would not induce T cell tolerance to foreign antigens in these sites. Consistent with this hypothesis, CCR9 has recently been implicated in pDC localization to the gut wall34. Our findings, however, imply a more widespread distribution and function of CCR9+ pDCs. Previous work has suggested that resting DCs have the capacity to sample tissue-specific antigens and carry them into the thymus where they induce clonal deletion of antigen-specific T cells38. While it would be attractive to propose a critical role for CCR9 on tolerogenic pDCs in this context, in our studies GVHD is mediated by adoptively transferred mature effector T cells, ruling out central tolerance as an important mechanism of GVHD suppression. Thymic generation of regulatory T cells might play a role, but seems unlikely in the timeframe required for suppression of the acute graft-versus-host response. Moreover, others found no role for CCL25 in peripheral DC localization to the thymus38. Taken together, these findings rule out the importance of thymic mechanisms in the tolerogenicity of CCR9+ DCs in our model. Rather our data are more consistent with a mechanism involving CCR9+ pDC-induced development of Foxp3+ regulatory T cells from the mature peripheral T cell pool.
A notable finding in our studies was the long-term suppression of disseminated GVHD by CCR9+ DCs. Previous studies have shown that lethal GVHD is initiated predominantly by alloreactive CD4+ donor T cells39, but that disease can be inhibited by the co-transfer of CD4+ CD25+ regulatory T cells of donor origin40,41. These regulatory T cells have to recognize alloantigens of the recipient in order to mediate their protective effects. In our studies transferred donor CCR9+ DCs are potent inducers of allogeneic Foxp3+ regulatory T cells both in vitro and in vivo. We also see suppressed T cell proliferation in vitro and an altered ratio of IL-17 to IFN-γ production by effector T cells in vivo. Using a lymphopenic T cell-mediated mouse model of systemic autoimmunity that resembles GVHD, IL-17 was shown to be a key mediator of autoimmune pathology whereas IFN-γ had a protective effect42. Furthermore regulatory T cells in this setting were shown to inhibit the accumulation of effector T cells and attenuate disease42. Other studies have also suggested an inhibitory role of IFN-γ in polyclonal models of GVHD43,44 and the cross regulation between TH1 responses and IL-17 production has also been demonstrated in vitro45,46. This result might explain the protective effects of high IFN-γ and low IL-17 production on GVHD seen after co-transfer of tolerogenic CCR9+ pDCs. In summary, our data suggest that donor T cell recognition of host alloantigens on CCR9+ DCs induces regulatory T cells that inhibit the accumulation of IL-17-producing effector T cells and thereby contribute to potent and prolonged disease suppression.
Previous studies have shown that a variety of DC populations can inhibit immune responses2,15 and can display therapeutic effects in animal models of transplantation4 and autoimmunity47–50. While direct comparisons are complicated by the diversity of animal models and DC sources employed, two potentially related features distinguish our studies. First, tolerogenic effects reported previously have been impermanent and generally partial, contrasting with the prolonged GVHD suppression we observe. Second, no previous study has used CCR9, or indeed any rigorous sorting method based on DC activation markers, to purify a tolerogenic subset from immune stimulatory DCs prior to therapeutic transfer. Studies on tolerogenic properties of adoptively transferred mDCs5 or pDCs20,21 have instead employed total DCs generated in vitro from bone marrow cells cultured with GM-CSF or Flt3L, respectively; or entire DC populations expanded in vivo with Flt3L used in our system, GM-CSF or G-CSF13,51. Full tolerance across MHC barriers in these studies has only been achieved with additional interventions such as pharmacologic immunosuppression or antibody treatments to block costimulatory molecules4; and although experimentally quiescent DCs have been employed, these cells were not sorted based on immature phenotype. In contrast, by segregating immature pDCs based on their robust CCR9 expression, we achieved 100% survival of irradiated hosts after transfer of these cells with allogeneic bone marrow- and effector T cells. Transfer of CCR9− pDCs instead, or no pDC transfer at all, resulted in a vigorous alloimmune response and subsequent wasting due to the graft versus host response in the majority of mice. Survival of ~50% of control mice may reflect incomplete myeloablative conditions as seen in other studies40. Our results thus suggest that the suppressive effects of tolerogenic DCs may be counteracted by immune stimulatory effects of mature DCs present in most experimental DC populations. Our studies may also be related to a previous report describing the therapeutic use of in vivo derived CD8α+ CD11c+ ‘lymphoid-related’ DCs, expanded by Flt3L, whereby these cells prolonged allograft survival in adoptive recipients13. Interestingly, CD8α expression has been demonstrated on subsets of mouse pDCs, varying according to tissue source and state of activation18,52,53. We consider that these CD8α+ DCs might comprise CCR9+ as well as CCR9− pDCs, representing therefore a heterogenous population of pDCs ranging from immature to activated cells13. It will be important to compare the efficiency and duration of immune suppression by sorted immature versus mature pDC populations in this and other models in the future.
In conclusion, we have used phenotypic criteria, in particular CCR9 expression, to segregate in vivo-derived tolerogenic pDCs from other DCs; and have shown that this purified subset was remarkably effective at suppressing GVHD. The phenotypic characterization and isolation of tolerance-inducing DC subsets may be of therapeutic benefit in adoptive immunotherapy against a wide range of inflammatory disorders, including autoimmunity, allergic disorders and transplantation.
Methods
Mice
C57BL/6 (CD45.2), congenic CD45.1 (B6.SJL-Ptprca Pep3b/BoyJ) and BALB/cJ mice were purchased from the Jackson Laboratory. BALB/C.Thy1.1 congenic mice were bred in the VMU facility of the Veterans Affairs Palo Alto Health Care Systems (VAPAHCS). Mice were housed under specific pathogen-free conditions and were used according to the guidelines set forth by the animal committee of the VAPAHCS.
Flow Cytometric Analysis
Samples were first incubated with the 2.4.G2 anti-Fc receptor antibody (BD Biosciences) for the DC studies to prevent non-specific mAb binding. The following mAbs were used for staining: B220-PerCP (RA3-6B2), CD11c-PE (HL3), CD3-PECy7 (145-2C11), CD19-PECy7 (1D3), IA/IE-biotin (2G9), IAb-FITC (AF6-120.1) CD25-APC (PC61), CD4-PE (RM4-5), CD4-PerCP-Cy5.5 (RM4-5), Thy1.1-biotin (OX-7) and CD3-PerCP-Cy5.5 (145-2C11) from BD Biosciences and CD40-FITC (HM40-3), CD80-FITC (16-10A1) and CD86-FITC (GL1) from eBioscience. CCR9-APC (242503) was purchased from R&D Systems and used according to the manufacturer’s recommendations. Secondary reagents for the visualization of biotinylated mAbs included Streptavidin-Pacific Blue (Invitrogen).
DC isolation and sorting
DCs were isolated from lymphoid tissues of normal C57BL/6 and BALB/c mice using Collagenase IV (Worthington Biochemical Corp) and DNaseI (Sigma) in protein-free media at a final concentration of 2 mg/ml and 1U/ml for 1–2 h at 37 °C. Tissues were resuspended and passed over a wire mesh, washed, enumerated and stained with conjugated mAbs. For the isolation of Flt3L-expanded DCs, C57BL/6 mice were injected subcutaneously with 5 × 106 Flt3L-secreting B16 melanoma cells that promote the expansion of DCs in vivo54. After 14 days designated lymph nodes were isolated and passed through a 70-micron nylon mesh. For sorting pure populations of CCR9+ and CCR9− pDCs, the cells were first enriched using CD11c microbeads (Miltenyi) followed by sorting Lin− (CD3−CD19−) CD11cint B220+ cells based on their CCR9 expression.
Chemotaxis Assays
Pooled lymph node cell suspensions from C57BL/6 mice transplanted with Flt3L-secreting B16 melanoma cells, were resuspended in 100 μl of complete RPMI-1640 medium and loaded into collagen-coated Transwells (Corning 3421; 5 μm pore size) that were placed in 24-well plates containing 600 μl medium or medium supplemented with 250–500 nM of CCL25, 100 nM CCL21, or 50 nM CXCL12(R&D Systems). After 2 h of incubation at 37 °C, a constant number of Polysyrene beads (Polysciences, Inc.) were added to each sample to control for recovery of cells from different wells. The migrated cells were collected, counted, and stained with mAb to determine the number of migrated pDC andmDC by flow cytometry. The ratio of the number of pDC that migrated in the presenceof chemokine vs. the number of cells that migrated to control mediawas calculated and is given as the percentage of migrated cells relative to the input.
DC stimulation with TLR ligands
0.2–0.5 × 106 MACS-purified and CCR9 sorted pDCs from pooled peripheral lymph nodes of Flt3L-treated B6 mice were cultured in 200 μl of complete RPMI 1640 medium supplemented with 10% FCS for 8–12 h in the absence or presence of LPS (1 ng/ml), R848 (10 μg/ml) and ODN1826 CpG (1 μM) (Invivogen). Following stimulation, DCs were stained for their expression of MHC class II (IA/IE) and CD80, CD86 or CD40.
Intracellular Foxp3 and cytokine assays
Single cell suspensions of lymph node cells and RBC-free splenocytes were stimulated in vitro at 37 °C for 4 h with 5 ng/ml of Phorbol Myristate Acetate (PMA) (Sigma) and 1 μg/ml of ionomycin (Sigma). Brefeldin A (eBioscience) was added 2 h after the addition of PMA-ionomycin to a final concentration of 1 μg/ml. Cells were harvested, and stained for surface CD4-PerCPCy5.5 and Thy1.1-biotin followed by the secondary reagent Streptavidin-Pacific Blue (Invitrogen). For the visualization of Foxp3, cells were not stimulated. Following surface staining, cells were washed, fixed and permeabilized according to the manufacturer’s recommendation (eBioscience). Cells were then stained in permeabilization buffer (eBioscience) with fluorochrome labeled mAbs for: IFN-γ-FITC (XMG1.2) (eBioscience), IL-17-PE (TC11-18H10) (BD Bioscience,) and IL-10-APC (JES5-16E3) (BD Bioscience) or Foxp3-FITC (FJK-16s) (eBioscience) for the visualization of regulatory T cells. Cells were washed in permeabilization buffer and resuspended in staining buffer for analysis on the flow cytometer. Supernatants from overnight (16 h) pDC cultures stimulated with TLR ligands were examined for the presence of IFN-α using a standardized kit (PBL Biomedical Laboratories) or TNF-α using Luminex Bead Technology with a standardized kit (Millipore).
In vitro T cell stimulation and suppressor T cell assays
CD4+ T cells were enriched from spleens of BALB/c mice using the CD4+ T cell isolation kit (Miltenyi) and cultured with CCR9+ and CCR9− pDCs, sorted from pooled lymph nodes of Flt3L-treated B6 mice, at a 5:1 ratio. To determine T cell proliferation, cultures were setup in 96-well flat-bottom microtiter plates using 2 × 105 sorted T cells and 0.4 × 105 DCs and stimulated with a dose range of pOVA for 3 days prior to the addition of 3H-thymidine (1 μCi/well). After a further 18 h, cultures were harvested and 3H-thymidine incorporation measured using a liquid scintillation β-counter (Wallac). Results are expressed as mean cpm of triplicate cultures. For T cell suppression assays, cultures were setup using larger numbers of sorted T cells (5 × 106) and DCs (106) for 5–7 days. Aliquots of T cells were analyzed for CD25 and Foxp3 expression as previously described. The remaining cells were cocultured with CD4+CD25− effector T cells isolated from spleens of BALB/c mice by negative selection over LD columns (Miltenyi) using the CD4+CD25+ regulatory T cell isolation kit (Miltenyi). Cultures were set up for 48 h in 96-well plates coated with anti-CD3 (3 μg/ml, 2C11 clone) (eBioscience) and anti-CD28 (3 μg/ml, 37.51 clone) (eBioscience) prior to the addition of 3H-thymidine and subsequent determination of T cell proliferation. Results are expressed as mean cpm of triplicate cultures.
Adoptive transfer assays and immunizations
CCR9+ and CCR9− pDCs were sorted from pooled lymph node cells from Flt3L-treated B6 mice and cultured for 2–4 h with 50 μM of ovalbumin peptide 323–339 (pOVA) prior to i.v. administration (0.5 × 106 DCs/mouse) to naive C57BL/6 mice. Recipient mice were boosted 1 week later with the same Ag-loaded pDCs (0.5 × 106 DCs/mouse) and immunized s.c. one week after the final boost with 20 μg pOVA emulsified in CFA (Sigma). After 10 days, cell suspensions from draining lymph nodes were stimulated with a dose range of pOVA for 72 hrs in 96-well plates at 0.5 × 106 LN cells/well prior to the addition of 3H-thymidine and subsequent determination of cellular proliferation. Results are expressed as mean cpm of quadruplicate cultures.
GVHD model
C57BL/6 hosts were given total body irradiation two times from a 131Cs source, 4 h apart at 450 rads per dose for a cumulative dose of 900 rads. Irradiated mice were injected with donor cells i.v. within 24 h. All mice received 2 × 106 T-cell depleted (TCD) bone marrow with 0.5–1 × 106 splenic CD4+ CD25− donor T cells both from BALB/c mice. Bone marrow T cells were depleted using anti-Thy1.2 microbeads followed by negative selection through LD columns (Miltenyi). CD4+ CD25− effector T cells were enriched using the CD4+ CD25+ regulatory T cell isolation kit (Miltenyi) by purifying total CD4+ T cells, followed by negative selection of CD4+ CD25+ T cells through LD columns (Miltenyi). MACS bead enrichment of CD4+ CD25− T cells and TCD bone marrow resulted in >99 % elimination of potential CD4+ CD25+ regulatory and CD3+ effector T cells respectively. Designated groups received in addition to T cells and bone marrow, 0.2–0.5 × 106 sorted CCR9+ or CCR9− pDCs from pooled lymph nodes of Flt3L-treated B6 mice. In studies involving the analysis of effector T cells post-transfer, CD4+ CD25− effector T cells were isolated from BALB/c.Thy1.1 congenic mice. Mice were kept on antibiotic water for the first month. The survival and appearance of mice were monitored daily and body weight was measured weekly. For the analysis of effector T cell responses in vivo, mice were evaluated at d10, d20 and d30 for cytokine production and regulatory T cell induction in lymphoid tissues.
Statistical Analysis
Data are presented as mean values ± standard error of the mean (SEM) unless otherwise indicated. Statistical significance between sets of data was assessed using the two-tailed unpaired Student’s t-test for comparison of two groups. Significance between survival curves of different groups in the GVHD studies were assessed using the logrank test. P-values <0.05 were considered statistically significant.
Acknowledgments
We thank L. Rott for assistance with flow cytometry and cell sorting, M. BenBarak for assistance with cytokine determination using Luminex technology and B. Zabel for helpful discussions. H. Hadeiba is a recipient of an Investigator Career Award from the Arthritis Foundation and was a fellow under the National Institutes of Health Training Grant AI07290. The work was supported in part by NIH grants and by a Merit Award from the Veterans Administration to ECB.
Footnotes
Author Contributions
H.H. designed and performed most of the experiments and wrote the manuscript; E.C.B. designed experiments and wrote the manuscript; T.S. prepared the irradiated mice, bone marrow and effector T cells in the GVHD studies and monitored the mice; A.H. was involved in the initial microarray studies and flow cytometry characterization of pDCs; C.O. assisted with the TLR-activated pDC cytokine assays and J.P. helped with the RNA preparation and microarray data analysis; all authors discussed the results, read and provided comments on the manuscript.
References
- 1.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology. 2006;211:627–632. doi: 10.1016/j.imbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 5.Charbonnier LM, et al. Immature dendritic cells suppress collagen-induced arthritis by in vivo expansion of CD49b+ regulatory T cells. J Immunol. 2006;177:3806–3813. doi: 10.4049/jimmunol.177.6.3806. [DOI] [PubMed] [Google Scholar]
- 6.Fu F, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–665. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastellini C, et al. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60:1366–1370. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, et al. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166:3499–3505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Kim S, Oligino TJ, Robbins PD. Effective treatment of established mouse collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express FasL. Mol Ther. 2002;6:584–590. [PubMed] [Google Scholar]
- 10.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 11.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell PJ, et al. Immature and mature CD8alpha+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002;168:143–154. doi: 10.4049/jimmunol.168.1.143. [DOI] [PubMed] [Google Scholar]
- 14.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 15.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 16.Kuwana M, Kaburaki J, Wright TM, Kawakami Y, Ikeda Y. Induction of antigen-specific human CD4(+) T cell anergy by peripheral blood DC2 precursors. Eur J Immunol. 2001;31:2547–2557. doi: 10.1002/1521-4141(200109)31:9<2547::aid-immu2547>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Martin P, et al. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 18.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–492. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arpinati M, et al. Role of plasmacytoid dendritic cells in immunity and tolerance after allogeneic hematopoietic stem cell transplantation. Transpl Immunol. 2003;11:345–356. doi: 10.1016/S0966-3274(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 20.Fugier-Vivier IJ, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201:373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 22.Kohrgruber N, et al. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol. 1999;163:3250–3259. [PubMed] [Google Scholar]
- 23.Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum Immunol. 2002;63:1156–1163. doi: 10.1016/s0198-8859(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 26.Salmi M, Jalkanen S. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev. 2005;206:100–113. doi: 10.1111/j.0105-2896.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 27.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilliet M, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanbervliet B, et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–830. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caux C, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–369. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 31.Dieu MC, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Wendland M, et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wurbel MA, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Zabel BA, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pabst O, et al. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–416. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonasio R, et al. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 39.Zeng D, et al. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.v99.4.1449. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy WJ, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 46.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morel PA, Vasquez AC, Feili-Hariri M. Immunobiology of DC in NOD mice. J Leukoc Biol. 1999;66:276–280. doi: 10.1002/jlb.66.2.276. [DOI] [PubMed] [Google Scholar]
- 48.Feili-Hariri M, et al. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes. 1999;48:2300–2308. doi: 10.2337/diabetes.48.12.2300. [DOI] [PubMed] [Google Scholar]
- 49.Popov I, et al. Preventing autoimmune arthritis using antigen-specific immature dendritic cells: a novel tolerogenic vaccine. Arthritis Res Ther. 2006;8:R141. doi: 10.1186/ar2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hochweller K, Anderton SM. Systemic administration of antigen-loaded CD40-deficient dendritic cells mimics soluble antigen administration. Eur J Immunol. 2004;34:990–998. doi: 10.1002/eji.200324782. [DOI] [PubMed] [Google Scholar]
- 51.Maraskovsky E, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 53.O’Keeffe M, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mach N, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]